Abstract
Background
Advanced glycation end products (AGEs) are a heterogeneous group of glycosylated proteins (of which carboxymethyl‐lysine (CML) is the most common) which accumulate during ageing processes and play an important role in the pathogenesis of a variety of chronic diseases. Impaired hepatic function might result in elevated levels of AGEs, as the liver represents the major site of AGE metabolism. The actions of AGEs are mediated by various receptors, among which the AGE‐receptor complex (including galectin‐3 as an essential part) is thought to have a cytoprotective effect, and receptor for advanced glycation end product (RAGE) a cytotoxic effect.
Aim
To assess the relationship between CML and expression of galectin‐3 and RAGE in different histological structures in biopsy specimens from patients with varying degrees of liver impairment.
Method
Immunohistochemical staining of 164 biopsies from patients with varying degrees of liver impairment was performed to determine the levels of CML, galectin‐3 and RAGE in hepatocytes, Kupffer cells and bile ducts by a semiquantative score.
Results
Independent of diagnosis, CML and RAGEs were detected in hepatocytes, whereas galectin‐3 was present only in hepatocytes of cirrhotics. By contrast, CML and galectin‐3 were highly expressed in Kupffer cells (well correlating levels, highest scores in cholestasis) whereas expression of RAGEs was not significant. All three assessed biochemical markers showed their highest levels of expression/detection in bile ducts.
Conclusion
These findings indicate an increased susceptibility of hepatocytes to the detrimental effects of AGEs and underline the protective function of Kupffer cells. Furthermore, the biliary system seems to play an important role in the disposition of AGEs.
Advanced glycation end products (AGEs) are a diverse class of glycosylated proteins which accumulate during ageing processes.1 The formation of AGEs begins with a Maillard reaction, in which sugars (eg, glucose, ribose, fructose) are reduced with amino residues of proteins to form Schiff bases and later, after undergoing the Amadori process, more stable ketoamines (Amadori products). Through further modification of these early products, they convert into non‐reversible derivatives called AGEs.2
Since their discovery, the number of known AGEs has been growing. Prominent representatives are Nε‐carboxymethyl‐lysine (CML), pyrraline, pentosidine and imidazolone, of which CML was shown to be the most common in vivo.3,4,5,6,7,8 Increased AGE levels are found in individuals with diabetes, chronic renal failure and liver cirrhosis.9 Dietary intake and smoking seem to play a relevant role as exogenous contributors to AGE‐levels.10,11,12 Strong evidence suggests that AGEs are a major factor in the pathogenesis of a variety of chronic diseases like diabetic nephro‐/retino‐ and neuropathy, cataract, atherosclerosis, β2‐microglobulin amyloidosis and Alzheimer's disease.13
These pathogenic effects are on one hand due to trapping/crosslinking of macromolecules, and involve on the other hand binding of AGEs to cell surface receptors. Known receptors for AGEs are RAGE, macrophage scavenger receptors classes A and B, AGE‐R1 (OST‐48), AGE‐R2 (80K‐H) and AGE‐R3 (galectin‐3).15,16,17,18 These multi‐functional/‐ligand receptors are upregulated by elevated AGElevels and mediate different biological effects. RAGE, for example, seems to trigger a proinflammatory cellular activation via NF‐κB, whereas macrophage scavenger receptors lead to AGE degradation.19
AGE‐R3 or galectin‐3, a multifunctional protein of the carbohydrate‐binding lectin family, plays a crucial role in an AGE‐receptor complex (consisting of AGE R1–R3) which mediates protection towards AGE‐induced tissue injury.18,19 This effect seems to be accomplished through removal of AGEs by endocytosis and following degradation.19,20 Galectin‐3 expression has been found in activated macrophages, eosinophils, neutrophils, mast cells, epithelium of gastrointestinal and respiratory tracts, some sensory neurones and many malignant tumours.21
Interestingly, galectin‐3 was also reported to be expressed in hepatic tissue, and the liver has been identified as the main site of metabolism of circulating AGEs.22,23 Furthermore, AGE‐R1 (OST‐48) and AGE‐R2 (80K‐H), both membrane proteins and co‐players of galectin‐3 in the AGE‐receptor complex, have been isolated from rat liver tissue.17 As mentioned above, impaired liver function has already been shown to lead to higher levels of AGEs in patients with liver cirrhosis.9
This study focused on the presence of CML and the expression of galectin‐3 and RAGE in specific cell types and histological structures of human liver biopsy specimens from patients with varying degrees of hepatic impairment. The aim was to further characterise the relationships between these biochemical entities and medical/histological diagnoses by immunohistochemistry.
Methods
Patients and liver specimens
A total of 164 liver specimens were obtained from the department of pathology of the Robert Bosch Hospital (patients with varying diagnoses connected to liver impairment) and from the Institute of Forensic Medicine, Tübingen (cases of suicide, control group). All control specimens were taken from subjects with normal hepatic function who had undergone sudden deaths, either from natural causes or due to traumas.
The computerised database contained information about the age of the patients, sex, clinical diagnosis and routine laboratory screening. Additional information (eg, patient history) was obtained by a review of hospital charts.
Ninety cases dropped out due to diabetes (n = 18), renal insufficiency (n = 4) and the overlap of various histological diagnosis (n = 68).
Immunohistochemistry
Immunostaining was performed in 5 μm thick sections of formalin‐fixed and paraffin‐embedded tissues. The paraffin wax was removed by washing in Microclear (Micron Environmental Industries, Fairfax, Virginia, USA), and then rehydrated in decreasing concentrations of alcohol. For antigen retrieval, the sections were treated by microwaves twice for 5 min in a citrate solution. The endogenous peroxidase activity was blocked by exposing the sections to a 0.03% H2O2 solution containing 0.1% sodium azide. After brief washing, the sections were incubated with the primary mouse antibody (dilution 1:200, Novocastra, Newcastle, UK) at 25°C for 60 min. Following further washing steps to remove unbound marker, the sections were treated with the secondary antibody, a goat anti‐mouse peroxidase conjugate (Dako, Carpinteria, California, USA). For the staining, we used a robotic system (Techmate 1000, Dako, Carpinteria, California, USA) with the EnVision System. To secure specificity of the staining reaction, negative (omission of the primary antibody, omission of EnVision System, omission of both) and positive (cytokeratin 18 and 19 staining) controls were performed. For the detection of galectin‐3 we used the NCL‐GAL3 antibodies (Novocastra, Newcastle, UK), for AGEs the anti‐CML IgGs (MAK 4G9, Roche, Penzberg, Germany) and for RAGE the goat polyclonal antibodies AB5484 (Chemicon, Temecula, USA).
Assessment of immunostaining
On the basis of the stained percentage of selected histological structures (hepatocytes, Kupffer cells and bile ducts), values of 0–3 were assigned to each tissue section. 0, no stained structures; 1, <33% stained; 2, 33–66% and 3: >66%. Likewise intensity of staining was graded: 0, no staining; 1, weak staining; 2, medium and 3, strong. By multiplying both values, a semiquantative staining score was obtained.
Statistical analysis
All results were evaluated by Wilcoxon's signed rank test, Kruskal–Wallis test and Dunn's multiple comparison test, setting the significance level to p<0.05 (GraphPad Prism Software, San Diego, California, USA).
Results
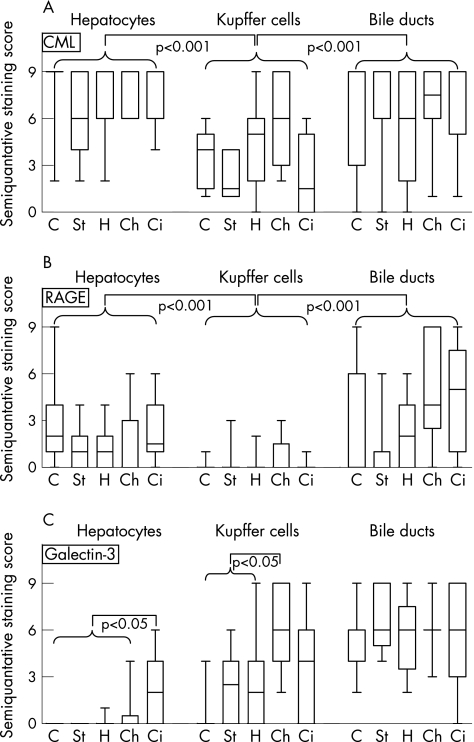
Figure 1 summarises the semiquantative staining scores of CML, RAGE and galectin‐3 in the examined histological structures (hepatocytes, Kupffer cells and bile ducts) from subjects of different diagnoses (healthy control, steatosis hepatis, hepatitis, cholestasis and cirrhosis). Based on the Kruskal–Wallis test, some significant differences could be observed between the different sites and patient populations.
Figure 1 Box and whiskers blots of a semiquantative immunohistochemical staining score (0–9) for carboxymethyl‐lysine (CML) (A), receptor for advanced glycation end product (RAGE) (B) and galectin‐3 (C) in controls (C, n = 15), and patients with steatosis hepatis (St, n = 20), hepatitis (H, n = 15), cholestasis (Ch, n = 15) and cirrhosis (Ci, n = 10), focusing on hepatocytes, Kupffer cells and bile ducts. Significant differences as tested by Kruskal–Wallis test are marked in the respective graphs.
Immunohistochemical staining patterns in healthy patients
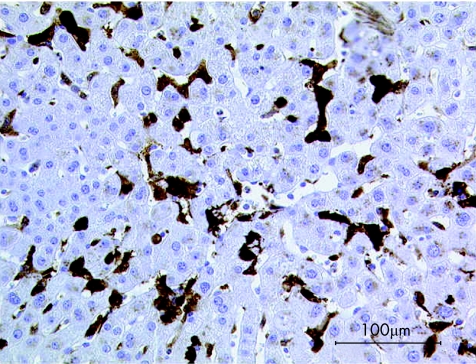
Hepatocytes and bile ducts of controls showed strong signals for CML, which were weaker (p<0.001) in Kupffer cells. Also, staining for RAGE was strongest in hepatocytes and bile ducts, whereas Kupffer cells were almost devoid of a signal (p<0.001). Figure 2 presents a typical example for staining of galectin‐3. In the control group, hepatocytes and Kupffer cells were free of staining (p<0.05), but positive bile ducts (with varying degrees of staining intensity) were seen in all examined cases.
Figure 2 Immunohistochemical staining of galectin‐3 (brown colour) in a liver biopsy sample of a healthy subject; no expression of galectin‐3 in hepatocytes or Kupffer cells; strong signal in bile ducts.
Immunohistochemical staining patterns in patients with hepatic dysfunction
Staining for CML was strong in hepatocytes and bile ducts independent of diagnoses. In Kupffer cells, signals were weaker (p<0.001) but continuously present. Marked levels of RAGE were detected in hepatocytes, with no difference between diagnoses. Bile ducts showed even stronger signals, except in steatosis hepatis. Kupffer cells had negative to weak staining for RAGE, independent of the kind of hepatic impairment.
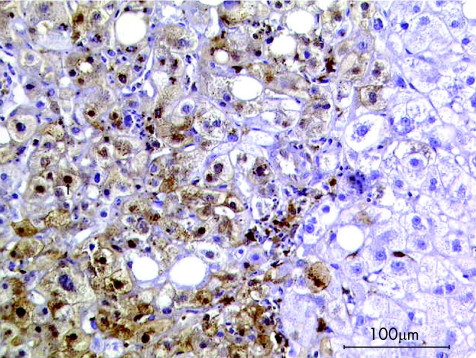
In steatosis, hepatis and hepatitis, hepatocytes showed little or no expression of galectin‐3. In cholestasis, positive signals occurred more often, although they were limited both in extent and intensity. Big nodules of strong positive signals were seen only in cases of cirrhosis (fig 3). Kupffer cells were shown to express galectin‐3 throughout all diagnoses, although signals were strongest in cases of cholestasis (fig 4). Independent of the diagnosis, the highest scores were detected in bile ducts.
Figure 3 Immunohistochemical staining of galectin‐3 (brown colour) in hepatocytes of a biopsy sample from a patient with cirrhosis.
Figure 4 Immunohistochemical staining of galectin‐3 (brown colour) in a biopsy sample from a patient with cholestasis; no expression of galectin‐3 in hepatocytes, strong signal in Kupffer cells.
Discussion
The underlying rationale of this study is an association between impaired liver function and consecutively rising AGE levels triggering an overexpression of galectin‐3 in the liver as part of an AGE–receptor complex.9 Binding of AGEs to this complex is thought to lead to their internalisation and degradation, and thereby to tissue protection.19,20
Following this concept, a trend towards an increased presence of CML in subjects with hepatic impairment as opposed to controls should be expected. Surprisingly, our results indicate no difference between the tested groups, independent of the histological structure examined. This finding could indicate that higher levels of circulating AGEs (as expected in patients with hepatic dysfunction) do not cause a stronger internalisation in hepatocytes, Kupffer cells and/or bile ducts. On the other hand, stronger internalisation could also trigger a higher rate of AGE degradation, keeping internalised levels equal.
Nonetheless, the observed high staining scores of hepatocytes of patients with liver cirrhosis could indicate a relationship between hepatic dysfunction, subsequent rising of AGE levels and galectin‐3 upregulation. In agreement with our data, Hsu et al22 showed 1999 that galectin‐3 is absent in normal hepatocytes, but is abundantly expressed in cirrhotic liver and frequently in hepatocellular carcinoma. The authors speculated that upregulated galectin‐3 expression is due to the high mitotic index of proliferating hepatocytes or part of a malignant transformation process. On the other hand, Iacobini et al23 recently showed the protective effect of galectin‐3 against AGE‐induced organ damage, using a galectin‐3 knockout mouse model.
Independent of hepatic impairment, the absence or low presence of RAGEs, which transmit the detrimental effects of AGEs versus the marked levels of cytoprotective galectin‐3 in Kupffer cells, is supportive for the protective nature of this cell type.
By contrast, hepatocytes were more or less devoid of galectin‐3 (except for subjects with cirrhosis), but showed strong stainings for RAGEs. This finding could indicate a susceptibility of hepatocytes regarding the toxic potential of AGEs.
Compared with the above‐discussed cell types, bile ducts showed the highest levels for CML, RAGEs and galectin‐3. This indicates that the biliary system is an important site for the disposition of AGEs. It could be speculated whether AGEs are, apart from intracellular degradation, also subject to biliary excretion. Similar to our findings, Shimonishi et al24 showed a constitutive (but weak) galectin‐3 expression in bile ducts, with a marked rise in staining intensity in case of biliary obstruction. Even though we were unable to reproduce this mechanism (staining scores of bile ducts in cholestasis did not significantly exceed scores in other diagnoses), their data support the idea of a biliary excretion of AGEs. The evident galectin‐3 upregulation in cholestasis shown by Shimonishi could be due to elevated reabsorption of AGEs by the AGE‐receptor complex as a reaction to obstructed bile ducts.
In support of this hypothesis, we found a strong expression of galectin‐3 in Kupffer cells during cholestasis. Smedsrod et al25 have reported that after intravenous administration of AGE‐bovine serum albumin in rats, uptake of AGE‐bovine serum albumin in liver endothelial, Kupffer and parenchymal cells accounted for approximately 60%, 25% and 10–15%, respectively, of hepatic elimination. This further supports the contention that AGEs are excreted in the bile, which could trigger the Kupffer cells to upregulate galectin‐3 due to rising AGE concentration in cholestasis.
Our findings show a complex pattern in expression of RAGE and galectin‐3 in the examined histological structures of the liver. Furthermore, we showed some associations between different forms of functional liver impairment and galectin‐3 expression. These phenomena may be linked to the function of galectin‐3 in the hepatic disposition of AGEs. Further studies are needed to define more clearly the role of the biliary system in this context.
Take‐home messages
The biliary system seems to play an important role in the disposition of AGEs.
While galectin‐3 shows a complex expression pattern in the examined histological structures of the liver, some associations exist between different forms of functional liver impairment and galectin‐3 expression.
Kupffer cells seem to play a protective role against the detrimental effects of AGEs, while hepatocytes seem to show an increased susceptibility.
Abbreviations
AGE - advanced glycation end product
CML - carboxymethyl‐lysine
RAGE - receptor for advanced glycation end product
Footnotes
Funding: The financial support of the Robert Bosch Foundation and the Dr Robert Pfleger Foundation is gratefully acknowledged. Neither funding organisation was involved in study design, in the collection, analysis and interpretation of data, in the writing of the report and in the decision to submit the paper for publication.
Competing interests: None.
References
- 1.Kasper M, Funk R H. Age‐related changes in cells and tissues due to advanced glycation end products (AGEs). Arch Gerontol Geriatr 200132233–243. [DOI] [PubMed] [Google Scholar]
- 2.Horiuchi S. The liver is the main site for metabolism of circulating advanced glycation end products. J Hepatol 200236123–125. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed M U, Brinkmann Frye E, Degenhardt T P.et al N‐epsilon‐(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem J 1997324565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayase F, Nagaraj R H, Miyata S.et al Aging of proteins: immunological detection of a glucose‐derived pyrrole formed during maillard reaction in vivo. J Biol Chem 19892643758–3764. [PubMed] [Google Scholar]
- 5.Sell D R, Monnier V M. Structure elucidation of a senescence cross‐link from human extracellular matrix. Implication of pentoses in the aging process. J Biol Chem 198926421597–21602. [PubMed] [Google Scholar]
- 6.Lo T W, Westwood M E, McLellan A C.et al Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha‐acetylarginine, N alpha‐acetylcysteine, and N alpha‐acetyllysine, and bovine serum albumin. J Biol Chem 199426932299–32305. [PubMed] [Google Scholar]
- 7.Reddy S, Bichler J, Wells‐Knecht K J.et al N epsilon‐(carboxymethyl) lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry 19953410872–10878. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda K, Higashi T, Sano H.et al N (epsilon)‐(carboxymethyl)lysine protein adduct is a major immunological epitope in proteins modified with advanced glycation end products of the Maillard reaction. Biochemistry 1996358075–8083. [DOI] [PubMed] [Google Scholar]
- 9.Sebekova K, Kupcova V, Schinzel R.et al Markedly elevated levels of plasma advanced glycation end products in patients with liver cirrhosis—amelioration by liver transplantation. J Hepatol 20023666–71. [DOI] [PubMed] [Google Scholar]
- 10.Vlassara H, Fuh H, Makita Z.et al Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: a model for diabetic and aging complications. Proc Natl Acad Sci USA 19928912043–12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlassara H, Striker L J, Teichberg S.et al Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proc Natl Acad Sci USA 19949111704–11708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerami C, Founds H, Nicholl I.et al Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci USA 19979413915–13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh R, Barden A, Mori T.et al Advanced glycation end‐products: a review. Diabetologia 200144129–146. [DOI] [PubMed] [Google Scholar]
- 14.Stern D M, Yan S D, Yan S F.et al Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev 200211–15. [DOI] [PubMed] [Google Scholar]
- 15.Horiuchi S, Higashi T, Ikeda K.et al Advanced glycation end products and their recognition by macrophage and macrophage‐derived cells. Diabetes 199645S73–S76. [DOI] [PubMed] [Google Scholar]
- 16.Ohgami N, Nagai R, Ikemoto M.et al CD36, a member of class B scavenger receptor family, is a receptor for advanced glycation end products. Ann N Y Acad Sci 2001947350–355. [DOI] [PubMed] [Google Scholar]
- 17.Li Y M, Mitsuhashi T, Wojciechowicz D.et al Molecular identity and cellular distribution of advanced glycation endproduct receptors: relationship of p60 to OST‐48 and p90 to 80K‐H membrane proteins. Proc Natl Acad Sci USA 19969311047–11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vlassara H, Li Y M, Imani F.et al Identification of galectin‐3 as a high‐affinity binding protein for advanced glycation end products (AGE): a new member of the AGE‐receptor complex. Mol Med 19951634–646. [PMC free article] [PubMed] [Google Scholar]
- 19.Iacobini C, Amadio L, Oddi G.et al Role of galectin‐3 in diabetic nephropathy. J Am Soc Nephrol 200314S264–S270. [DOI] [PubMed] [Google Scholar]
- 20.Zhu W, Sano H, Nagai R.et al The role of galectin‐3 in endocytosis of advanced glycation end products and modified low density lipoproteins. Biochem Biophys Res Commun 20012801183–1188. [DOI] [PubMed] [Google Scholar]
- 21.Krzeslak A, Lipinska A. Galectin‐3 as a multifunctional protein. Cell Mol Biol Lett 20049305–328. [PubMed] [Google Scholar]
- 22.Hsu D K, Dowling C A, Jeng K C.et al Galectin‐3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int J Cancer 199981519–526. [DOI] [PubMed] [Google Scholar]
- 23.Iacobini C, Oddi G, Pugliese G.et al Development of age‐dependent glomerular lesions in galectin‐3/AGE‐receptor‐3 knockout mice. Am J Physiol Renal Physiol 2005289F611–21 e‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Shimonishi T, Miyazaki K, Kono N.et al Expression of endogenous galectin‐1 and galectin‐3 in intrahepatic cholangiocarcinoma. Hum Pathol 200132302–310. [DOI] [PubMed] [Google Scholar]
- 25.Smedsrod B, Melkko J, Araki N.et al Advanced glycation end products are eliminated by scavenger‐receptor‐mediated endocytosis in hepatic sinusoidal Kupffer and endothelial cells. Biochem J 1997322567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]