Abstract
Objectives
To evaluate morphological findings in repeat biopsies in patients with isolated high‐grade prostatic intraepithelial neoplasia (HGPIN) after a 6‐month course of bicalutamide (Casodex) 50 mg/day.
Methods
20 consecutive patients with isolated HGPIN in prostate biopsies were treated for 6 months with bicalutamide 50 mg/day. After treatment, the patients were resubmitted to prostate biopsy mapping. The control group included 22 untreated consecutive patients with isolated high‐grade PIN with repeat biopsies taken 6 months after the initial biopsies.
Results
In the initial biopsies of the treated group, HGPIN was monofocal in 12 patients and plurifocal in 8. In the repeat biopsies HGPIN was present in 2 patients, monofocal in both, whereas prostate adenocarcinoma (PCa) was discovered in one. In the control group, HGPIN was monofocal in 15 and plurifocal in 7. In the repeat biopsies HGPIN was present in six patients, being monofocal in three and plurifocal in the other three. PCa was present in one.
Conclusions
There was a lower incidence of HGPIN (treated group vs control: 10% vs 27.2%) after 6 months of bicalutamide. Reduction in its extent was also observed (treated group vs control: monofocal 100% vs 50%). Treatment did not affect the incidence of cancer (treated vs control: 5% vs 4.5%).
The incidence of high‐grade prostatic intraepithelial neoplasia (HGPIN) in needle biopsies varies according to the patient population under consideration and the number of biopsies obtained. The American Cancer Society National Prostate Cancer Detection Project identified prostatic intraepithelial neoplasia (PIN) and cancer in 17 (5.2%) and 58 (15.8%) men, respectively, from a series of 330 biopsies from men participating in an early detection project.1 Other studies have found high‐grade PIN in up to 16.5% of contemporary needle biopsy specimens in urology office practice.2,3,4 The diagnosis of HGPIN is predictive of subsequent cancer detection in 2.3–100% of patients.5,6
Due to the lower predictive value for cancer in recent years, studies have focused on HGPIN parameters in needle core biopsies that may be more useful in subsequent detection of cancer. It has been clearly shown that plurifocal HGPIN on prostatic biopsies is a factor predicting cancer detection on extended repeat biopsies.7
HGPIN identifies patients at high risk for PCa, and these are ideal target populations for chemoprevention. For prostate cancer, as for other cancer targets, development of chemopreventive strategies requires suitable cohorts, reliable biomarkers for evaluating chemopreventive efficacy and well‐characterised agents, such as antiandrogens.
Bicalutamide is a non‐steroidal antiandrogen developed for the treatment of prostate cancer. The dosage recommended by the manufacturer is 50 mg daily in combination with orchiectomy or a luteinising hormone realising hormone (LHRH) agonist or 150 mg daily as monotherapy. The frequent side effects of 150 mg bicalutamide are gynaecomastia and breast pain. Bicalutamide does not affect sexual function and the patient's well‐being. Information on the morphological changes induced by 150 mg bicalutamide on prostate tissue components and lesions has been obtained in radical prostatectomy specimens.8
The purpose of the present study is to evaluate the morphological findings after a 6‐month course of bicalutamide 50 mg/day in repeat biopsies in a small series of patients with isolated HGPIN.
Materials and methods
Twenty‐four consecutive men with isolated HGPIN were enrolled in a study whose statistical design followed the recommendations of early phase II studies.9 The patients signed a detailed informed consent and were invited to report any adverse event. Each patient received 50 mg per day of bicalutamide (Casodex 50) (Casodex is a trademark of AstraZeneca, Alderley Park, UK) for 6 months. Twenty patients (mean age: 62.4 years; range 49–74 years) completed the treatment period and were included in the present analysis.
Diagnosis of HGPIN was made by means of transrectal ultrasonography (TRUS)‐directed 18 G biopsies carried out approximately 1 month before the first dose of the study drug was given. The number of biopsy cores was related to the prostate volume (mean (SD) number of cores per patient: 8.55 (1.39)). Before performing the biopsies and after the treatment, the TRUS volume of the prostate was calculated using the ellipsoid volume formula. Within 15 days from the end of the treatment, the patients were re‐submitted to prostate biopsy mapping (number of cores per patient: 12). The repeat biopsy material was processed at the Institute of Pathological Anatomy, Polytechnic University of Marche Region, Ancona, Italy. All the material were examined by the same pathologist (R Montironi). Haematoxylin‐and‐eosin stained slides of the initial biopsies, processed at the Ospedale di Circolo in Varese, were kindly made available for comparison.
Exclusion criteria were: serum prostate specific antigen (PSA)>10 ng/ml or free/total ratio⩽0.10; positive digital rectal examination; previous diagnosis of prostate cancer; previous or concurrent radiotherapy, hormonal therapy or chemotherapy; previous (within 12 months) or concurrent use of finasteride or other 5‐α‐reductase inhibitor; previous prostate surgery; and inadequate performance status.
The control group included 22 untreated consecutive men (mean age: 65.7 years; range 50–74 years) with isolated high‐grade PIN and with repeat biopsies taken 6 months after the initial biopsies. The material was retrieved from the files of the Institute of Pathological Anatomy of the Polytechnic University of Marche Region. The patients were all evaluated at the Urology of the Polytechnic University of Marche Region (Chairman: Professor Giovanni Muzzonigro) using the same methods and exclusion criteria adopted in the treated group. The mean (SD) number of cores per patient in the initial biopsies and in the repeat series was 8.2 (1.11) and 12 (1.5), respectively.
Results
Clinical data
In the treated group, the mean (SD) basal total PSA was 6.57 (2.7) ng/ml. After treatment it was 1.98 (1.65) ng/ml. The mean (SD) prostate volume before treatment was 48.4 (17.80) ml. At the completion of the study it was 37.5 (13.4) ml. Grade 2 bilateral painless gynecomastia was seen in 8 out of 20 patients.
In the control group, the mean (SD) basal total PSA was 7.11 (1.7) ng/ml. At 6 months it was 6.21 (2.1) ng/ml. The mean (SD) prostate volume before treatment was 51.5 (12.8) ml. At the completion of the study, it was 52.7 (9.4) ml.
The initial serum PSA and prostate volume were slightly greater in the control group than in the treated one. The difference was not statistically significant. Bicalutamide treatment reduced PSA and prostate volume by 69% and 22%, respectively, in comparison with the figures observed at the baseline. The data are reported in table 1.
Table 1 Patients' characteristics.
| Treated (n = 20) | Controls (n = 22) | p Value** | |
|---|---|---|---|
| Age of the patients (years), mean (range) | 62.4 (49–74) | 65.7 (50–74) | 0.07 |
| PSA (ng/ml) | |||
| Initial* | 6.57 (2.7) | 7.11(1.7) | 0.438 |
| At six months | 1.98 (1.65) | 6.21 (2.1) | <0.001 |
| TRUS prostate volume (cm3) | |||
| Initial | 48.4 (17.8) | 51.5 (12.8) | 0.478 |
| At 6 months | 37.5 (13.4) | 52.7 (9.4) | 0.001 |
| No. of biopsies taken | |||
| Initial | 8.55 (1.39) | 8.2 (1.11) | 0.37 |
| At 6 months | 12 | 12 (1.5) | 1.0 |
PSA, prostate‐specific antigen; TRUS, transrectal ultrasonography
*Mean and standard deviation
**Statistics: Student's t test
Pathological data
Treated patients
In the baseline biopsies, HGPIN involved one biopsy core in 12 patients (monofocal HGPIN) (number of glands involved per patient: mean 2, range 1–4) and more than one core in eight (plurifocal HGPIN; range of cores involved per patient: 2–5 with a mean of 3) (number of glands involved per patient: mean 5, range 3–8). Plurifocal HGPIN was present in both lobes of the prostate in three patients. As far as the main architectural pattern in each case was concerned, this was flat in five, tufting in six and micropapillary in nine. There were no cases with a cribriform pattern. Cancer was not seen.
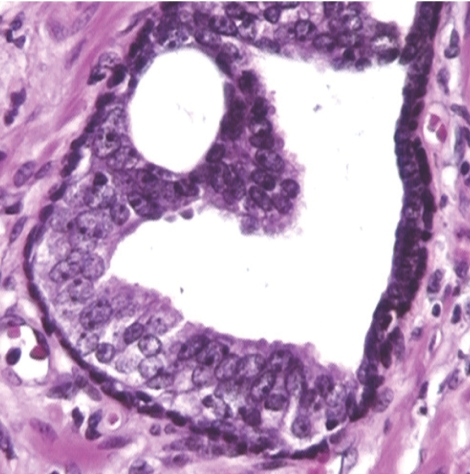
In the repeat biopsies, monofocal HGPIN was present in two patients (number of glands involved per patient: 2 and 3, respectively) (fig 1), whereas prostate adenocarcinoma was present in one, this was not the case with persistent HGPIN). One case of HGPIN and one of cancer had plurifocal HGPIN with a tufting pattern in the initial biopsies. Some degree of cell stratification and crowding was recognisable in the two HGPIN cases. The cancer, which occupied 30% of one biopsy, was composed of shrunken and collapsed acini. Due to the treatment effects, Gleason grading was not applied. Cystic and simple atrophy of the non‐neoplastic ducts and acini, often with chronic inflammation, was present in all cases. The cytological changes included nuclear chromatin condensation and cytoplasmic clearing. Prominent nucleoli were occasionally seen in HGPIN and cancer.
Figure 1 Morphological changes due to bicalutamide. The left half of this duct/acinus shows architectural and cytological features of high‐grade prostatic intraepithelial neoplasia whereas the right part is atrophic.
Control patients
In the initial biopsies, HGPIN was monofocal in 15 patients (mean number of glands involved per patient: 2, range 1– 4) and plurifocal in seven (mean number of cores involved per patient: 4 range 2–6 with a mean of 4) (mean number of glands involved per patient: 6, range 3–9). Plurifocal HGPIN was present in both lobes of the prostate in four patients. The main architectural pattern was flat in 4, tufting in 8 and micropapillary in 10.
HGPIN was present in the repeat biopsies of six patients, being monofocal in three (mean number of glands involved per patient: 2, range 1 –3) and plurifocal in the other three (mean number of cores involved per patient: 4, range 2–7) (mean number of glands involved per patient: 7, range 4 –9). The HGPIN architectural pattern and focality (ie, monofocal vs plurifocal) were similar to those observed in the initial biopsies. PCa was seen in one patient at 6 months and was not associated with persistent HGPIN. The Gleason score was 3+3 = 6. It was present in a single biopsy core, where it occupied 50% of its length.
Comparison between treated and control groups
There was significantly lower incidence of HGPIN (treated group vs control: 10% vs 27.2% of patients, respectively) following 6 months of bicalutamide treatment. There was also a reduction in the extent. In fact, the two HGPIN cases were both monofocal (ie 100% of patients), whereas in the control group 50% were monofocal and the other 50% plurifocal. Treatment did not affect the incidence of cancer (treated vs control: 5% vs 4.5% of patients, respectively) (tables 2–4).
Table 2 Comparison between treated and control groups (no of patients).
| Initial biopsies | No of patients | Repeat biopsies | ||
|---|---|---|---|---|
| Monofocal HGPIN | Plurifocal HGPIN | PCa | ||
| Monofocal HGPIN | ||||
| Treated group | 12 | 1 | 0 | 0 |
| Controls | 15 | 3 | 0 | 0 |
| Plurifocal HGPIN | ||||
| Treated group | 8 | 1 | 0 | 1 |
| Controls | 7 | 0 | 3 | 1 |
| Total no of patients | ||||
| Treated group | 20 | 2 (10%) | 0 | 1 (5%) |
| Controls | 22 | 3 (13.6%) | 3 (13.6%) | 1 (4.5%) |
HGPIN, high‐grade prostatic intraepithelial neoplasia; PCa, prostate adenocarcinoma;
Table 3 Comparison between treated and control groups (no of cores per patient).
| Initial biopsies | No of cores | Repeat biopsies | |
|---|---|---|---|
| Monofocal HGPIN | Plurifocal HGPIN | ||
| Monofocal HGPIN | |||
| Treated group | 1 | 1 | 0 |
| Controls | 1 | 1 | 0 |
| Plurifocal HGPIN | |||
| Treated group | 3 (2–5)* | 1 | 0 |
| Controls | 4 (2–6)* | 0 | 4 (2–7)* |
HGPIN, high‐grade prostatic intraepithelial neoplasia; PCa, prostate adenocarcinoma;
*Mean and range
Table 4 Comparison between treated and control groups (no of glands per patients).
| Initial biopsies | No of glands | Repeat biopsies | |
|---|---|---|---|
| Monofocal HGPIN | Plurifocal HGPIN | ||
| Monofocal HGPIN | |||
| Treated group | 2 (1–4)* | 2 | 0 |
| Controls | 2 (1–4)* | 2 (1–3)* | 0 |
| Plurifocal HGPIN | |||
| Treated group | 5 (3–8)* | 3 | 0 |
| Controls | 6 (3–9)* | 0 | 7 (4–9)* |
HGPIN, high‐grade prostatic intraepithelial neoplasia;
*Mean and range
Table 5 shows the comparison of the histological features of benign tissue in the control and treated groups in the repeat biopsy. There were no appreciable differences in the normal seen in the two groups in the initial biopsies (data not shown).
Table 5 Morphology of the benign tissue at 6 months.
| Benign tissue | Treated (n = 20) | Controls (n = 22) | p Value* |
|---|---|---|---|
| Stroma/gland ratio† | 2.11 (1.1) | 1.56 (0.61) | 0.049 |
| % of cases with atrophic epithelium | 55% | 15% | 0.013 |
| % of cases with basal cell hyperplasia | 45% | 8% | 0.002 |
| % of cases with transitional cell metaplasia | 35% | 5% | 0.036 |
| % of cases with chronic inflammation | 60% | 10% | 0.002 |
*Statistics: Student's t and χ2 tests.
†Visual estimation of the stroma:epithelium ratio, mean (SD)
Discussion
The current study showed that there was a lower incidence and decreased extent of HGPIN after 6 months of treatment with bicalutamide. Treatment did not affect the incidence of cancer. Cells with regressive changes were present in the case of cancer as well as in the ducts with HGPIN. The non‐neoplastic epithelium was atrophic.
The literature reports that there is a decrease in the prevalence and extent of HGPIN after androgen deprivation therapy, as compared with untreated prostates. Maximum androgen blockade (MAB; castration plus an antiandrogen) has been shown to reduce the prevalence and extent of HGPIN.10,11,12,13 Three months of neoadjuvant MAB (luteinising hormone realising hormone agonist and flutamide) reduced the extent of HGPIN by 50%.12 Neoadjuvant therapy with the steroidal antiandrogen cyproterone acetate (300 mg/day) for 12 weeks also reduces the incidence of HGPIN in men with clinically localised prostate cancer, probably as a result of cytological alterations occurring in the neoplastic cells.11 However, as both MAB and cyproterone have major side effects, especially on sexuality, neither can be recommended to treat isolated HGPIN.14,15 Blockade of 5α‐reductase with finasteride has a minimal effect on HGPIN,16 although a 6‐ to 10‐week course of high‐dose dutasteride (5 mg/day compared with the usual 0.5 mg/day dose for benign prostatic hyperplasia) has been shown to decrease HGPIN volume by 40%.17
Non‐steroidal antiandrogens such as bicalutamide are better tolerated than castration‐based therapies or steroidal antiandrogens, and are more attractive for the treatment of HGPIN. Bicalutamide is the most extensively studied non‐steroidal antiandrogen in early prostate cancer.18,19,20,21,22 In locally advanced disease, bicalutamide 150 mg provides a similar survival outcome as castration, with significant quality‐of‐life benefits with respect to sexual interest and physical capacity, as well as preservation of bone mineral density.20,21 In a recent study on the pathological changes of HGPIN and prostate cancer in radical prostatectomies after bicalutamide 150 mg monotherapy, the tumour volume in the treated group was found to be 38% lower than in the control group whereas the volume of HGPIN was significantly lower (45%) in the bicalutamide group than in the controls. In addition, involution and epithelial shrinkage of prostate cancer and HGPIN were evident after neoadjuvant bicalutamide 150 mg treatment.8
A number of papers have dealt with the cytological changes in high‐grade PIN due to androgen manipulation.10,23,24 A certain degree of secretory cell type stratification is always present. However, crowding is less evident than in the untreated high‐grade PIN. The cells show cytoplasmic clearing and enlargement by coalescence of vacuoles and rupture of cell membranes. The nuclei have different degrees of chromatin changes which range from a mild condensation—which barely allows the distinction between coarse chromatin granules (corresponding to heterochromatin) and finely dispersed chromatin (corresponding to euchromatin)—to a tightly condensed state close to that observed in apoptosis. Similar to treated PCa, apoptotic bodies are identifiable in all epithelial cell layers.23 In the treated cases, the nucleoli often become inconspicuous.10 The basal‐cell layer is easily recognisable in most instances. There seems to be some correspondence between the type of treatment and the degree of regressive changes.8
In the present study, cancer was present in one out of 20 treated patients. The regressive changes seen in the cancer as well as in the two HGPIN cases are similar to those documented by one of us in radical prostatectomy specimens in patients with prostate cancer treated with 150 mg of bicalutamide for 3 months before operation.8 The pattern of atrophy of the non‐neoplastic epithelium is similar to that described by Eri et al25 after bicalutamide treatment. Our findings should be interpreted with caution, at least for three reasons. First of all, it cannot be excluded that the lower incidence and extent of HGPIN in the repeat biopsies in the treated group could also be related to the fact that the morphological changes are as such to mask the possibility of recognising HGPIN. Second, it cannot be excluded that our findings are not all related to the treatment but more simply to the biopsy procedures. For instance, Postma et al26 observed a reduction in frequency of HGPIN during repeat biopsy in untreated patients. Lastly, there are limitations in our preliminary study: it is not randomised, the duration of treatment is short and the number of patients is small.
Take‐home messages
HGPIN identifies patients at high risk PCa, and these are ideal target populations for chemoprevention.
There is a lower incidence and extent of HGPIN following 6 month treatment with bicalutamide.
Reduction in its extent is also observed.
Our findings should be interpreted with caution: not all of our findings relate to the treatment, but simphy to the biopsy procedures.
In conclusion, the current study showed that there was a lower incidence and extent of HGPIN after 6 months of treatment with bicalutamide. Reduction in its extent was also observed. Treatment did not affect the incidence of cancer. Our findings should be interpreted with caution and also in consideration of the limitations of our current study.
Abbreviations
HGPIN - high‐grade prostatic intraepithelial neoplasia
MAB - Maximum androgen blockade
PIN - prostatic intraepithelial neoplasia
PSA - prostate specific antigen
TRUS - transrectal ultrasonography
Footnotes
Competing interests: None declared.
References
- 1.Mettlin C, Lee F, Drago J.et al The American Cancer Society National Prostate Cancer Detection Project: findings on the detection of early prostate cancer in 2425 men. Cancer 1991672949–2958. [DOI] [PubMed] [Google Scholar]
- 2.Bostwick D G. Prostatic intraepithelial neoplasia. Curr Urol Rep 2000160–70. [DOI] [PubMed] [Google Scholar]
- 3.Bostwick D G, Qian J, Frankel K. The incidence of high‐grade prostatic intraepithelial neoplasia in needle biopsies. J Urol 19951541791–1794. [PubMed] [Google Scholar]
- 4.Sakr W A, Grignon D J, Haas G P.et al Epidemiology of high‐grade prostatic intraepithelial neoplasia. Pathol Res Pract 1995191838–841. [DOI] [PubMed] [Google Scholar]
- 5.Bishara T, Ramnani D M, Epstein J I. High‐grade prostatic intraepithelial neoplasia on needle biopsy: risk of cancer on repeat biopsy related to number of involved cores and morphologic pattern. Am J Surg Pathol 200428629–633. [DOI] [PubMed] [Google Scholar]
- 6.Gokden N, Roehl K A, Catalona W J.et al High‐grade prostatic intraepithelial neoplasia in needle biopsy as a risk factor for detection of adenocarcinoma. Current level of risk in a screening population. Urology 200565538–542. [DOI] [PubMed] [Google Scholar]
- 7.Roscigno M, Scattoni V, Freschi M.et al Monofocal and plurifocal high‐grade prostatic intraepithelial neoplasia on extended prostatic biopsies: factors predicting cancer detection on extended repeat biopsy. Urology 2004631105–1110. [DOI] [PubMed] [Google Scholar]
- 8.Scattoni V, Montironi R, Mazzucchelli R.et al Casodex (bicalutamide) 150‐mg monotherapy decreases the amount of prostate cancer and high‐grade prostatic intraepithelial neoplasia. Br J Urol Int 20069854–58. [DOI] [PubMed] [Google Scholar]
- 9.Chen T T. Optimal three‐stage designs for phase II cancer clinical trials. Stat Med 1997162701–2711. [DOI] [PubMed] [Google Scholar]
- 10.Balaji K C, Rabbani F, Tsai H.et al Effect of neoadjuvant therapy on prostatic intraepithelial neoplasia and its prognostic significance. J Urol 1999162753–757. [DOI] [PubMed] [Google Scholar]
- 11.Bullock M J, Srigly J R, Klotz L H.et al Pathologic effects of neoadjuvant cyproterone acetate on nonneoplastic prostate, prostatic intraepithelial neoplasia, and adenocarcinoma: a detailed analysis of radical prostatectomy specimens from a randomized trial. Am J Surg Pathol 2002261400–1413. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson J, Zincke H, Ellison E.et al Decrease of prostatic intraepithelial neoplasia following androgen deprivation therapy in patients with stage T3 carcinoma treated by radical prostatectomy. Urology 19944491–95. [DOI] [PubMed] [Google Scholar]
- 13.Vailancourt L, Tetu B, Fradet Y.et al Effect of neoadjuvant endocrine therapy (combined androgen blockade) on normal prostate and prostatic carcinoma. Am J Surg Pathol 19962086–93. [DOI] [PubMed] [Google Scholar]
- 14.Holzbeierlein J M, Castle E, Thrasher J B. Complications of androgen deprivation therapy: prevention and treatment. Oncology 200418303–309. [PubMed] [Google Scholar]
- 15.Mcleod D G. Hormonal therapy: historical perspective to future direction. Urology 2003613–7. [DOI] [PubMed] [Google Scholar]
- 16.Bostwick D G, Qian J. High‐grade prostatic intraepithelial neoplasia. Mod Pathol 200417360–379. [DOI] [PubMed] [Google Scholar]
- 17.Andriole G, Bostwick D, Civantos F.et al The effect of 5‐alpha‐reductase inhibitors on the natural history, detection and grading of prostate cancer: current state of knowledge. J Urol 20051742098–2104. [DOI] [PubMed] [Google Scholar]
- 18.Henderson A, Langley S E, Laing R W. Is bicalutamide equivalent to goserelin for prostate volume reduction before radiation therapy? A prospective, observational study. Clin Oncol 200315316–317. [DOI] [PubMed] [Google Scholar]
- 19.Iversen P. Antiandrogen monotherapy: indication and results. Urology 20026064–71. [DOI] [PubMed] [Google Scholar]
- 20.Iversen P, Tyrrell C J, Kaisary A V.et al Bicalutamide monotherapy compared with castration in patients with nonmetastatic locally advanced prostate cancer: 6.3 years of followup. J Urol 20001641579–1582. [PubMed] [Google Scholar]
- 21.Smith M R, Goode M, Zietman A L. Bicalutamide monotherapy versus leuprolide monotherapy for prostate cancer: effects on bone mineral density and body composition. J Clin Oncol 2004222546–3253. [DOI] [PubMed] [Google Scholar]
- 22.Wirth M P, See W A, McLeod D.et al Bicalutamide 150 mg in addition to standard care in patients with localized or locally advanced prostate cancer: results from the second analysis of the early prostate cancer program at median followup of 5.4 years. J Urol 20041721865–1870. [DOI] [PubMed] [Google Scholar]
- 23.Montironi R, Schulman C C. Pathological changes in prostate lesions after androgen manipulation. J Clin Pathol 1998515–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montironi R, Pomante R, Diamanti L.et al Evaluation of prostatic intraepithelial neoplasia after treatment with a 5‐alpha‐reductase inhibitor (finasteride). A methodologic approach. Anal Quant Cytol Histol 199618461–470. [PubMed] [Google Scholar]
- 25.Eri L M, Svindland A, Tveter K J. The effect of Bicalutamide on prostate histology. Prostate 200146275–280. [DOI] [PubMed] [Google Scholar]
- 26.Postma R, Roobol M, Schroeder F H.et al Lesions predictive for prostate cancer in a screened population: first and second screening round findings. Prostate 200461260–266. [DOI] [PubMed] [Google Scholar]