Abstract
Magnetic resonance images of the brain were obtained from 2 gorillas (Gorilla gorilla gorilla), 4 orangutans (Pongo pygmaeus), 14 chimpanzees (Pan troglodytes), and 4 bonobos (Pan paniscus). The region on the motor cortex of humans identified as responsible for motor skill of the hand (the “knob”) was identified in this sample on consecutive 1-mm axial scans. The shape of the knob area was traced on each scan from both hemispheres, and the area from all scans was summed to calculate the knob volume. The width of the knob was also measured and correlated highly with knob volume. A significant population-level leftward asymmetry in the volume and width of the knob was revealed (p < .05). Species differences in knob asymmetry and overall volume were not significant, but the variability in overall volume between species was substantial. Selection for the evolution of a neuroanatomical representation of the hand in primates and an evolutionary trend toward population-level right handedness are discussed.
Recent studies in human subjects have identified a neuroanatomical landmark referred to as the “knob,” which extends from the precentral gyrus into the central sulcus and has been described as exhibiting the shape of an inverted omega (Ω) or a horizontal epsilon (ε) in the axial plane (Boroojerdi et al., 1999; Yousry et al., 1997). Functional imaging studies have revealed that the knob represents the location of the hand and digit control on the motor strip (Boroojerdi et al., 1999; Pizzella, Tecchio, Romani, & Rossini, 1999; Yousry et al., 1997). Additional structural and functional imaging studies in human subjects have indicated that the knob is larger and more active in the hemisphere contralateral to the subject's preferred hand, which in most cases is the right hand (Triggs, Calvanio, & Levine, 1997). Taken together, the data suggest that the knob represents the neuroanatomical substrate of the hand in humans and may correlate with functional asymmetries associated with preferential hand use and skill.
Primates are unique in that they have evolved hands (Napier, 1962; Napier & Napier, 1985). Within the order Primate, there has been selection toward increasing neural control of individual digits, which presumably reflects greater motor skill and dexterity in more recently evolved species such as the great apes (Preuschoft & Chivers, 1993). Increasing motor skill and dexterity has been proposed to underlie the evolution of a number of human traits including language, speech, and advanced cognitive abilities such as tool use and handedness (Bradshaw & Rogers, 1993; Christel, 1993). There has been a significant amount of research describing both the morphology and function of the hands in different primate species (Connolly, 1998). In terms of the function of the hand, the overwhelming emphasis has been on the relationship between morphology and grip preferences. For example, the hands of different primate species have clearly adapted morphologically to accommodate different locomotor and foraging demands (Fragaszy, 1998). Another area of research has focused on phylogenetic variation in the grip types used to pick up objects of different size and shape (e.g., Christel, 1993; Torigoe, 1985). Christel (1993) has described the distribution of different grip types used by apes and monkeys to pick up small foods. Of particular interest has been the relative use of precision grips, including tip-to-tip prehension using the thumb and index finger. Christel (1993) suggests that the use of fine motor prehension increases in species that are more closely related to humans. For example, she reports that gibbons exhibited the least amount of variability in precision gripping, whereas orangutans showed the most. Christel (1993) also reported that chimpanzees, when compared with the other apes, showed the most “individuality” in their gripping techniques.
Given the importance of the hand in primate evolution, the purpose of this study was to examine whether the knob could be localized and quantified in different nonhuman primates. In addition, we were interested in assessing asymmetries in the knob area. As noted previously, motor thresholds in response to cortical stimulation are lower in the dominant hand, which is usually the right hand in humans (Triggs et al., 1997). There has been a recent resurgence of interest in the evolution of handedness in nonhuman primates that both supports and negates evidence of population-level asymmetries in various nonhuman primate species (for reviews, see Bradshaw & Rogers, 1993; Hopkins, 1996, 1999; McGrew & Marchant, 1997). One problem with the existing comparative data is the lack of comparisons on identical measures of hand use in various primate species. However, for quadrupedal and bipedal reaching, as well as coordinated bimanual actions, great apes appear to be more right-handed compared with Old and New World monkeys and prosimians (Hopkins, 1995; Ward, Milliken, & Stafford, 1993; Westergaard, Kuhn, & Suomi, 1998; Westergaard & Suomi, 1996). There is also recent evidence that great apes have smaller ratios in the size of the corpus callosum relative to brain volume (Rilling & Insel, 1999) and that these smaller ratios are associated with increasing neuroanatomical asymmetries (Hopkins & Rilling, 2000). Taken together, these data suggest that great apes have more asymmetric brains than other nonhuman primates, but it is not clear how this is associated with the expression of functional asymmetries such as handedness. If the knob represents the neural substrate of the hand and, potentially, hand preference, then it is possible that this anatomical region will be larger in the left hemisphere compared with the right hemisphere.
Method
Subjects
Magnetic resonance images (MRI) of the brain were collected in a sample of 24 great apes, including 2 gorillas (Gorilla gorilla gorilla), 4 orangutans (Pongo pygmaeus), 14 chimpanzees (Pan troglodytes), and 4 bonobos (Pan paniscus). Four chimpanzee brains were scanned postmortem (specimens were removed from skull and preserved in 10% Formalin: 2 brains were less than 1 month postmortem, and 2 were approximately 5 years postmortem); the remaining subjects were alive and healthy at the time of data collection.
Hand Preference
The knob asymmetries were correlated with two hand preference measures that were previously collected from the chimpanzees at the Yerkes Regional Primate Research Center (YRPRC) and have been described in detail elsewhere (Hopkins, 1994, 1995; Hopkins & Pearson, 2000). The first task is referred to as the “tube” task and was designed to assess coordinated bimanual actions. Peanut butter is smeared on the inside edges of both ends of a polyvinyl chloride pipe (approximately 15.0 cm in length and 2.5 cm in diameter) so that the subjects may not obtain all the reward with their tongue but must use their fingers. The pipe is handed to the subjects in their home cages, and a focal sampling is used to collect individual data from each subject. The experimenter records the hand used to extract the peanut butter. Data were collected until the subject dropped the tube or returned it to the experimenter. Each subject received at least two test sessions separated by at least 2 days, and a minimum of 25 responses was obtained from each subject. A test–retest correlation indicated reliable hand use between test sessions (r = .66, p < .01).
The second measure of hand preference is referred to as the “feed” task and is intended to assess independent bimanual hand use. The chimpanzees were observed during their regular afternoon feeding at YRPRC, at which they received handfuls of fruits and vegetables. As the caregivers approached the front of the cages and handed the food through the mesh slots (5 cm in diameter), the chimpanzees took the food and positioned themselves apart from their cage mates. Typically, the subjects used one hand to feed and the other to hold the remaining food items. The feeding hand (interpreted as the preferred hand) was recorded only if the opposite hand was holding the remaining food portions, not if the opposite hand was used in postural support. The subject was also required to feed with the preferred hand for at least 3 s. Over the entire colony, there was an average of 32 responses per chimpanzee over a 60-day period. A test–retest correlation indicated that the feed measures were reliable over the 60-day period (r = .73, p < .001).
MRI Procedure
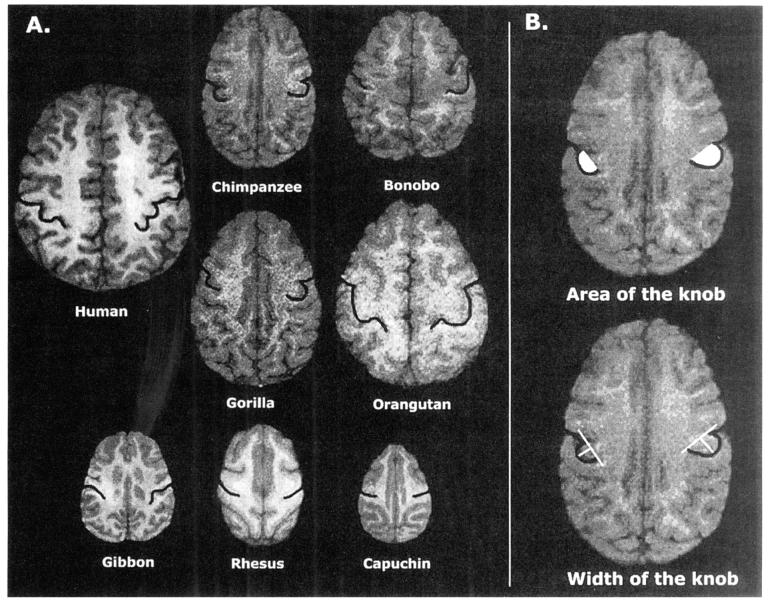
The collection of MRI scans followed procedures described in detail elsewhere (Hopkins, Marino, Rilling & MacGregor, 1998). Briefly, all brain scans were collected with two 1.5 Tesla superconducting magnets. For all subjects, T1-weighted images were collected in the transverse plane by means of a gradient echo protocol (pulse repetition = 19.0 ms, echo time = 8.5 ms, slice thickness = 1.2 mm, slice overlap = 0.6 mm, number of signals averaged = 8, matrix = 256 × 265). The raw images were reformatted with Easy Vision multiplanar formatting software (Philips Medical Systems, 1998) in the axial plane. Bicommissural lines were used to align the images in all three planes before cutting into 1-mm slices, which were archived and transferred to YRPRC for image analyses. The knob was localized in serial 1-mm slices in the axial plane by the use of procedures similar to those used in human brain specimens (Yousry et al., 1997). Two procedures were used to assess asymmetries in the size of the knob, which has been described as shaped like a horizontal epsilon or inverted omega, projecting into the postcentral gyrus. Both methods of quantification were made with NIH Image software that was adapted for use in the Windows platform (Scion Corporation, Frederick, MD). The dorsal and ventral edges of the knob served as markers for defining the boundaries of the area. In the first method, the area of the entire knob was traced on each slice and hemisphere by using a mouse-driven pointer (see Figure 1). To derive a volume of the knob for each hemisphere, the area traced on all scans was summed and multiplied by the number of axial slices in which the knob could be identified (ranging from 5 to 13 slices in each hemisphere). The second technique used to quantify asymmetry was similar to the method used in human subjects by Yousry and colleagues (1997). With the use of the area measurements described above, the scans with the largest knob area from the left and right hemisphere were selected. A straight line was then drawn from the dorsal and ventral boundaries, or the edges of the protruding knob, which define its length. At the greatest width of the protruding knob, a second straight line was drawn perpendicular to the first line (Figure 1). The length of this line was quantified to represent knob width.
Figure 1.
A: Axial views of nonhuman primate specimens with the central sulcus (and knob) traced in black. On initial evaluation of the magnetic resonance imaging scans, quantification of the knob was not possible in the Cebidae, Cercopithecidae, or Hylobatidae specimens. This was due to the fact that there was no protrusion of the precentral gyrus into the central sulcus, which defines this morphological landmark; thus, quantifying the knob was restricted to the Pongidae sample. B: A visual representation of the methods used to quantify area (white shaded region) and width (white line bisecting central sulcus) of the knob.
Data Analysis
A handedness index was derived to reflect direction of hand preference for both the tube and feed task. The handedness index score was determined by subtracting the number of left-hand responses from the number of right-hand responses and dividing by the total number of responses (R−L)/([R+L] × 0.05). Positive values reflected right-hand biases, and negative values reflected left-hand biases. The absolute values represent strength in hand use.
To assess asymmetries in knob volume, we subtracted right hemisphere volumes from left hemisphere volumes and divided by the total volume, and multiplied by .05 (R−L)/([R+L] × 0.5), to derive an asymmetry quotient (AQ). A positive AQ reflected a right hemisphere bias, and a negative AQ represented a left hemisphere bias. The absolute value reflected the magnitude in volume asymmetry. To assess asymmetry in width of the knob, the right hemisphere value was subtracted from the left hemisphere value; thus, a negative value represents a leftward bias, and a positive value reflects a rightward bias. For descriptive analyses, and consistent with previous studies (Hopkins et al., 1998), subjects were classified as left- or right-hemisphere dominant if they had AQ values with a magnitude greater than .025, regardless of direction. Subjects with a magnitude of asymmetry smaller than .025 were classified as having no bias.
Last, intra- and interrater reliability for measuring the volume and width of the knob for 5 randomly selected subjects (which included 1 bonobo, 1 gorilla, 1 orangutan, and 2 chimpanzees) were examined. The second rater was unaware of the orientation of the brain and the subject names. The interrater correlation coefficients for the volume and width of the knob were .843 and .834, respectively. The intrarater correlation coefficients were .868 and .783, for knob volume and width, respectively.
Results
Population Effects
In terms of descriptive analysis of the ape sample, single omega-shaped knobs were seen in only 10 of the 24 left hemispheres and 15 of the 24 right hemispheres. For the remaining 14 left hemispheres and 9 right hemispheres, the knob transitioned between an omega- and an epsilon-shaped knob. There was not a single hemisphere that exhibited only an epsilon-shaped knob throughout all axial slices. A McNemar test did not, however, reveal a significant difference in the distribution of omega- and epsilon-shaped knobs between the two hemispheres.
Additional descriptive analyses revealed that a significant proportion of the directional AQ values for knob volumes were leftward. Of the 24 subjects, 19 showed a leftward asymmetry, 2 showed a rightward asymmetry, and 3 had no bias in knob volume, a distribution that differs significantly from chance, χ2(2, N = 24) = 22.75, p < .01 (see Table 1). A one-sample t test indicated that the leftward asymmetry in knob volume was also significant at the population level, t(23) = −3.54, p < .01. When descriptive analyses were completed for the knob width asymmetry values, 18 apes exhibited a leftward asymmetry, 5 had a rightward asymmetry, and 1 showed no asymmetry. Population-level effects were also evident for this leftward asymmetry in knob width, as shown by a one-sample t test, t(23) = −3.19, p < .01. In addition, the two measures of knob asymmetry were positively correlated, r(23) = .595, p < .01, indicating that both approaches assess similar degrees of asymmetry at the individual level.
Table 1.
Individual Knob Asymmetry Data in Great Ape Sample
| Species and name |
Sex | Area (mm3) | Volume AQ |
Width difference (mm) |
|---|---|---|---|---|
| Pan paniscus | ||||
| Bosondjo | M | 416.94 | −.104 | −1.15 |
| Brian | M | 294.37 | −.114 | −2.34 |
| Jill | F | 370.87 | −.121 | −1.60 |
| Lorel | F | 661.42 | −.271 | −6.28 |
| Pan troglodytes | ||||
| Cheri (cdvr) | F | 437.29 | −.128 | −1.30 |
| Ada (cdvr) | F | 562.76 | −.059 | −2.01 |
| Donald | M | 645.55 | −.102 | −3.29 |
| Jimmy Carter | M | 620.00 | −.027 | −1.68 |
| Jeannie | F | 706.39 | −.217 | −3.23 |
| Hoboh (cdvr) | M | 580.38 | −.264 | −4.53 |
| Kengee | F | 548.18 | −.220 | −0.67 |
| Lana | F | 450.51 | −.026 | 0.00 |
| Lazarus | M | 479.34 | .001 | 0.50 |
| Lulu | F | 306.81 | .214 | 0.08 |
| Mary | F | 275.91 | .132 | 4.33 |
| Merv | M | 581.24 | .019 | −1.02 |
| Panzee | F | 606.30 | .023 | −2.98 |
| Storer (cdvr) | M | 461.76 | −.080 | −0.73 |
| Pongo pygmaeus | ||||
| Hati | F | 1,176.40 | −.146 | 1.19 |
| Mentubar | M | 243.73 | −.441 | −8.05 |
| Minyak | M | 2,125.70 | −.062 | −2.66 |
| Molek | M | 716.68 | −.101 | −2.75 |
| Gorilla gorilla gorilla | ||||
| Kekla | M | 992.13 | −.125 | 1.26 |
| Kinyani | F | 347.82 | −.098 | −0.53 |
Note. AQ = asymmetry quotient; M = male; F = female; cdvr = postmortem specimen.
Sex and Species Differences
An independent samples t test failed to reveal significant differences between males and females for the directional asymmetry values of knob width, knob volume, or the absolute AQ values of knob volume. Using a one-way analysis of variance, we also compared the orangutans, chimpanzees, and bonobos on both the asymmetry (AQ) and the total area of the knob. Gorillas were omitted from this analysis because only 2 specimens were available. No significant differences were found between species in either overall volume or asymmetry measures of the knob. However, inspection of Table 1 clearly indicates that orangutans showed relatively larger values compared with the remaining ape species.
Handedness Data
For a subsample of the great apes, hand preference data were available for the tube (n = 16) and feed (n = 19) tasks. Hand preferences for the two measures do not correlate with each other and therefore may assess different aspects or dimensions of hand use in chimpanzees (Hopkins & Pearson, 2000). Correlation coefficients between the tube task and the knob area and knob width AQ scores were .040 and .374, respectively, neither of which was significant at p < .05. The correlation coefficients between the feed measure and the knob area and knob width AQ values were −.070 and −.230, respectively. Neither of these coefficients reached conventional levels of statistical significance. Last, we correlated the absolute degree of asymmetry for the feed and tube tasks with the absolute values for the knob area and knob width AQ scores. None of these correlation coefficients approached statistical significance.
Discussion
The data reported here are the first evidence that the morphology of the motor hand area can be located and quantified in the great apes and that this neuroanatomical region is asymmetric. It is interesting that this same knoblike structure is depicted in many figures of the chimpanzee brain that identify the area that is localized for hand and thumb movement when stimulated by electrical current (for review, see Bailey, Bonin, & McCulloch, 1950). Unfortunately, these investigators did not quantify this knob area directly but instead focused on the motor responses involved in response to stimulation of this area. On the basis of these early brain stimulation experiments, the hand is similarly represented in the same region of the precentral gyrus in chimpanzees and humans (for review, see Bailey et al., 1950).
In this sample of great apes, there was a significant population-level leftward asymmetry in the width and volume of the knob area. Although there has been considerable investigation of neuroanatomical asymmetries in nonhuman primate brains (for reviews, see Falk et al., 1990; LeMay, 1985), as far as we know, this is the first report on the identification and asymmetry in the knob in nonhuman primates. At present, what the functional correlates of this neuroanatomical asymmetry are is not clear. The knob asymmetry does appear to correlate with hand use, at least on the basis of the preliminary results reported in this study, but this conclusion should be considered tentative given the small sample size. Moreover, in the study by Triggs and colleagues (1997) in humans, the difference in right and left motor thresholds established with transcranial magnetic stimulation correlated higher with hand dominance for tasks of independent finger movement, such as the peg board or finger-tapping, as opposed to a task that required the whole-hand grip strength. Unfortunately, both the handedness tasks for which we have data at the YRPRC measure, to some extent, whole-hand manipulation, not independent finger movements. Although the tube task requires extraction of the peanut butter by a single digit, it does not require any prehension or opposition of the index finger with other digits, notably the thumb. Thus, until measures that assess motor skill and independent finger control are developed, the potential functional correlates of the knob will remain unresolved.
It is clear that there are individual differences in both the total area of the knob and degree of asymmetry of the knob in our sample, but the origin of these differences is not clear. Subjects with larger brain volumes have larger total knob area, r(17) = .69, p < .03, but neither brain volume, r(17) = −.09, ns, nor total knob area, r(22) = −.07, ns, were correlated with the size of the knob asymmetries. Of course, brain volume is associated with body size; this is relevant for our sample because the largest subjects, after adjusting for age and sex, were clearly the orangutans. Thus, the particularly large knob of the orangutans may simply be due to having a sample of subjects that were relatively large in body size. Notwithstanding, these findings are consistent with at least one other report suggesting that the motor cortex of the orangutan is relatively large compared with that of other great apes (Semendeferi, Damasio, Frank, & Van Hoesen, 1997). We would also emphasize that we cannot rule out the possibility that the size differences between the orangutans and other great apes have functional consequences that are presently unknown. Two immediate possibilities could include the degree of manipulative propensities or differences in the motor systems involved in locomotion and positional behavior.
The absence of a morphologically definable knob in the lesser apes, as well as Old World and New World monkeys, warrants some discussion in light of the fact that monkeys, particularly capuchin monkeys, use their hands extensively for manipulation. The most parsimonious explanation is that there is simply not enough gyrification in the precentral gyrus of the lesser ape and monkey brains to create the formation of a knoblike structure. Great apes have larger gyrification indices than lesser apes and monkeys (Zilles, Armstrong, Moser, Schleicher, & Stephan, 1989), and the presence of the knob may be a manifestation of this overall effect in this taxonomic family. Differences in morphological structures used to quantify asymmetries in different areas of the brain are not unique to the knob. For example, the planum temporale can be morphologically defined in great apes, but not in lesser apes and monkeys (see Hopkins et al., 1998), and this creates a similar problem for comparative studies of the posterior portion of the temporal lobe in apes and monkeys. What should be emphasized is that the differences between apes and monkeys in morphology of specific regions of the brain do not generalize into differences in function of the homologous areas of the brain. Rather, they reflect the extent to which similar morphological landmarks can be used in comparative studies of neuroanatomy.
In conclusion, the neuroanatomical substrate of the hand can be anatomically defined in great apes (but not monkeys or lesser apes) by using landmarks that define the knob in the human brain. Furthermore, the knob is larger in the left hemisphere in great apes, suggesting that functional asymmetries associated with hand preference or skill may be evident in great apes, although it is unlikely that the tube or feed tasks represent the hand skill localized in the knob (but see McGrew & Marchant, 1997). Last, there was some variation in the size of the knob in different great ape species, and this variation may be due to inherent differences in hand morphology, motor skill, or adaptations of the hand to different locomotor systems. The current findings provide for a novel approach to any future assessments of the neuroanatomical substrates associated with the evolution of the hand and associated motor skills from a phylogenetic perspective.
Acknowledgments
This work was supported in part by National Institutes of Health Grants RR-00165, NS-29574, NS-36605, and HD-38051. We thank Jim Rilling and Tom Insel for many hours of dedication spent in collecting significant proportions of the magnetic resonance imaging scans. We also thank Brent Swenson and the rest of the veterinary staff for assisting in the care of the apes during scanning.
Contributor Information
William D. Hopkins, Department of Psychology, Berry College and Division of Psychobiology, Yerkes Regional Primate Research Center, Atlanta, Georgia
Dawn L. Pilcher, Division of Psychobiology, Yerkes Regional Primate Research Center
References
- Bailey P, Bonin, Von G, McCulloch WS. The isocortex of the chimpanzee. The University of Illinois Press; Urbana, IL: 1950. [Google Scholar]
- Boroojerdi B, Foltys H, Krings T, Spetzger U, Thron A, Topper R. Localization of the motor hand area using transcranial magnetic stimulation and functional magnetic resonance imaging. Clinical Neurophysiology. 1999;110:699–704. doi: 10.1016/s1388-2457(98)00027-3. [DOI] [PubMed] [Google Scholar]
- Bradshaw J, Rogers LJ. The evolution of lateral asymmetries, language, tool use, and intellect. Academic Press; San Diego, CA: 1993. [Google Scholar]
- Christel M. Grasping techniques and hand preference in Hominoidea. In: Preuschoft H, Chivers D, editors. Hands of primates. Springer-Verlag; New York: 1993. pp. 93–108. [Google Scholar]
- Connolly KJ. The psychobiology of the hand. Cambridge University Press; Cambridge, MA: 1998. [Google Scholar]
- Easy Vision (Version 4.2) [Computer software] Philips Medical Systems; Eindhoven, the Netherlands: 1998. [Google Scholar]
- Falk D, Hildebolt C, Cheverud J, Vannier M, Helmkamp C, Konigsberg L. Cortical asymmetries in frontal lobes of rhesus monkeys (Macaca mulatta) Brain Research. 1990;512:40–45. doi: 10.1016/0006-8993(90)91167-f. [DOI] [PubMed] [Google Scholar]
- Fragaszy D. How nonhuman primates use their hands. In: Connolly KJ, editor. The psychobiology of the hand. Cambridge University Press; Cambridge, MA: 1998. pp. 77–96. [Google Scholar]
- Hopkins WD. Hand preference for bimanual feeding in a sample of 140 chimpanzees (Pan troglodytes): Ontogenetic and developmental factors. Developmental Psychobiology. 1994;27:395–408. doi: 10.1002/dev.420270607. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees: Cross-sectional analysis. Journal of Comparative Psychology. 1995;109:291–297. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Chimpanzee handedness revisited: 54 years since Finch (1941) Psychonomic Bulletin and Review. 1996;3:449–457. doi: 10.3758/BF03214548. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Heritability of hand preference in chimpanzees (Pan troglodytes): Evidence from a partial interspecies cross-fostering study. Journal of Comparative Psychology. 1999;113:307–313. doi: 10.1037/0735-7036.113.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Marino L, Rilling JK, MacGregor L. Planum temporale asymmetries in great apes as revealed by magnetic resonance imaging (MRI) NeuroReport. 1998;9:2913–2918. doi: 10.1097/00001756-199808240-00043. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Pearson K. Chimpanzee (Pan troglodytes) handedness: Variability across multiple measures of hand use. Journal of Comparative Psychology. 2000;114:115–125. doi: 10.1037/0735-7036.114.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Rilling JK. A comparative MRI study of the relationship between neuroanatomical asymmetry and interhemispheric connectivity in primates: Implication for the evolution of functional asymmetries. Behavioral Neuroscience. 2000;114:739–748. doi: 10.1037//0735-7044.114.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMay M. Asymmetries of the brains and skulls of nonhuman primates. In: Glick SD, editor. Cerebral lateralization in nonhuman species. Academic Press; New York: 1985. pp. 223–245. [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: Current issues in and meta-analyses of the behavioral laterality of hand function in nonhuman primates. Yearbook of Physical Anthropology. 1997;40:201–232. [Google Scholar]
- Napier JR. The evolution of the hand. Scientific American. 1962;207:156–162. doi: 10.1038/scientificamerican1262-56. [DOI] [PubMed] [Google Scholar]
- Napier JR, Napier PH. The natural history of primates. MIT Press; Cambridge, MA: 1985. [Google Scholar]
- Pizzella V, Tecchio F, Romani GL, Rossini PM. Functional localization of the sensory hand area with respect to the motor central gyrus knob. NeuroReport. 1999;10:3809–3814. doi: 10.1097/00001756-199912160-00016. [DOI] [PubMed] [Google Scholar]
- Preuschoft H, Chivers DJ. Hands of primates. Springer-Verlag; New York: 1993. [Google Scholar]
- Rilling JK, Insel TR. Differential expansions of neural projection systems in primate brain evolution. NeuroReport. 1999;10:1453–1459. doi: 10.1097/00001756-199905140-00012. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Damasio H, Frank R, Van Hoesen GW. The evolution of the frontal lobes: A volumetric analysis based on three-dimensional reconstructions of magnetic resonance scans of human and ape brains. Journal of Human Evolution. 1997;32:375–388. doi: 10.1006/jhev.1996.0099. [DOI] [PubMed] [Google Scholar]
- Torigoe T. Comparison of object manipulation among 74 species of nonhuman primates. Primates. 1985;26:182–194. [Google Scholar]
- Triggs WJ, Calvanio R, Levine M. Transcranial magnetic stimulation reveals a hemispheric asymmetry correlate of intermanual differences in motor performance. Neuropsychologia. 1997;35:1355–1363. doi: 10.1016/s0028-3932(97)00077-8. [DOI] [PubMed] [Google Scholar]
- Ward JP, Milliken GW, Stafford DL. Patterns of lateralized behavior in prosimians. In: Ward JP, Hopkins WD, editors. Primate laterality: Current behavioral evidence of primate asymmetries. Springer-Verlag; New York: 1993. pp. 43–76. [Google Scholar]
- Westergaard GC, Kuhn HE, Suomi SJ. Bipedal posture and hand preference in humans and other primates. Journal of Comparative Psychology. 1998;112:56–63. doi: 10.1037/0735-7036.112.1.55. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Suomi SJ. Hand preference for a bimanual task in tufted capuchins (Cebus apella) and rhesus macaques (Macaca mulatta) Journal of Comparative Psychology. 1996;110:406–411. doi: 10.1037/0735-7036.110.4.406. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus: A new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Moser KH, Schleicher A, Stephan H. Gyrification in the cerebral cortex of primates. Brain, Behavior and Evolution. 1989;34:143–150. doi: 10.1159/000116500. [DOI] [PubMed] [Google Scholar]