Abstract
Background: The use of needle core biopsy (NCB) as part of triple assessment for non‐operative evaluation and diagnosis of breast lesions is now routine practice. Trauma to breast tissue during NCB may result in displacement of breast epithelium and may lead to diagnostic difficulty in subsequent excision specimens.
Methods: The cases of seven mammographically detected breast lesions in which epithelial displacement due to NCB was identified and caused problems in confirmation of tumour size, assessment of surgical margins, and interpretation of possible invasive carcinoma and lymphovascular invasion are reported here.
Conclusion: Previous observations that epithelial displacement is more likely to occur when the interval between NCB and surgical excision is short are supported.
The use of needle core biopsy (NCB) as part of triple assessment for non‐operative evaluation and diagnosis of breast lesions is now standard of care. This offers significant advantages to the patient, obviating the need for open diagnostic biopsy and permitting treatment planning with the patient before surgery. It is recognised that trauma to breast tissue during the NCB procedure may result in displacement of breast epithelium1 and may lead to diagnostic difficulty in subsequent excision specimens. Douglas‐Jones and Verghese, reporting in the Journal of Clinical Pathology, described diagnostic difficulties in relation to intracystic papillary carcinoma following NCB.2 In another study, displaced carcinomatous fragments were identified in 28% of breast excision specimens following 14‐guage NCB.3
We report our experience of diagnostic uncertainty caused by the presence of epithelial cells within the NCB tract in a series of seven patients with mammographically detected breast lesions, categorised according to the R‐coding classification system (R1: negative mammogram, R2: benign changes, R3: probably benign, short follow‐up advised, R4: suspicious lesion, biopsy advised, R5: highly suggestive of malignancy, appropriate action advised).4 In accordance with our patient charter, we aim to offer all patients definitive surgery within 21 days of diagnosis. We explore the possibility that a short time interval between NCB and definitive surgery may be an important contributory factor to the presence of displaced epithelium.
Case studies
Case 1
A diagnosis of radial scar with associated ductal carcinoma in situ (DCIS) was established on ultrasound‐guided 14‐gauge NCB in a 58‐year‐old woman who had an irregular code 5 spiculate lesion in the right breast on screening mammogram. At 16 days after NCB, the patient underwent wide local excision (WLE) of the right breast (76 g). Histological examination showed the presence of a radial scar/complex sclerosing lesion with associated cysts, sclerosing adenosis, epithelial hyperplasia and apocrine adenosis. High‐grade DCIS was evident within the lesion and in the surrounding breast tissue, extending over 3 cm. A collection of malignant epithelial cells (2 mm) was present within the lesion, within granulation tissue interpreted as the NCB tract. Cytokeratin studies confirmed the presence of epithelial cells arranged singly and in very small groups. It was considered not possible to confidently distinguish between epithelial displacement caused by NCB and a small focus of invasion. The diagnostic uncertainty was explained to the patient who subsequently underwent sentinel lymph node biopsy (SLNB), which was negative on multiple H&E levels and on cytokeratin immunohistochemistry (CK IHC).
Case 2
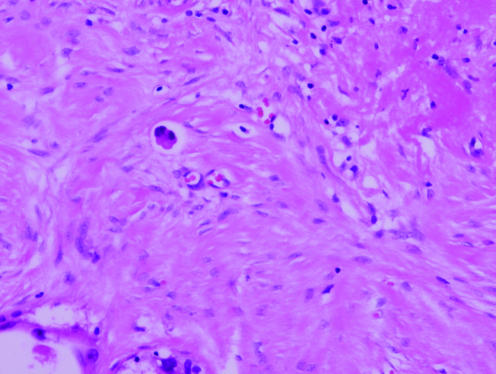
A 64‐year‐old woman underwent a non‐diagnostic 14‐gauge stereotactic NCB for evaluation of screen‐detected code 4 calcification in her right breast. At 18 days after NCB, a right needle localised diagnostic excision biopsy (25.5 g) was performed. Histological examination revealed a 4 cm area of intermediate grade DCIS, micropapillary and cribriform, with necrosis and calcification. A few clusters of malignant epithelial cells were identified in the centre of the specimen, in an area showing florid post‐NCB change (fig 1). The cells were not adjacent to ducts affected by DCIS, and there was no evidence of microinvasion or invasion elsewhere in the specimen. The changes were interpreted as epithelial displacement.
Figure 1 Cluster of malignant epithelial cells within an area of florid post‐needle core biopsy change, interpreted as displaced epithelium (case 2).
Case 3
A 66‐year‐old woman had a code 5 lesion in the right breast on screening mammogram. Stereotactic 14‐gauge NCB was performed on two occasions, which revealed non‐specific benign breast change. In view of the radiological concern, a right needle localised diagnostic excision biopsy (36 g) was carried out 9 days after the second NCB. An area of firm white tissue was identified macroscopically corresponding to a variety of benign changes microscopically, including florid epithelial hyperplasia, cyst formation with apocrine metaplasia and papillomatosis. Detached epithelial cells were identified within the NCB tract. These were cytologically bland and, considering the absence of atypia or malignant change within the specimen, were interpreted as displaced epithelium.
Case 4
A diagnosis of invasive ductal carcinoma (IDC) was established on stereotactic 14‐gauge right NCB in a 52‐year‐old woman who had a radiological code 5 lesion in her right breast on screening mammogram. At 21 days after NCB, the patient underwent right WLE (180 g) and SLNB. Histological examination revealed a circumscribed 2.7 cm IDC with accompanying high‐grade DCIS and lymphovascular invasion. Two sentinel lymph nodes were present, both of which were positive for metastatic carcinoma. The discrete tumour was clear of all radial margins by at least 1 cm, but small collections of tumour cells were identified within the NCB tract, 1.5 cm from the main tumour mass and 4 mm from the inferior margin. In view of the fact that the cells were clearly within the NCB tract and separate from the main tumour that was well circumscribed, the cells were interpreted as displaced tumour cells rather than tumour foci, and re‐excision of the margin was not performed.
Case 5
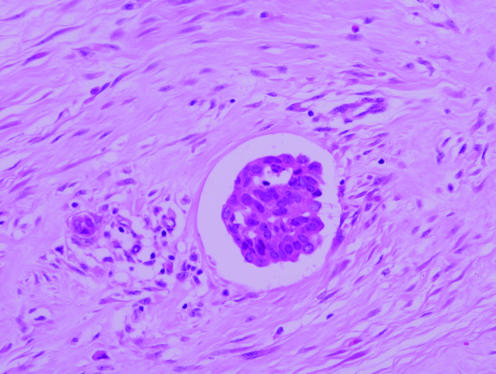
A diagnosis of DCIS was established on stereotactic 14‐gauge NCB in a 52‐year‐old woman who underwent screen‐detected code 5 calcification in her right breast. At 23 days after NCB, a right mastectomy and SLNB were performed. Histological examination revealed high‐grade DCIS, solid and cribriform, with necrosis and calcification, extending over 5 cm and clear of all margins by 1 cm. There was cancerisation of lobules but no diagnostic evidence of invasion. Focally, ducts involved by DCIS were disrupted and a group of malignant epithelial cells was noted in a space, with appearances suggestive of lymphovascular invasion (fig 2). There was post‐NCB change in the tissue surrounding the space, and immunohistochemical vascular markers were negative. The cluster of malignant cells was interpreted as displaced epithelium. The SLNB was negative on multiple H&E levels and on CK IHC.
Figure 2 Malignant epithelial cells within an area of florid needle core biopsy in a space suspicious for lymphovascular invasion, interpreted as displaced epithelium (case 5).
Case 6
A diagnosis of IDC was established on left 14‐gauge NCB in a 62‐year‐old female with code 5 calcification in her left breast on screening mammogram. At 19 days after NCB, a left WLE and SLNB were performed. Histological examination revealed a grade 2, IDC with associated intermediate grade DCIS, solid and cribriform. The invasive tumour measured 13 mm. However, nests of tumour cells were identified outside the main lesion within an area of prominent NCB change. These cells were not in continuity with the main tumour, which was well‐circumscribed. The tumour cells were interpreted as displaced epithelial cells which had been implanted in the NCB tract. Had these cells been deemed to represent tumour extension, the estimate of invasive tumour size would have increased by 2 mm, to 15 mm. SLNB was negative.
Case 7
A diagnosis of IDC with accompanying DCIS was established on 14‐gauge NCB in a 59‐year‐old woman who had code 5 calcification in her left breast on screening mammogram. At 8 days after NCB, a left WLE (82 g) and SLNB were performed. Histological examination revealed a predominantly well‐circumscribed, grade 2 IDC, with accompanying DCIS with a micropapillary architecture focally. The tumour was partly disrupted peripherally, and there was prominent post‐NCB change. Small groups of tumour cells were identified within the NCB tract, in continuity with the main tumour. The proximity of the cell groups to the tumour and the focal micropapillary architecture of the tumour rendered interpretation difficult. Ultimately, the cells were interpreted as an extension of the main lesion, increasing invasive tumour size by 3 mm, from 15 mm to 18 mm.
Owing to extensive accompanying DCIS and compromised surgical margins, a completion left mastectomy was performed, which contained foci of residual DCIS. SLNB showed a micrometastasis which was identified on both H&E and CK IHC. A subsequent axillary lymph node clearance yielded 27 negative lymph nodes.
Discussion
We have outlined a series of seven cases that illustrate the diagnostic difficulties and potential management problems associated with interpretation of epithelial cells within NCB tracts. Current thinking suggests that this finding represents a biologically non‐significant displacement phenomenon in which benign or malignant epithelial cells become dislodged and implanted along the line of the needle during the NCB procedure.5 Interpretative difficulties in pathology excision specimens are frequent and may affect the assessment of biological parameters, including tumour size, the presence of invasion, lymphovascular invasion and compromised surgical margins with consequent management implications. Recognition of displaced epithelial cells is assisted by awareness of a previous NCB procedure and appreciation of the typical post‐NCB histological changes including haemorrhage, granulation tissue, fat necrosis with accumulation of vacuolated macrophages, acute and chronic inflammation, and haemosiderin‐laden macrophages.6
Invasive tumour size is a powerful prognostic parameter, a component of TNM staging, a determinant of patient treatment and a surrogate marker for the efficacy of mammographic screening. Despite detailed guidelines,7 external quality assurance schemes and observer studies8 have demonstrated huge interobserver variation in assessment of tumour size. The reasons for this are not clear and are likely to be multifactorial. We illustrate two cases (cases 6 and 7) in which estimation of invasive tumour size was complicated by the presence of collections of tumour cells outside the main lesion. In case 6, the collection of cells appeared to be clearly within the NCB tract and separate from the main tumour that was well circumscribed, facilitating an interpretation of displaced tumour cells. In case 7, the cells were in continuity with the main lesion and, although associated with NCB change, were ultimately assessed as an extension of the tumour. This problem has been highlighted by Youngson et al,3 who identified displaced carcinomatous fragments outside the main tumour mass in 12 of 43 cases (28%), following needling procedures. This is a notably high incidence and the authors emphasise that the findings may have been influenced by additional needling procedures including administration of local anaesthetic using a 25‐gauge needle.
Lymphovascular invasion is an independent prognostic indicator in breast cancer, and its presence may influence the decision to offer chemotherapy to patients who are lymph node negative. Consistency in pathology reporting of lymphovascular invasion is important, but is hampered by difficulties in evaluating retraction artefact and epithelial displacement. We illustrate one case (case 5) in which clusters of displaced epithelium mimicked lymphovascular invasion in a patient with DCIS. There was extensive core biopsy change in the area, and the interpretation of the findings as displaced epithelium was further assisted by the fact that the lesion had been thoroughly sampled and showed DCIS only with no evidence of invasion. Fragments of breast epithelium in artefactual spaces or in lymphovascular channels, when associated with evidence of a needle tract, should be interpreted as displaced epithelium.3
The diagnosis of microinvasion and early invasive carcinoma in DCIS is often difficult, but may be further complicated by the presence of displaced epithelium.4 Pathological features that argue against invasion include the presence of epithelial fragments in artefactual spaces, NCB change and the absence of a desmoplastic reaction.9 Youngson et al1 caution that a malignant diagnosis should not be based solely on the presence of epithelial cells within stroma since epithelial displacement has been observed following needling procedures in benign breast lesions, notably papillomatosis and intraduct papilloma. We have also encountered this problem as illustrated in case 3.
The presence of tumour cells at or near a surgical resection margin may lead to the decision to re‐excise that margin or to treat with radiotherapy, and the misinterpretation of displaced epithelium as invasive tumour may have significant consequences. We illustrate one case (case 4) in which clusters of cytologically malignant cells were identified within 5 mm of a radial resection margin, while the main tumour was 10 mm from this margin. These cell clusters were clearly located in the NCB tract and were interpreted as displaced epithelium. No further surgery was undertaken.
Histological changes induced by needling procedures depend on the time interval between the NCB and definitive surgery. In our study, the average time interval between NCB and surgery was 15.9 days (range = 8–23). Displaced epithelium is most likely to occur when the interval is <10 days, and may be observed in up to 30% of excision specimens. The incidence of epithelial cell displacement in excision specimens decreases to 10% when the interval exceeds 28 days.6 These observations suggest that tumour cells do not ultimately survive displacement, and that it may be pertinent to take into consideration the time interval between NCB and surgical excision when making the decision to interpret clusters of tumour cells as displaced epithelium.
The biological significance of epithelial displacement following needling procedures remains unclear and there are little data to address this issue. A popular current view is that this represents a benign phenomenon supported by the observation that epithelial displacement is more common when definitive surgery is carried out soon after NCB, suggesting that the cells do not survive long term. However, sporadic cases of metastases due to tumour seeding following needling procedures have been postulated.10 Youngson et al3 suggest that tumour cell dissemination following needling procedures represents a theoretical risk, that the potential viability of these cells is not known and that further long‐term follow‐up is required.
A similar controversy surrounds the finding of small clusters of epithelial cells in the subcapsular sinuses of sentinel lymph nodes.11 The relative infrequency of epithelial cells in the sentinel lymph nodes of patients undergoing prophylactic mastectomy suggests that this may not be a random event.12 It is generally held that epithelial cells in the sentinel lymph node result from displacement of tumour cells during NCB or breast massage during the SLNB procedure.13,14 The current TNM International Union Against Cancer classification system15 advises that these so‐called isolated tumour cells be classified as lymph node negative. Hansen et al16 observed an increase in sentinel lymph node metastases following manipulation of an intact tumour by diagnostic needling procedures. However, this study predates the most recent TNM classification,15 and the lymph node tumour cells have not been subcategorised into micrometastases and isolated tumour cells. In case 7 of our own series, there was evidence of displaced epithelium within the WLE specimen with partial disruption of tumour. SLNB contained a deposit of tumour cells, the size and characteristics of which exceeded the definition of isolated tumour cells. The deposit was considered to represent a micrometastasis and the patient was regarded as lymph node positive.
Conclusion
Epithelial displacement following NCB of breast, although likely to be biologically insignificant, is an important issue that may lead to overstaging of tumours, overdiagnosis of microinvasion/early invasive carcinoma in DCIS, lymphovascular invasion, compromised surgical resection margins and ultimately to overtreatment of patients. It is important that pathologists are aware of this phenomenon and that care is taken in the interpretation of clusters of epithelial cells outside the main lesion. It is vital that all clinical information is available to the pathologist. In particular, the time interval between NCB and surgical excision appears to be relevant and this information may be helpful in difficult cases.
Take‐home messages
Epithelial displacement may occur during needle core biopsy (NCB) and is more likely to cause diagnostic difficulty in excision specimens when the time interval between NCB and surgery is short.
Misinterpretation of epithelial displacement may lead to overstaging of tumours, over‐diagnosis of invasive carcinoma and lymphovascular invasion, and false positive surgical margins.
The use of immunohistochemical vascular markers may assist the identification of true lymphovascular invasion.
Awareness of the phenomenon of epithelial displacement and appreciation of the typical histological features of post‐NCB change facilitate accurate recognition of epithelial displacement in histological sections.
Abbreviations
CK IHC - cytokeratin immunohistochemistry
DCIS - ductal carcinoma in situ
IDC - invasive ductal carcinoma
NCB - needle core biopsy
SLNB - sentinel lymph node biopsy
WLE - wide local excision
Footnotes
Competing interests: None.
References
- 1.Youngson B J, Cranor M, Rosen P P. Epithelial displacement in surgical breast specimens following needling procedures. Am J Surg Pathol 199418896–903. [DOI] [PubMed] [Google Scholar]
- 2.Douglas‐Jones A G, Verghese A. Diagnostic difficulty arising from displaced epithelium after core biopsy in intracystic papillary lesions of the breast. J Clin Pathol 200255780–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Youngson B J, Liberman L, Rosen P P. Displacement of carcinomatous epithelium in surgical breast specimens following stereotaxic core biopsy. Am J Clin Pathol 1995103598–602. [DOI] [PubMed] [Google Scholar]
- 4.European guidelines for quality assurance in mammography screening 3rd edn 2001149
- 5.Tavassoli F A, Pestaner J P. Pseudoinvasion in intraductal carcinoma. Mod Pathol 19958380–383. [PubMed] [Google Scholar]
- 6.Tardivon A A, Guinebretiere J M, Dromain C.et al Histological findings in surgical specimens after core biopsy of the breast. Eur J Radiol 20024240–45. [DOI] [PubMed] [Google Scholar]
- 7.The Royal College of Pathologists Minimum dataset for breast cancer histopathology report. London: The Royal College of Pathologists, 1998
- 8.Sloane J P, Ellman R, Anderson T J.et al Consistency of histopathological reporting of breast lesions detected by screening. Findings of the U.K. National External Quality Assessment (EQA) scheme. U.K. National Co‐ordinating Group for Breast Screening Pathology. Eur J Cancer 199430A1414–1419. [DOI] [PubMed] [Google Scholar]
- 9.Diaz N, Rigberg Mayes J, Vrcel V. Breast epithelial cells in dermal angiolymphatic spaces: a manifestation of benign mechanical transport. Hum Pathol 200536310–313. [DOI] [PubMed] [Google Scholar]
- 10.Glaser K S, Weger A R, Schmid K W.et al Is fine needle aspiration of tumours harmless? Lancet. 1987;1: 620 [letter], [DOI] [PubMed]
- 11.Moore K H, Thaler H T, Tan L K.et al Immunohistochemically detected tumour cells in the sentinel lymph nodes of patients with breast carcinoma. Biological metastasis or procedural artefact? Cancer 2004100929–934. [DOI] [PubMed] [Google Scholar]
- 12.King T A, Ganaraj A, Fay J F.et al Cytokeratin‐positive cells in sentinel lymph nodes in breast cancer are not random events. Experience in patients undergoing prophylactic mastectomy. Cancer 2004101926–933. [DOI] [PubMed] [Google Scholar]
- 13.Rosser R J. A point of view: trauma is the cause of occult micrometastatic breast cancer in sentinel axillary lymph nodes. Breast J 20006209–212. [DOI] [PubMed] [Google Scholar]
- 14.Carter B A, Jensen R A, Simpson J F.et al Benign transport of breast epithelium into axillary lymph nodes after biopsy. Am J Clin Pathol 2000113259–265. [DOI] [PubMed] [Google Scholar]
- 15.International Union Against Cancer (UICC) Breast tumours. In: Sobin LH, Wittekind Ch, eds. TNM classification of malignant tumours. 6th edn. New York: Wiley‐Liss, 1998131–142.
- 16.Hansen N M, Ye X, Grube B J.et al Manipulation of the primary breast tumour and the incidence of sentinel node metastases from invasive breast cancer. Arch Surg 2004139634–640. [DOI] [PubMed] [Google Scholar]