Abstract
This study examined intertask consistency in handedness across multiple measures of hand use in a sample of 187 chimpanzees (Pan troglodytes). Hand preferences for 2 to 6 measures were collected from the sample, and hand preference scores were derived on the basis of the individual hand preferences for each measure. Seven of 15 possible intratask correlations were significant, with some degree of clustering depending on the motor demands of the tasks. Two overall measures of handedness revealed population-level right-handedness in the chimpanzees, although the degree of bias was reduced for chimpanzees tested on more than 3 measures of hand use. The results are interpreted in the context of several recent studies that proposed theoretical models of handedness in nonhuman primates.
Approximately 90% of humans report themselves as being right-handed (Annett, 1985; Porac & Coren, 1981). The historical view has been that nonhuman animals, particularly nonhuman primates, do not exhibit population-level handedness (see Ettlinger, 1988; Lehman, 1993; Warren, 1980). In recent years, behavioral research in a variety of nonhuman primate species using a multitude of tasks has revealed that population-level handedness can be found in some species for certain measures (Bradshaw & Rogers, 1993; Hopkins, 1996; Ward & Hopkins, 1993). In addition to handedness, evidence of perceptual and cognitive asymmetries in a host of animal species has been reported (Bisazza, Rogers, & Vallortigara, 1998; Bradshaw & Rogers, 1993). These cumulative data clearly challenge the historical view purporting the uniqueness of hemispheric specialization to humans.
With the emergence of evidence of hemispheric specialization and handedness in animals, a fundamental question has arisen: Is the manifestation of handedness in animals the same as in humans? Corballis (1992) has suggested that nonhuman primate handedness reflects a “weaker” form of handedness than is evident in humans. Roney and King (1993) have made a similar argument with respect tO differences between human and nonhuman primate handedness. More recently, McGrew and Marchant (1994, 1997) have dichotomized laterality of hand functions on the basis of consistency in use within and between subjects and tasks. Specifically, according to McGrew and Marchant (1997), hand preference refers to within-task and within-subject variability in hand preference. Task specialization refers to situations in which most individuals within a sample show the same hand preference for one and only one measure. Manual specialization refers to circumstances in which the same individual uses the same hand across task, but all individuals within that sample show inconsistent preferences. Finally, true handedness reflects consistent hand use across all measures and individuals in a sample. McGrew and Marchant 1997) argued that most studies in nonhuman primates have failed to meet their definition of true handedness, and most fall into the categories of either task specialization or hand preference (but see Byrne & Byrne, 1991; Diamond & McGrew, 1994). Thus, according to McGrew and Marchant (1997), the evidence of comparable forms of hand preference in nonhuman primates compared with humans remains highly suspect.
Unfortunately, it is difficult to evaluate McGrew and Marchant's (1997) model of laterality because few handedness studies in nonhuman primates have assessed laterality by means of multiple measures (see Beck & Barton, 1972; Boesch, 1991; Byrne & Byrne, 1991; Fagot & Vauclair, 1991; Hopkins & Morris, 1993; Marchant & McGrew, 1991). The purpose of this study was to assess intertask variability in hand use in a sample of captive chimpanzees for which multiple measures of hand use have been obtained over the past several years. Specifically, in a series of studies, my colleagues and I have reported hand preferences for throwing, reaching, feeding, coordinated bimanual hand use, and tool use (Hopkins, 1993, 1994, 1995a, 1995b; Hopkins, Bard, Jones, & Bales, 1993; Hopkins & Rabinowitz, 1997). With the exception of certain forms of reaching, chimpanzees exhibit a population-level right-hand bias for each of the measures. Of interest to this study was the consistency in hand use across these different measures and the distribution of hand preference when configured on the basis of multiple measures rather than individual ones. According to McGrew and Marchant's (1994, 1997) model of handedness, if task-specific handedness is the norm for nonhuman primate populations, then combining measures of hand preference across multiple measures should yield a sample of ambidextrous subjects and a lack of population-level handedness. In contrast, if true handedness is the norm for nonhuman primate populations, then combining measures of hand preference across multiple measures should yield either population-level left- or right-handedness.
Method
Subjects
A total of 187, 107 females and 80 males, captive chimpanzees (Pan troglodytes) housed at the Yerkes Regional Primate Research Center (YRPRC) were the subjects in this study. Of those, 103 chimpanzees were nursery-reared, and 84 chimpanzees were mother-reared. Chimpanzees that stayed with their mothers 30 days or more were considered mother-reared. Chimpanzees that entered the YRPRC nursery before 30 days of age were considered nursery-reared (see Bard, 1994, for a description of nursery-rearing practices of chimpanzees at the YRPRC).
Hand Preference Measures
The behavioral tasks used to assess hand preferences in the chimpanzees have been described in detail elsewhere (Hopkins, 1993, 1994, 1995a, 1995b). Listed below is a brief description of each task and the general procedure used to assess hand preference.
Bimanual feeding (feed)
Each afternoon, the primates housed at the YRPRC received fruits and vegetables as part of their daily diet. Each chimpanzee usually received two oranges, one banana, some celery stalks, and/or carrots. After retrieving the food, the chimpanzees typically moved to a seating place and consumed the food, holding the extra pieces of food with one hand and feeding with the opposite hand. Hand use was recorded when the chimpanzees fed with one hand for a minimum of 3 s and the nonfeeding hand held the remaining portions of food. The dominant hand was recorded as the feeding hand.
Bipedal reaching (biped)
For this measure, food items were placed on the outside edge of the cage. The height of the food placement varied randomly between 0.5 and 1.0 m above the floor of the cage. Chimpanzees were required to locomote to the position of the food item and stand upright while reaching for the food item. The hand used to retrieve the food was recorded for each trial.
Quadrupedal reaching (quad)
For this measure, a piece of food (a peanut or raisin) was randomly thrown into the chimpanzee's cage. Chimpanzees had to locomote to the location and pick up the food. The hand used to pick up the food was recorded. A trial was only counted when the chimpanzees maintained a tripedal posture while reaching (i.e., one upper limb and two hind limbs were on the floor).
Simple reaching (reach)
Peanuts were placed 16 cm from the cage on a cement edge adjacent to the outdoor cage 10 cm above the ground. To retrieve the peanuts, chimpanzees had to reach through the cage mesh and grasp the food item and pull it back into the cage. The hand used to pick up the peanut was scored each trial.
Coordinated bimanual tube task (tube)
Polyvinyl chloride (PVC) tubes (24–31 cm long, 2.5 cm wide) with peanut butter smeared on the inside were given to the chimpanzees in their cage. The digit and hand used to remove the peanut butter were recorded each time a chimpanzee inserted its finger, removed peanut butter from the tube, and placed its finger in its mouth. Observations continued until the chimpanzee stopped showing interest in the tube (usually when it had eaten all of the peanut butter), dropped the tube for at least 10 s, or pushed the tube back out of its cage through the cage mesh.
Coordinated bimanual ball task (ball)
The motor demands of this task were similar to the tube task, only the materials were different. In symmetrical plastic balls 22 cm in diameter, 2.5-cm holes were cut on opposite sides of the stimulus. Peanut butter was then placed inside the ball on the underlying edge of the holes cut on each side. Similar to the tube task, the chimpanzee had to hold the ball with one hand and remove the peanut butter with a finger with the opposite hand. The hand used to extract the peanut butter was recorded each time a chimpanzee inserted its finger in the hole, removed its finger, and ate the peanut butter. Observations continued until the chimpanzee stopped showing interest in the ball (usually when it had eaten all of the peanut butter) or dropped the ball for at least 10 s.
Procedure
Not every chimpanzee was tested on each behavioral measure. The number of chimpanzees tested on the reach, quad, biped, feed, tube, and ball tasks were 70, 74, 55, 176, 188, and 53, respectively. For all of the measures, individual data were collected in the chimpanzees' home cage, including the indoor and outdoor portions of the enclosure. The home cages varied in size and shape depending on the housing assignment of each chimpanzee, but all cages were constructed of 5-cm wire cage mesh. In addition, the number of chimpanzees in each home cage varied from 2 to 16 animals. For each measure, focal animal-sampling techniques were used. Thus, the chimpanzees were not separated from their social groups for the purposes of data collection. At least 15 responses were recorded for each chimpanzee and each measure. The average number of responses for the tube, ball, feed, reach, biped, and quad tasks were 58, 56, 33, 56, 59, and 44, respectively. The number of chimpanzees tested on 2, 3, 4, 5, or 6 tasks were 43, 70, 33, 37, and 4, respectively.
Two general procedural controls were followed for all measures. First, the hand used by the experimenter in placing food items or handing test materials to the chimpanzees was always randomized across trials. This randomization was done to ensure that the chimpanzees were not imitating the hand use of the experimenters. Second, the presentation of food items, particularly for the biped, quad, and reach measures, was displaced temporally and spatially over time. That is, we placed items in different locations, and we tested chimpanzees over many days rather than on a single day of testing. Moreover, chimpanzees were required to reposition themselves 3 m or more away from the cage mesh and the experimenter before another trial was administered. This repositioning was done to ensure that each trial was not influenced by the hand used on the previous trial (see Lehman, 1993; Marchant & McGrew, 1991, for discussion). For the feed task, one observation was made on a given day, and observations were made over a 60-day period (see Hopkins, 1994, for description). However, one trial was not obtained every day for each chimpanzee because of factors such as the visibility of the chimpanzee or whether it had assumed the operational posture defined in the feed task. Thus, 60 data points were not necessarily obtained for all chimpanzees. Finally, for the ball and tube tasks, data were collected during two test sessions separated by at least 1 day. Each time the chimpanzees extracted food from either the PVC pipe or ball, the hand they used was recorded. No attempt was made to code for bouts of responses rather than the individual motor events. Some have argued that this is critical in evaluating individual hand preferences because there is a lack of independence of data points (McGrew & Marchant, 1997), but there is no empirical evidence to support this contention (Hopkins, 1999). The order of task presentation was randomized across chimpanzees and days.
Data Analysis
Several different means of characterizing chimpanzees' lateral bias were used in this study. First, for each chimpanzee, a z score based on the total frequency of right- and left-hand responses was determined for each measure. On the basis of the z scores, chimpanzees were classified as being either left-handed (z ≤ −1.64), right-handed (z ≥ 1.64), or nonpreferent (z > −1.64 and z < 1.64) for each measure. On the basis of these data, we adopted procedures, which are used in human-handedness studies (see Porac & Coran, 1981), to characterize each chimpanzee's composite handedness index. Specifically, chimpanzees with left, right, or nonsignificant hand preferences were given a score of −1, 1, or 0, respectively, for each measure. The categorical scores were then averaged across all measures (HISUM1), resulting in values ranging from −1.0 to 1.0. Negative values represented left-hand preferences, and positive values represented right-hand preferences. A value of 0 reflected an ambidextrous hand preference. In addition, a handedness index (HI) score was assessed for each chimpanzee and measure. The HI value was derived by subtracting the number of left-hand responses (#L) from the number of right-hand responses (#R) and dividing by the total number of responses, HI = (#R − #L)/(#R + #L). A second overall handedness score (HISUM2) was derived by calculating the average HI across all measures. As with the HISUM1 measure, negative values represented left-hand preferences, and positive values represented right-hand preferences. For both the HISUM1 and HISUM2 scores, strength in lateral bias was determined by taking the absolute value of the calculated value. Finally, a variable was created that reflected the number of tasks that were used to derive the HISUM1 and HISUM2 scores. Categorical data were analyzed by using nonparametric statistics (chi-square), and the continuous data were analyzed by using parametric statistics (correlations, t tests, and analysis of variance).
Results
Intertask Correlations and Individual Distributions in HI Scores
Table 1 depicts the intercorrelations in HI values between measures. Biped, quad, reach, and feed measures all significantly and positively correlated with each other. The tube and ball tasks also significantly and positively correlated, but neither of these measures correlated with the remaining four measures of hand use. Table 2 depicts the mean HI values for each measure, the standard errors, and associated t values. One-sample t tests revealed significant population right-hand biases for four of the six measures, including the tube, ball, feed, and biped measures. Quad and reach did not reach conventional levels of statistical significance.
Table 1.
Intercorrelations in Hand Use Between Different Measures
| Measure | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. Ball | — | |||||
| 2. Tube | .4616** (53) |
— | ||||
| 3. Feed | −.2385 (51) |
−.0040 (160) |
— | |||
| 4. Reach | .2582 (10) |
−.0235 (67) |
.3617** (63) |
— | ||
| 5. Biped | −.1116 (17) |
−.1531 (49) |
.3394* (53) |
.5889** (18) |
— | |
| 6. Quad | −.0913 (23) |
.0255 (65) |
.3162** (71) |
.6846** (19) |
.3785** (54) |
— |
Note. Numbers in parentheses indicate the number of pairs of scores.
p < .05.
p <. 01.
Table 2.
One-Sample t Tests for Each Measure of Hand Preference
| Measure | n | M | SE | t | p |
|---|---|---|---|---|---|
| Ball | 53 | .2710 | .079 | 3.43 | .001 |
| Tube | 188 | .1449 | .041 | 3.53 | .001 |
| Feed | 176 | .1118 | .035 | 3.19 | .002 |
| Reach | 70 | .1694 | .092 | 1.85 | .068 |
| Biped | 55 | .2094 | .065 | 3.20 | .002 |
| Quad | 73 | −.0251 | .045 | −0.55 | .582 |
Cumulative Measures of Hand Preference
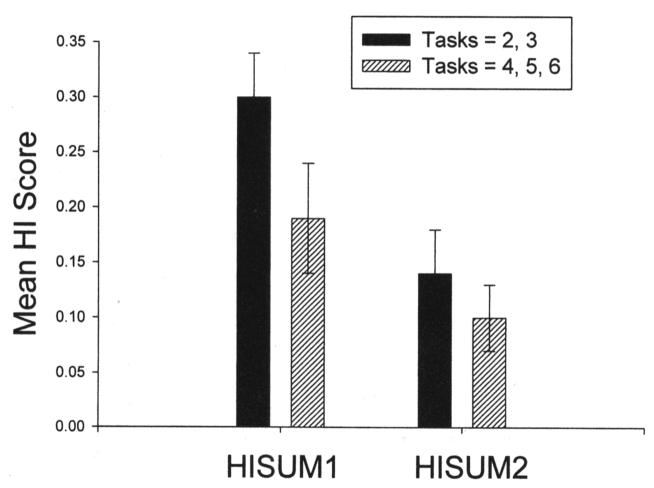
One-sample t tests indicated significant population-level right-handedness for the HISUM1, t(186) = 6.36, p < .001 (M = .239), and HISUM2, t(186) = 5.62, p < .001 (M = .152), measures. Pearson product–moment correlations revealed a significant negative correlation between the number of measures and the HISUM1 score, r(185) = −.155, p < .04, but no significant association for the HISUM2 score, r(185) = −.116, ns. For the HISUM1 score, the correlation indicated that chimpanzees that had been assessed on a greater number of tasks had lower HISUM1 values. The modal number of tasks assessed per chimpanzee was three; therefore, separate one-sample t tests were performed on chimpanzees with either two or three tasks and were contrasted with one-sample t tests for chimpanzees with either four, five, or six assessments. The mean HISUM1 and HISUM2 scores for each of these subgroups are displayed in Figure 1. As can be seen, the HISUM1 and HISUM2 scores are lower for the subgroup assessed on four, five, or six measures, compared with the chimpanzees assessed on two or three measures. Notwithstanding, in both subsamples, the means deviated significantly from zero, indicating population-level right-handedness.
Figure 1.
Mean HISUM1 and HISUM2 scores for each subsample of scores, depending on the number of measures deriving the individual score. For each distribution, one-sample t tests revealed significant population-level right-hand preferences (ts = 6.25, 5.07, 2.43, and 2.57 from left to right). Error bars indicate standard errors of the means. HI = handedness index.
As an alternative means of considering consistency across tasks, separate cumulative HI values were derived for the two and three most frequent measures of hand preference for which data had been collected from all chimpanzees. These included the feed, tube, and reach tasks. One measure (SUB-HI2), which included 160 chimpanzees, was based solely on the mean HI scores of the tube and feed measures. The second measure (SUB-HI3), which included 60 chimpanzees, was based on the mean HI scores of the tube, feed, and reach measures. For both the SUB-HI2, t(159) = 3.92, p < .001, and SUB-HI3, t(59) = 2.82, p < .009, scores, the means differed significantly from zero (M HI scores = .118 and .200, respectively), indicating population-level right-handedness.
Categorical Assessment of Hand Preference
In addition to the one-sample t tests, chimpanzees were categorized as either left-handed, right-handed, or ambidextrous on the SUB-HI2 and SUB-HI3 measures on the basis of the possible scores that could be derived for each compilation of categorical values (see Table 3). Specifically, on a given measure, a chimpanzee could be classified as left-, right-, or ambiguously handed, which derives an expected probability of .33. When multiplying .33 by the number of measures used to derive a specific hand preference classification, the expected probabilities of being classified into each category were calculated as (.33 × .33) for the two tasks (feed and tube) and (.33 × .33 × .33) for the three measures (feed, tube, and reach). The expected probabilities were then calculated for each possible outcome of hand preference on the basis of the number of possible outcomes multiplied by the probability of occurrence. Chi-square and goodness-of-fit tests were used to assess population asymmetries against a distribution of expected values on the basis of the probabilities of different outcomes in hand use.
Table 3.
Outcomes of Hand Preference Derived From a Categorical Scale of Measurement
| Measure | Outcome | ||||
|---|---|---|---|---|---|
| Hand use for two measures | |||||
| Average HI score | −1.0 | −.50 | 0 | +.50 | +1.0 |
| Observed distribution | 14 | 21 | 48 | 46 | 31 |
| Hand use combinations | L,L | L,A | L,R | R,A | R,R |
| A,L | R,L | A,R | |||
| A,A | |||||
| Hand use for three measures | |||||||
| Average HI score | −1.0 | −.67 | −.33 | 0 | +.33 | +.67 | +1.0 |
| Observed distribution | 2 | 5 | 7 | 16 | 11 | 9 | 10 |
| Hand use combinations | L,L,L | L,L,A | L,L,R | L,A,R | R,L,R | R,A,R | R,R,R |
| A,L,L | L,R,L | L,R,A | R,R,L | R,R,A | |||
| A,L,L | R,L,L | R,L,A | L,R,R | A,R,R | |||
| L,A,A | R,A,L | R,A,A | |||||
| A,L,A | A,A,A | A,R,A | |||||
| A,A,L | A,L,R | A,A,R | |||||
| A,R,L | |||||||
Note. HI = handedness index; L = left-handedness; A = ambidextrous; R = right-handedness.
Table 3 depicts observed frequencies of hand preference classifications on the basis of the two and three most common measures. For both measures, SUB-HI2, χ2(4, n = 160) = 28.06, p < .001, and SUB-HI3, χ2(6, n = 60) = 31.89, p < .001, the distribution of observed values differed significantly from the expected values. For subsequent analyses and to increase statistical power, chimpanzees with positive values were classified as right-handed and those with negative values were classified as left-handed. Chimpanzees with weighted scores of 0 were classified as ambidextrous. For the SUB-HI2 measure, the number of right-handed chimpanzees was significantly greater than the number of left-handed chimpanzees, χ2(1, n = 112) = 15.75, p < .001, and ambidextrous chimpanzees, χ2(1, n = 125) = 6.73, p < .01. Similarly, for the SUB-HI3, the number of right-handed chimpanzees was significantly greater than the number of left-handed chimpanzees, χ2(1, n = 44) = 5.80, p < .03, and ambidextrous chimpanzees, χ2(1, n = 46) = 4.66, p < .05.
Sex and Rearing Effects on Strength and Direction in Hand Preference
For these analyses, the HISUM1 and absolute value of HISUM1 were used as dependent measures in an analysis of covariance (ANCOVA). Sex and rearing history served as independent variables, and age and the number of measures served as covariates. For the HISUM1 score, no significant main effects or interactions were found. Similarly, no significant main effects or interactions were found for strength in hand preference in relation to rearing history and sex.
Comparisons With Other Species and Chimpanzees in Other Settings
The mean HI score for the YRPRC chimpanzees was .239. The extent to which these observed findings apply to other great ape species and to chimpanzees in other settings is a question of central interest to the interpretation of these findings and to the issue of whether nonhuman primates exhibit handedness in any form comparable to humans. To address this question, we assessed the degree of handedness for multiple measures of hand use on the basis of the existing published literature on great apes. To be included in the subsequent analyses, one or more studies had to provide at least two individual measures of hand preference for a given sample or individual subject. The data did not necessarily have to come from the same study, but subject identification in studies with overlapping subjects had to be clearly demarcated in the article. Studies in which individual data were not provided were not included in this analysis, although multiple measures of hand use may have been collected for a number of subjects (e.g., Aruguete, Ely, & King, 1992; Marchant & McGrew, 1996).
Using these criteria, we obtained data from a total of 343 great apes, which included 130 gorillas (Gorilla gorilla), 108 chimpenzees, 66 orangutans (Pongo pygmalus), and 39 bonobos (Pan paniscus). Listed in Table 4 are the studies that were included in this analysis, as well as the great ape species studied, the mean HI score for the sample, and the standard error of the HI values. From the available data, the sex, rearing history, and age of each subject were recorded. With respect to rearing history, subjects were numerically coded in the data file as wild, captive born, or having an unknown rearing history. Regarding age, the subjects were numerically classified as adults, adolescents, or juveniles. Categorical age classifications were used instead of numerical ages because not all authors provided exact ages of their subjects in quantitative values.
Table 4.
Studies Used in Meta-Analysis of Handedness in Great Apes
| Study | Rearing | n | M HI | SE |
|---|---|---|---|---|
| Chimpanzee | ||||
| Boesch (1991) | W | 20 | .083 | .10 |
| Sugiyama, Fushimi, Sakura, & Matsuzawa (1993) | W | 15 | .078 | .11 |
| McGrew, Marchant, Wrangham, & Klein (1999) | W | 8 | .313 | .31 |
| Colell, Segarra, & Sabater-Pi (1995) | W,C | 21 | −.021 | .09 |
| Marchant (1983) | W,C,U | 23 | −.094 | .17 |
| Heestand (1986) | U | 20 | .200 | .15 |
| Bresard & Bresson (1983) | C | 1 | 1.00 | |
| Bonobo | ||||
| Hopkins & de Waal (1995) | W,C,U | 21 | .047 | .04 |
| Shafer (1997) | C | 13 | .175 | .09 |
| De Vleeschouwer, Van Elsacker, & Verheyen (1995) | U | 5 | −.061 | .06 |
| Gorilla | ||||
| Byrne & Byrne (1991) | W | 37 | .207a | .13 |
| Heestand (1986) | U | 24 | .379 | .17 |
| Shafer (1993) | W,C | 47 | .234 | .08 |
| Olson, Ellis, & Nadler (1990) | W,C | 12 | .360 | .16 |
| Fagot & Vanclalr (1991) | W,C | 10 | −.108a | .20 |
| Orangutan | ||||
| Colell et al. (1995) | W | 2 | .585 | .09 |
| Heestand (1986) | U | 7 | .857 | .17 |
| Hopkins (1999) | W,C | 8 | .229 | .26 |
| Cunningham, Forsythe, & Ward (1989) | C | 1 | .143 | |
| Bresard & Bresson (1983) | C | 1 | .800 | |
| Olson et al. (1990) | W,C | 11 | .121 | .11 |
| Rogers & Kaplan (1996) | U | 36 | −.124 | .05 |
Note. HI = handedness index score; W = wild; C = captive; U = unknown.
Values were reverse scored in order to be on the same scale of measurement as the remaining studies.
For each subject in each study, the scores of −1, 1, and 0 were entered into a spreadsheet corresponding to the reported significance of hand preference for a given measure used in the study, just as was done with the YRPRC chimpanzees. For example, Boesch (1991) reported individual hand preferences for a portion of subjects for up to four measures of hand use (reaching, picking, grooming, and nut-cracking). As with our own data, subjects with a left-hand preference, right-hand preference, or no preference were given values of −1, 1, or 0, respectively, for each measure. The individual handedness score was derived by averaging the categorical scores across all measures. This approach was used rather than the raw HI value because the exact number of left- and right-hand responses were not available for all subjects, and this was a simpler approach that allowed for use of a larger number of subjects. Also, as with the previous analysis, for each subject, the number of measures that were used to derive an individual handedness score was recorded.
Analyses
In the initial analysis, the HI scores were compared between species by using an ANCOVA. Species was the independent variable, and the HI score was the dependent variable. Age and the number of measures variable served as the covariates. Depicted in Figure 2 are the weighted mean HI scores for each species. Included in the chimpanzee sample were the 187 chimpanzees housed at the YRPRC. Despite considerable variation between species, no significant differences were found between species. One-sample t tests on the HI values for each species revealed population-level right-handedness for the gorillas, t(129) = 4.17, p < .01, and chimpanzees, t(294) = 5.32, p < .001, with a borderline significant effect for the bonobos, t(38) = 1.85, p <. 10.
Figure 2.
Mean handedness index (HI) scores for each great ape species in the meta-analysis. Note that the means are slightly different from those that can be derived from Table 4. This is because the mean HI scores are derived after weighting the scores for variation in the age of the subject and in the number of measures used to derive the HI value. Error bars indicate standard errors of the mean.
Next, we compared the HI scores for all the great apes as a function of their rearing history. This was done to test whether captive-born apes were more right-handed compared with either wild-born apes or apes of unknown origin. McGrew and Marchant (1997) have suggested that population-level right-handedness may be specific to captive primates because of the biased nature of their environments. For this analysis, another ANCOVA was performed with rearing history and sex serving as between-groups variables. As with the previous analysis, the number of measures and age served as covariates, and the HI score served as the dependent variable. Although the mean HI scores for captive-born apes (M = .236) were higher than for wild-born apes (M = .121) and apes of unknown origin (M = .126), the ANCOVA did not reveal a significant difference. However, it did approach conventional levels of significance, F(2, 523) = 2.42, p < .10. Notwithstanding, one-sample t tests performed on each subsample revealed significant population-level right-handedness for the apes that were captive-born, t(256) = 6.56, p < .01; wild-born, t(174) = 2.69, p < .01; and of unknown origin, t(101) = 2.21, p < .01.
In the final analysis, the HI scores for the YRPRC chimpanzee colony were compared with the HI scores for all other chimpanzees. This was done to test whether the population-level right-handedness reported for the YRPRC chimpanzees was unique or whether it was consistent with other reports when using a common means of characterizing hand preference. For this analysis, a variable was created that reflected dummy codes for YRPRC chimpanzees contrasted with all other chimpanzees. Number of measures and age served as covariates. The mean HI scores for the YRPRC and the comparison groups were .220 and .102, respectively, which approached statistical significance, F(1,293) = 3.57, p < .06. This result suggests that the YRPRC chimpanzees were more right-handed than the data reported for other captive and wild chimpanzees. However, for both sample populations, the mean HI scores differed significantly from chance, as revealed by one-sample t tests. Thus, the borderline significant difference reflects one of degree rather than a qualitative difference in the distribution of hand preference.
Discussion
Four significant findings emerged from this study. First, significant intertask correlations were found. Seven out of a possible 15 correlations were both significant and positive. Second, cumulative handedness scores based on either mean individual HI scores or on the mean weighted scores revealed significant population-level right-handedness. The number of measures were negatively correlated with the cumulative handedness scores, indicating that chimpanzees that were assessed on a greater number of tasks had lower handedness scores. Notwithstanding, dividing the sample into chimpanzees that were assessed on only two or three measures contrasted with chimpanzees assessed on four, five, or six measures still revealed population-level right-handedness for the samples. Third, limiting the analyses to chimpanzees that had been tested on the same two or three measures revealed significant population-level right-handedness by means of both a continuous scale of measurement as well as categorical data. Fourth, a comparison of the findings between the YRPRC chimpanzees, other great apes, and chimpanzees in other settings suggests that the effects are not an anomaly of this colony. Although the mean HI scores are higher in the YRPRC colony, data from other great apes species in various settings suggest that population-level right-handedness is evident.
True or Task-Specific Handedness Within the YRPRC Colony?
One of the central premises of McGrew and Marchant's (1997) model is that true handedness reflects consistent hand use across all measures. The degree to which this theory is or is not supported depends on how one interprets the cumulative results of this study. On the one hand, the analyses of the HISUM1 and HISUM2 scores revealed significant population-level right-handedness and, therefore, suggest evidence of true rather than task-specific handedness. Moreover, by using a categorical rather than a continuous scale of measurement to characterize hand preferences, we found that the chimpanzees similarly had population-level right-handedness. More important, the proportion of completely right-handed chimpanzees was higher in contrast to completely left-handed chimpanzees when compared with samples of subjects that had been tested on the same two or three measures of hand use (see Table 3). Like the HISUM1 and HISUM2 values, these results support the interpretation of true handedness in chimpanzees. However, only 7 of a possible 15 intertask correlations were significant, and the definition of true handedness as outlined by McGrew and Marchant (1997) should predict significant correlations between all measures. Thus, these results do not fully support the interpretation of true handedness in chimpanzees.
Given the mixed results in this study with respect to McGrew and Marchant's (1997) model of handedness, it is important to recognize that other models of handedness have been proposed that may support the findings of this study more concisely because they do not assume that all measures of hand preference are correlated with each other. Recall that the chimpanzee hand preferences did cluster in specific ways for some tasks. Specifically, the tube and ball tasks significantly correlated with each other, but neither of these measures correlated with the remaining four measures, which all significantly correlated with each other. These findings are similar to findings using factor analysis of human handedness measures (Annett, 1985; Healey, Liederman, & Geschwind, 1986; Porac & Coren, 1981; Steenhuis & Bryden, 1989) and are consistent with the hypothesis that handedness is not unidimensional and that different measures of hand preference may involve different motor or neural systems. For example, Healey et al. (1986) had participants fill out a 52-item questionnaire in which they indicated their hand use on a 5-point Likert-type scale as 1 (right always), 2 (right preferred but sometimes use left), 3 (no preference), 4 (left preferred but sometimes use right), or 5 (left always). Weighted scores of −2, −1, 0, +1, and +2 were assigned to each qualitative response, and the weighted values were subjected to a factor analysis to determine the clustering of responses for specific items on the questionnaire. From their analysis, Healey et al. reported four clusters of responses, or factors, that they subsequently characterized by the motor, cognitive, and neurological systems involved in their execution. It could be argued that the motor and neurological demands of the ball and tube tasks are different than those involved in the remaining four tasks, and this is why they are unrelated by way of the correlation analysis.
The ball and tube tasks have different motor demands from the other tasks in that they require the use of the index finger to extract the food and they involve the coordinated use of the hands rather than independent actions by each hand. Moreover, neurologically, previous studies in monkeys have reported that use of the fine motor, sequential movements involving distal musculature typically involve greater use of the contralateral hemisphere compared with the ipsilateral hemisphere (Brinkman & Kuypers, 1972a, 1972b). In light of the fact that the ball and tube tasks would involve distal musculature (index finger), it may be that these tasks more strongly elicit activation of the contralateral hemisphere compared with the other tasks. In the absence of any neurofunctional data, this interpretation remains speculative but does warrant further investigation.
As an alternative explanation for these findings, some researchers have proposed a distinction between measures of manual specialization and measures of handedness (Fagot & Vauclair, 1991; Young, Segalowitz, Corter, & Trehub, 1983). According to this model, manual specialization is manifest under conditions in which the motor task is complex and somewhat novel to the subjects, whereas handedness is characterized simply as repetitive movements that are neither complex nor novel to the subjects. This model predicts that greater asymmetries are manifest in tasks that assess manual specialization as compared with tasks that assess handedness (Vauclair & Fagot, 1993). Both the ball and tube tasks were relatively novel to the subjects and arguably more complex because they required coordinated use of the hands. In contrast, the remaining measures were all relatively simple movements and highly ritualized behaviors. Thus, the clustering of the tasks may reflect the degree to which the different measures assessed manual specialization rather than handedness.
The individual HI scores were negatively correlated with the number of measures used to derive the overall handedness measure. Moreover, the mean HI scores for subjects tested on four or more tasks were lower than for those tested on only two or three. These data further support the interpretation of task-specific handedness rather than true handedness in chimpanzees. It should be emphasized, however, that a similar pattern of results would be obtained if the same analysis was applied to data from humans. The best evidence in support of this argument comes from individual variation in the proportion of right-handedness found across various measures of hand use in humans. Some measures, such as writing or throwing, elicit a high proportion of right-handedness (e.g., 95%) compared with other measures, such as reaching or picking up a dime (65%–70%; Annett, 1985). Calculating an HI score based on just writing and throwing responses would clearly yield higher HI values than one that included measures of reaching or picking up a dime.
Comparative Handedness Data From Cumulative Handedness Scores
The data from the YRPRC were compared with the existing data on multiple measures of hand preference in other great apes and to chimpanzees from other settings. After statistically controlling for variation due to rearing history and the age of the subjects, we found that gorillas and chimpanzees exhibited population-level right-handedness. Bonobos had a borderline significant population-level right-hand bias, and the results from orangutans did not approach statistical significance. When considering all the great apes as one group, subjects reared in captivity were significantly more right-handed than wild apes and apes of unknown origin (in terms of rearing). Notwithstanding, subjects from all three rearing histories exhibited population-level right-handedness. Finally, although not significant at conventional levels of significance (p < .05), the mean HI scores for the YRPRC sample were higher than those for chimpanzees from other settings. These results, coupled with the significantly greater degree of right-handedness in captive-born apes, suggest that the rearing of apes in a human environment may enhance an existing right-hand bias in apes. This, in turn, may explain the greater prevalence of right-handedness that has been reported in captive apes than in wild apes, particularly chimpanzees.
Notwithstanding, it is important to emphasize that population-level asymmetries are evident in wild apes and apes of unknown origin. Thus, an explanation based solely on rearing environment cannot explain all of the existing data. In addition, nearly all studies of hand preference in great apes in which multiple measures of hand use were made and a cumulative handedness score was derived reveal positive HI values suggesting more right- than left-handedness in the samples. For example, in Table 4, it can be seen that the mean HI score is greater than zero in 73% (16 out of 22) of the studies. Unfortunately, the standard errors for each sample are tremendously large and are clearly influenced by the sample size within a given study. Thus, collapsing the data across studies reveals a population-level asymmetry because the sample size is increased and, therefore, there is a reduction in the standard error. Recall that the sample size for the YRPRC colony was 187, which is at least 3 times greater than any single published study on great apes. Thus, it could be argued that any study of wild or captive populations that has a sample size that approaches that of the YRPRC colony would obtain significant population-level hand preferences. It is hoped that such data will become available in the near future.
The comparison between the YRPRC data and data from other great apes rests on the assumption that similar handedness constructs are being derived despite that fact that there is variation between studies in the individual hand preference measures. This is an obvious drawback to the analyses performed in this study, and ideally one would want comparable measures across all studies. With a continued emphasis on comparative studies of handedness, perhaps more concise and methodologically sound analyses can be performed on various species.
Conclusion
In summary, chimpanzees exhibit population-level right-handedness when using cumulative hand-preference scores derived from multiple measures of hand use. Chimpanzees also exhibit some, but not exclusive, consistency in hand use across. multiple measures, a finding that is consistent with some previous reports on other primates (e.g., Beck & Barton, 1972; Byrne & Byrne, 1991; Spinozzi & Truppa, 1999), but not in others (Diamond & McGrew, 1994). Attempts to link functional asymmetries with neuroanatomical and physiological asymmetries in different primate species may shed important light on the issue of task versus true handedness in human and nonhuman primates. Previous studies with humans have reported that some measures of hand preference are more closely related to specific asymmetries in certain regions of the brain (Cowell, Kertesz, & Denenberg, 1993; Yousry et al., 1997), which suggest different neural substrates for specific motor functions. A similar dissociation may be evident in nonhuman primates, and this differentiation may allow for a neurological distinction between various handedness tasks used within the same sample of subjects or between different species. With the increasing use of neural imaging technologies with nonhuman primates, such as structural magnetic resonance imaging (Hopkins & Marino, 2000; Hopkins & Rilling, in press; Hopkins, Marino, Rilling, & MacGregor, 1998; Zilles et al., 1996), testing this hypothesis seems feasible in the immediate future.
Acknowledgments
This research was supported by National Institutes of Health Grants NS-29574, NS-36605, and RR-00165. The Yerkes Regional Primate Research Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care. American Psychological Association guidelines for the ethical treatment of animals were adhered to during all aspects of this study.
References
- Annett M. Left, right, hand, and brain: The right-shift theory. Erlbaum; London: 1985. [Google Scholar]
- Aruguete MS, Ely EA, King JE. Laterality of spontaneous motor activity in chimpanzees and squirrel monkeys. American Journal of Primatology. 1992;27:177–188. doi: 10.1002/ajp.1350270303. [DOI] [PubMed] [Google Scholar]
- Bard KA. Evolutionary roots of intuitive parenting. Maternal competence in chimpanzees. Early Development and Parenting. 1994;1:19–28. [Google Scholar]
- Beck CHM, Barton RL. Deviation and laterality in hand preference in monkeys. Cortex. 1972;8:339–363. doi: 10.1016/s0010-9452(72)80001-7. [DOI] [PubMed] [Google Scholar]
- Bisazza A, Rogers LJ, Vallortigara G. The origins of cerebral asymmetry: A review of evidence of behavioural and brain lateralization in fishes, reptiles, and amphibians. Neuroscience and Biobehavioral Reviews. 1998;22:411–426. doi: 10.1016/s0149-7634(97)00050-x. [DOI] [PubMed] [Google Scholar]
- Boesch C. Handedness in wild chimpanzees. International Journal of Primatology. 1991;6:541–558. [Google Scholar]
- Bradshaw B, Rogers L. The evolution of lateral asymmetries, language, tool-use and intellect. Academic Press; San Diego, CA: 1993. [Google Scholar]
- Bresard B, Bresson F. Handedness in Pongo pymaeus and Pan troglodytes. Journal of Human Evolution. 1983;12:659–666. [Google Scholar]
- Brinkman C, Kuypers HGJM. Cerebral control of contralateral and ipsilateral arm, hand and finger movements in the split-brain monkey. Brain. 1972a;96:653–674. doi: 10.1093/brain/96.4.653. [DOI] [PubMed] [Google Scholar]
- Brinkman C, Kuypers HGJM. Split-brain monkeys: Cerebral control of ipsilateral and contralateral arm, hand and finger movements. Science. 1972b September 12;176:536–539. doi: 10.1126/science.176.4034.536. [DOI] [PubMed] [Google Scholar]
- Byrne RW, Byrne JM. Hand preferences in the skilled gathering tasks of mountain gorillas (Gorilla gorilla berengei) Cortex. 1991;27:521–536. doi: 10.1016/s0010-9452(13)80003-2. [DOI] [PubMed] [Google Scholar]
- Colell M, Segarra MD, Sabater-Pi J. Manual laterality in chimpanzees (Pan troglodytes) in complex tasks. Journal of Comparative Psychology. 1995;109:298–307. doi: 10.1037/0735-7036.109.3.298. [DOI] [PubMed] [Google Scholar]
- Corballis MC. The lopsided ape: Evolution of the generative mind. Oxford University Press; New York: 1992. [Google Scholar]
- Cowell PE, Kertesz A, Denenberg VH. Multiple dimensions of handedness and the human corpus callosum. Neurology. 1993;43:2353–2357. doi: 10.1212/wnl.43.11.2353. [DOI] [PubMed] [Google Scholar]
- Cunningham D, Forsythe C, Ward JP. A report on behavioral lateralization in an infant orangutan. Primates. 1989;30:249–253. [Google Scholar]
- De Vleeschouwer K, Van Elsacker L, Verheyen RF. Effect of posture on hand preferences during experimental food reaching in bonobos (Pan paniscus) Journal of Comparative Psychology. 1995;109:203–207. doi: 10.1037/0735-7036.109.2.203. [DOI] [PubMed] [Google Scholar]
- Diamond AC, McGrew WC. True handedness in the cotton-top tamarin (Saguinus oedipus) Primates. 1994;35:69–77. [Google Scholar]
- Ettlinger G. Hand preference, ability, and hemispheric specialization: How far are these factors related in the monkey? Cortex. 1988;24:389–398. doi: 10.1016/s0010-9452(88)80002-9. [DOI] [PubMed] [Google Scholar]
- Fagot J, Vanclair J. Manual laterality in nonhuman primates: A distinction between handedness and manual specialization. Psychological Bulletin. 1991;109:76–89. doi: 10.1037/0033-2909.109.1.76. [DOI] [PubMed] [Google Scholar]
- Healey JM, Liederman J, Geschwind N. Handeduess is not a unidimensional trait. Cortex. 1986;22:33–53. doi: 10.1016/s0010-9452(86)80031-4. [DOI] [PubMed] [Google Scholar]
- Heestand J. Behavioral lateralization in four species of ape. University of Washington; Seattle: 1986. Unpublished doctoral dissertation. [Google Scholar]
- Hopkins WD. Posture and reaching in chimpanzees (Pan) and orangutans (Pongo) Journal of Comparative Psychology. 1993;17:162–168. doi: 10.1037/0735-7036.107.2.162. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preference for bimanual feeding in a sample of 140 chimpanzees (Pan troglodytes): Ontogenetic and developmental factors. Developmental Psychobiology. 1994;27:395–408. doi: 10.1002/dev.420270607. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees (Pan troglodytes): Cross-sectional analysis. Journal of Comparative Psychology. 1995a;105:178–190. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences in juvenile chimpanzees: Continuity in development. Developmental Psychology. 1995b;31:619–625. [Google Scholar]
- Hopkins WD. Chimpanzee handeduess revisited: 54 yeats since Finch (1941) Psychonomic Bulletin and Review. 1996;3:449–457. doi: 10.3758/BF03214548. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. On the other hand: Statistical issues in the assessment and interpretation of hand preference data in nonhuman primates. International Journal of Primatology. 1999;20:851–866. [Google Scholar]
- Hopkins WD, Bard KA, Jones A, Bales S. Chimpanzee hand preference for throwing and infant cradling: Implications for the origin of human handedness. Current Anthropology. 1993;34:786–790. [Google Scholar]
- Hopkins WD, de Waal FBM. Behavioral laterality in captive bonobos (Pan paniscus): Replication and extension. International Journal of Primatology. 1995;16:261–276. [Google Scholar]
- Hopkins WD, Marino LA. Cerebral width asymmetries in nonhuman primates as revealed by magnetic resonance imaging. Neuropsychologia. 2000;38:493–499. doi: 10.1016/s0028-3932(99)00090-1. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Marino L, Rilling J, MacGregor LA. Planum temporale asymmetries in great apes as revealed by magnetic resonance imaging (MRI) NeuroReport. 1998;9:2913–2918. doi: 10.1097/00001756-199808240-00043. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Morris RD. Handedness in great apes. A review of findings. International Journal of Primatology. 1993;14:1–25. [Google Scholar]
- Hopkins WD, Rabinowitz DM. Manual specialization and tool-use in captive chimpanzees (Pan troglodytes): The effect of uni-manual and bimanual strategies on hand preference. Laterality. 1997;2:267–278. doi: 10.1080/713754273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Rilling J. A comparative MRI study of the relationship between neuroanatomical asymmetry and interhemispheric connectivity in primates: Implication for the evolution of functional asymmetries. Behavioral Neuroscience. doi: 10.1037//0735-7044.114.4.739. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman RAW. Manual preference in prosimians, monkeys, and apes. In: Ward JP, Hopkins WD, editors. Primate laterality: Current behavioral evidence of primate asymmetries. Springer-Verlag; New York: 1993. pp. 107–124. [Google Scholar]
- Marchant LF. Hand preferences among captive island groups of chimpanzees. Rutgers, The State University of New Jersey; New Brunswick: 1983. Unpublished doctoral dissertation. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of function in apes: A meta-analysis of methods. Journal of Human Evolution. 1991;21:425–438. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of limb function in chimpanzees in wild chimpanzees of Gombe National Park: Comprehensive study of spontaneous activities. Journal of Human Evolution. 1996;30:427–443. [Google Scholar]
- McGrew WC, Marchant LF. Primate ethology: A perspective on human and nonhuman handedness. In: Bock PK, editor. Hand-book of psychological anthropology. Greenwood Press; Westport, CT: 1994. pp. 171–184. [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: Current issues in and meta-analysis of the behavioral laterality of hand function in nonhuman primates. Yearbook of Physical Anthropology. 1997;40:201–232. [Google Scholar]
- McGrew WC, Marchant LF, Wrangham RW, Klein H. Manual laterality in anvil use: Wild chimpanzees cracking strychnos fruits. Laterality. 1999;4:79–88. doi: 10.1080/03069887600760101. [DOI] [PubMed] [Google Scholar]
- Olson DA, Ellis JE, Nadler RD. Hand preferences in captive gorillas, orangutans and gibbons. American Journal of Primatology. 1990;20:83–94. doi: 10.1002/ajp.1350200203. [DOI] [PubMed] [Google Scholar]
- Porac C, Coren S. Lateral preferences and human behavior. Springer; New York: 1981. [Google Scholar]
- Rogers LJ, Kaplan G. Hand preferences and other lateral biases in rehabilitated orangutans. Animal Behaviour. 1996;51:13–25. [Google Scholar]
- Roney LS, King JE. Postural effects on manual reaching laterality in squirrel monkeys (Samiri sciureus) and cotton-top tamarins (Saguinus oedipus) Journal of Comparative Psychology. 1993;107:380–385. doi: 10.1037/0735-7036.107.4.380. [DOI] [PubMed] [Google Scholar]
- Shafer D. Patterns of hand preference in gorillas and children. In: Ward JP, Hopkins WD, editors. Primate laterality: Current behavioral evidence of primate asymmetries. Springer-Verlag; New York: 1993. pp. 267–283. [Google Scholar]
- Shafer DD. Hand preference behaviors shared by two groups of captive bonobos. Primates. 1997;38:303–313. [Google Scholar]
- Spinozzi G, Truppa V. Hand preferences in different tasks by tufted capuchins (Cebus apella) International Journal of Primatology. 1999;20:827–848. [Google Scholar]
- Steenhuis RE, Bryden MP. Different dimensions of hand preference that relate to skilled and unskilled activities. Cortex. 1989;25:289–304. doi: 10.1016/s0010-9452(89)80044-9. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Fushimi T, Sakura O, Matsuzawa T. Hand preference and tool use in wild chimpanzees. Primates. 1993;34:151–159. [Google Scholar]
- Vauclair J, Fagot J. Manual specialization in gorillas and baboons. In: Ward JP, Hopkins WD, editors. Primate laterality: Current behavioral evidence of primate asymmetries. Springer-Verlag; New York: 1993. pp. 193–205. [Google Scholar]
- Ward JP, Hopkins WD. Primate laterality: Current behavioral evidence of primate asymmetries. Springer-Verlag; New York: 1993. [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiological Psychology. 1980;8:351–359. [Google Scholar]
- Young G, Segalowitz SJ, Corter CM, Trehub SE. Manual specialization and the developing brain. Academic Press; New York: 1983. [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- Zilles K, Dabringhaus A, Geyer S, Amunts K, Qu M, Schleicher A, Gilissen E, Schlaug G, Steinmetz H. Structural asymmetries in the human forebrain and the forebrain of non-human primates and rats. Neuroscience and Biobehavioral Reviews. 1996;20:593–605. doi: 10.1016/0149-7634(95)00072-0. [DOI] [PubMed] [Google Scholar]