Abstract
The most common renal tumours are clear cell, papillary, chromophobe and collecting duct renal cell carcinomas (RCCs), and benign oncocytomas and angiomyolipomas. Tumours with hybrid features between some of these entities have been recognised; in particular, tumours with features of both chromophobe RCC and oncocytoma. Case reports describing one distinct type of primary renal tumour actually within another are very rare. The incidental finding of a papillary RCC located in an oncocytoma in a nephrectomy specimen from a 75‐year‐old man is described. Morphological criteria for each tumour type were completely satisfied and fluorescence in situ hybridisation detected the expected number of copies of chromosome 7 in the cells of each tumour type. The cells in the papillary tumour contained three copies, whereas the oncocytoma cells contained only two per nucleus. To our knowledge, this is the first report of a papillary RCC being identified within an oncocytoma.
The current World Health Organization classification of renal epithelial tumours recognises malignant lesions such as, clear cell, papillary, chromophobe and collecting duct renal cell carcinomas (RCCs), and benign entities such as oncocytoma and angiomyolipoma.1 Each of these neoplasms has characteristic histological and/or immunophenotypic features and genetic/chromosomal alterations specific to each type have been identified.2 Oncocytomas are characterised by large cells with mitochondria‐rich eosinophilic cytoplasm that occur in diffuse sheets or as islands of cells in an oedematous stroma.1 The cell of origin is thought to be the intercalated cell of the kidney tubules. Cytogenetic alterations in oncocytomas include the t(5;11)(q35;q13) translocation and loss of chromosomes 1 and Y.3,4,5 Oncocytomas are also a component of Birt–Hogg–Dube syndrome, a rare autosomal dominant condition characterised by benign skin tumours, pulmonary cysts and multiple renal tumours, particularly oncocytomas and chromophobe RCC.6 Chromophobe RCC may have the same cell of origin as oncocytoma, however these tumours are characterised by extensive allelic imbalance and loss of chromosomal material on chromosomes 1, 2, 6, 10, 13, 17 and 21.7
Papillary RCC has papillary or tubulopapillary architecture1 and two morphological subtypes are recognised. Type 1 tumours consist of papillae covered with a single layer of small cells with scanty cytoplasm and low‐grade nuclei. In type 2 tumours, the cells covering the papillae are pseudostratified, generally have eosinophilic cytoplasm and are usually of higher nuclear grade than the cells of type 1 tumours.8 The histogenesis of papillary RCC is unclear, with evidence suggesting that the cell of origin resides in the proximal or distal tubule.9,10 At the cytogenetic level, the most common karyotypic changes in papillary RCC are trisomy of chromosomes 7 and 17, and loss of chromosome Y.11
Composite renal tumours comprising cells with features of both chromophobe RCC and oncocytomas have been described,12,13 however, papillary RCC combined with oncocytoma has not been reported to our knowledge.
Case history
The patient was a 75‐year‐old man with long‐standing hypertension and diabetes mellitus who presented with normocytic anaemia. Haematuria was not a feature of the clinical presentation. A transabdominal ultrasound examination demonstrated bilateral renal masses. CT scanning showed two enhancing lesions in the right kidney (a 4 cm mass in the interpolar region, and a 2 cm mass in the inferior pole) and a 1.5 cm “exophytic” mass in the lower pole of the left kidney. Right total and left partial nephrectomies were performed.
Methods
The resection specimens were processed according to standard surgical pathology protocols. Briefly, they were fixed in 10% neutral buffered formalin for 24–36 h. Representative sections of tumour, 3–4 mm in thickness, were embedded in paraffin wax and sections of 5 µm were cut and stained with H&E for light microscopy. Fluorescence in situ hybridisation with flourescein isothiocyanate‐labelled peptide nucleic acid probes directed at the centromere of chromosome 7 was performed and scored using standard methods.14 The probe was hybridised to 5 μm sections from formalin‐fixed, paraffin‐wax‐embedded tissue to compare the number of copies of chromosome 7 in the oncocytoma and papillary tumour cells.
Results
Pathological findings
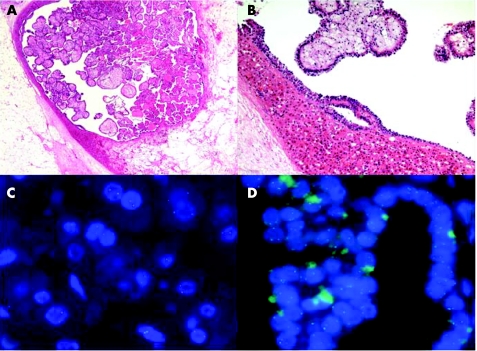
Both tumours in the right kidney had the gross and histological features of renal oncocytoma. The left partial nephrectomy contained a 1.5 cm lesion that was the same mahogany‐brown colour as the two lesions in the right kidney. However, unlike the other two tumours, the lesion in the left kidney contained a grossly distinct central whitish‐yellow area that measured 0.7 cm in maximum diameter. This central area was circumscribed and did not have the typical stellate appearance of the “central scar” often identified within oncocytomas. Histological examination of the left kidney lesion revealed that the outer mahogany‐brown portion was an oncocytoma comprising large cells with abundant eosinophilic, granular cytoplasm with large nuclei and visible nucleoli. The oncocytic cells were arranged in solid sheets and small islands scattered in an oedematous stroma (fig 1). The central area had papillary architecture with cuboidal cells showing mild nuclear pleomorphism and nucleoli that were visible at high magnification. Many of the papillary structures contained foamy macrophages within their cores. Based on current diagnostic criteria, the features are those of a type I papillary RCC (fig 1) of Fuhrman nuclear grade 2. There was no tumorous involvement of the renal capsule or perinephric fat, no vascular space invasion was identified and the margins of resection were clear.
Figure 1 (A) Histological appearance of the lesion, at low magnification, in the lower pole of the left kidney showing the oncocytoma surrounding the central papillary neoplasm (original magnification ×50). (B) Histological appearance of the lesion, at higher magnification, to demonstrate the morphological features of the oncocytoma and the papillary renal cell carcinoma (RCC) within it (original magnification ×200). (C) Fluorescence in situ hybridisation (FISH) using the centromere 7 probe to demonstrate the presence of two signals per nucleus in the oncocytoma portion of the lesion. (D) The same FISH probe revealing the presence of three signals per nucleus in the papillary RCC within the oncocytoma.
Cytogenetics
As shown in table 1 and fig 1, the majority of the cells in the papillary area had three signals per nucleus. The majority of cells in the classic oncocytoma area had only two signals per nucleus.
Table 1 Comparison of the number of copies of chromosome 7 in the papillary and oncocytoma areas of the left kidney lesion as determined by fluorescence in situ hybridisation using centromeric peptide nucleic acid probes.
| Number of signals/nucleus | Papillary area (% of nuclei) | Oncocytoma area (% of nuclei) |
|---|---|---|
| 0 | 2 | 1 |
| 1 | 9 | 19 |
| 2 | 31 | 65 |
| 3 | 53 | 13 |
| 4 | 5 | 2 |
Discussion
The finding of a papillary RCC actually within an oncocytoma has not been reported to our knowledge. This finding could be viewed as being of little clinical significance and nothing more than a pathologist's curiosity, however the case raises interesting points with respect to the aetiology of tumours within tumours, the relationship between different types of renal neoplasms, and the current criteria used to distinguish papillary RCC from papillary renal adenomas.
Extensive papillary architecture is considered to be an impermissible feature in renal oncocytoma.1,15 The tumour within the oncocytoma in our case consisted entirely of papillary architecture and was well demarcated (grossly and histologically) from the oncocytoma that surrounded it. Fluorescence in situ hybridisation studies revealed trisomy 7 in the papillary component, but not in the oncocytoma, consistent with the known cytogenetic profiles of each tumour type.
There is growing evidence that oncocytoma and chromophobe RCC may be related entities with oncocytoma representing the benign end of a spectrum and chromophobe RCC residing at the malignant end. Specifically, both may arise from the tubular intercalated cell, they share common histological features that can make it challenging to distinguish one from the other, hybrid oncocytoma–chromophobe RCC tumours have been described and there are cytogenetic aberrations common to both.5,7,12,13 There is no evidence to suggest that such a relationship exists between oncocytoma and papillary RCC. As such, it is unlikely that our finding represents divergent differentiation of either oncocytoma into papillary RCC or papillary RCC into oncocytoma. The patient in our report had bilateral oncocytomas, suggesting a predisposition to form oncocytic neoplasms. This raises the possibility of “oncocytosis”, a process whereby small oncocytic nodules coalesce to form a mass.16 It is tempting to speculate that, in our case, several oncocytic foci coalesced around an independently developing low‐grade papillary neoplasm.
One could argue that the papillary neoplasm we found could be called a papillary adenoma as opposed to papillary RCC. The two entities are histologically and cytogentically identical,17,18,19 and are currently separated based purely on size. Tumours ⩽5 mm in maximum dimension are believed to have limited growth potential and are designated as adenomas.17 The papillary neoplasm in our case was 0.7 mm in size with Fuhrman grade 2 nuclei and was, therefore, considered to be a papillary RCC. Interestingly, a recent study by Brunelli et al19 found no evidence for an accumulation of additional chromosomal aberrations in papillary RCC over papillary adenomas. This suggests that cytogenetic features are not able to replace the current size‐based method of distinguishing the two entities.
Take‐home messages
While rare, morphologically and cytogenetically distinct primary renal neoplasms can occur one within the other.
Extensive papillary architecture is on impermissable feature of renal oncocytoma.
Type I papillary renal cell carcinomas and papillary adenomas closely correspond both morphologically and cytogenetically and size ⩽5 mm is the feature that distinguishes the two entities.
In summary, we describe the unusual incidental finding of a papillary RCC within an oncocytoma. This has not been previously described to the best of our knowledge.
Abbreviations
RCC - renal cell carcinoma
Footnotes
Competing interests: None declared.
References
- 1.Eble J N, Sauter G, Epstein J I, Sesterhenn I A. eds. World Health Organization classification of tumours. Pathology and genetics of tumours of the urinary system and male genital organs. Lyon: IARC Press, 2004
- 2.Linehan W M, McClellan M W, Zbar B. The genetic basis of cancer of the kidney. J Urol 20031702163. [DOI] [PubMed] [Google Scholar]
- 3.Fuzesi L, Gunawan B, Braun S.et al Cytogenetic analysis of 11 renal oncocytomas: further evidence of structural rearrangements of 11q13 as a characteristic chromosomal anomaly. Cancer Genet Cytogenet 19981071–6. [DOI] [PubMed] [Google Scholar]
- 4.Kovacs G, Szucs S, Eichner W.et al Renal oncocytoma: a cytogenetic and morphologic study. Cancer 1987592071–2077. [DOI] [PubMed] [Google Scholar]
- 5.Crotty T B, Lawrence K M, Moertel C A.et al Cytogenetic analysis of six renal oncocytomas and a chromophobe renal cell carcinoma. Evidence that ‐Y, ‐1 may be a characteristic anomaly in renal oncocytomas. Cancer Genet Cytogenet 19926161–66. [DOI] [PubMed] [Google Scholar]
- 6.Pavolich C P, Walther M M, Eyler R A.et al Renal tumors in the Birt‐Hogg‐Dube syndrome. Am J Surg Pathol 2002261542–1552. [DOI] [PubMed] [Google Scholar]
- 7.Speicher M R, Schoell B, du Manoir S.et al Specific loss of chromosomes 1,2,6,10,13,17, and 21 in chromophobe renal cell carcinomas revealed by comparative genomic hybridization. Am J Pathol 1994145356–364. [PMC free article] [PubMed] [Google Scholar]
- 8.Delahunt B, Eble J N. Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol 199710537–544. [PubMed] [Google Scholar]
- 9.Reuter V E, Tickoo S K. Adult renal tumors. In: Mills SE, ed. Sternberg's diagnostic surgical pathology. 4th edn. Philadelphia: Lippincott Williams & Wilkins, 20041956
- 10.Skinnider B, Folpe A, Hennigar R.et al Distribution of cytokeratins and vimentin and adult renal neoplasms and normal renal tissue. Potential utility of a cytokeratin antibody panel in the differential diagnosis of renal tumors. Am J Surg Pathol 200529747–754. [DOI] [PubMed] [Google Scholar]
- 11.Kovacs G, Fuzesi L, Emanual A.et al Cytogenetics of papillary renal cell tumors. Genes Chromosomes Cancer 19913249–255. [DOI] [PubMed] [Google Scholar]
- 12.Noguchi S, Nagashima Y, Shuin T.et al Renal oncocytoma containing “chromophobe” cells. Int J Urol 19952279–280. [DOI] [PubMed] [Google Scholar]
- 13.Salido M, Lloreta J, Melero C.et al Insertion (8;11) in a renal oncocytoma with multifocal transformation to chromophobe renal cell carcinoma. Cancer Genet Cytogenet . 2005;163160–163. [DOI] [PubMed]
- 14.Beatty B G, Mai S, Squire J.FISH (fluorescence in situ hybridization). 4‐7:2002. Oxford: Oxford University Press
- 15.Amin M B, Crotty T B, Tickoo S K.et al Renal oncocytoma: a reappraisal of morphologic features with clinicopathologic findings in 80 cases. Am J Surg Pathol 1997211–12. [DOI] [PubMed] [Google Scholar]
- 16.Tickoo S K, Reuter V E, Amin M B.et al Renal oncocytosis: a morphologic study of fourteen cases. Am J Surg Pathol 1999231094–1101. [DOI] [PubMed] [Google Scholar]
- 17.Grignon D J, Eble J N. Papillary and metanephric adenomas of the kidney. Semin Diagn Pathol 19981541–53. [PubMed] [Google Scholar]
- 18.Kovacs G. The value of molecular genetic analysis in the diagnosis and prognosis of renal cell tumours. World J Urol 19941264–68. [DOI] [PubMed] [Google Scholar]
- 19.Brunelli M, Eble J N, Zhang S. Gains of chromosomes 7, 17, 12, 16, and 20 and loss of Y occur early in the evolution of papillary renal cell neoplasia: a fluorescent in situ hybridization study. Mod Pathol 2003161053–1059. [DOI] [PubMed] [Google Scholar]