Abstract
CpG oligodeoxynucleotides are potent immunostimulants. For parenterally delivered alum based vaccines, the immunostimulatory effect of CpG depends on the association of the CpG and antigen to the alum. We describe effects of buffer components on the binding of CPG 7909 to aluminum hydroxide (Alhydrogel), assays for measuring binding of CPG 7909 to alum and CPG 7909 induced dissociation of antigen from the alum. Free CPG 7909 is a potent inducer of IP-10 in mice. However the lack of IP-10 production from formulations containing bound CPG 7909 suggested that CPG 7909 does not rapidly dissociate from the alum after injection. It also suggests that IP-10 assays are not a good basis for potency assays for alum based vaccines containing CPG 7909.
Keywords: vaccine, formulation, CPG 7909, IP-10
1. Introduction
CpGs are oligodeoxynucleotides (ODN) containing unmethylated CpG dinucleotide motifs that possess immunostimulatory properties. They are potentially useful as adjuvants and are currently being evaluated in veterinary and human vaccines (Klinman et al., 2004). CpG ODN function through their activation of antigen presenting cells and B cells by binding to Toll-like receptor 9 (TLR9). The interaction of TLR9 with CpG motifs initiates a cascade of events resulting in the secretion of T helper (Th)1-type cytokines and chemokines. Production of the chemokine interferon-gamma-inducible protein-10 (IP-10) is an early indicator of Th1 response (Krieg et al., 2004; Blackwell and Krieg, 2003). Administration of CPG 7909 induces IP-10 that peaks in mouse plasma 2-4 h after injection (Krieg et al., 2004).
Activation of antibody response is most efficient when the CpG is chemically linked to (Tighe et al., 2000) or physically associated with the antigen (Aebig et al, submitted). In studies with the malaria vaccine candidate antigen Apical Membrane Antigen 1 bound to Alhydrogel, an enhanced antibody response elicited by CPG 7909 only occurred when the CpG was bound to the alum. Furthermore, injection of free CpG in addition to the bound CpG ablated the immunostimulation seen with the bound CpG.
In this report, we show that the amount of CpG bound to Alhydrogel critically depends on the buffer and also that CpG binding may dissociate previously bound antigen. We describe assays for measuring bound and free CpG in Alhydrogel formulations and on the use of IP-10 assays to assess formulations.
One CpG ODN, designated ODN 2006, produced by Coley Pharmaceutical Group is a 24-mer oligonucleotide that contains three CpG motifs (5′-GTCGTT-3′) in the sequence 5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′. It has been chosen for use in human vaccine trials under the trademark VaxImmune™. It enhances immune responses in a range of animals including primates (Hartmann et al., 2000), mice, rats and guinea pigs (Mullen et al., 2006). This B-type CpG (also referred to as K type) contains a wholly phosphorothioate backbone that makes the molecule resistant to nuclease attack, thus increasing its in vivo half-life (Klinman et al., 2004).
2. Materials and Methods
2.1. CPG 7909 description and characterization
Four lots of CPG 7909 (Coley Pharmaceutical Group, Wellesley, MA) were used: two clinical lots, 207-03-002 and PLI004-04, a gift under Clinical Trials Agreement from Coley, were supplied as 10 mg/ml in 6 mM monobasic sodium phosphate, 94 mM dibasic sodium phosphate, 154 mM sodium chloride (hypertonic phosphate buffer), and two preclinical lots, ACZ-01F-006-M and ACZ-01F-007-M, were purchased from Coley as 21.58 mg/ml and 21.29 mg/ml, respectively, in 10 mM Tris base, 1 mM disodium EDTA, pH 8 (TE). Lot ACZ-01F-007-M was derived from the same lyophilized batch as clinical lot 207-03-002. For some experiments, material from clinical lot 207-03-002 was dialyzed (Slide-A-Lyzer® 3.5K, Pierce) against purified water (Milli-Q System, Millipore Corp., Bedford, MA) to remove buffer components. Intact mass analyses were performed on clinical and preclinical CPG 7909 by negative ion electrospray ionization mass spectrometry using a quadrupole time of flight mass spectrometer QStarXL MS/MS System (Applied Biosystems/Sciex, Framingham, MA).
2.2. Vaccine formulations
AMA1-C1 is an equal mixture (by mass) of two recombinant allelic forms of P. falciparum apical membrane antigen 1 (FVO and 3D7 clones) expressed in Pichia pastoris. Synthetic gene design, recombinant protein expression, and purification have been described (Malkin et al., 2005a; Kennedy et al., 2002). MSP142-FVO and MSP142-3D7 are the E. coli-expressed 42 kDa C-terminal regions of P. falciparum merozoite surface protein 1 (FVO and 3D7 clones) (Singh et al., 2003). Pvs25H is a recombinant form of the 25 kDa ookinete surface protein of P. vivax produced in Saccharomyces cerevisiae (Miles et al., 2002). Vaccines were formulated to contain 10, 40, or 160 μg/ml protein adsorbed to aluminum hydroxide (2% Alhydrogel, Brenntag Biosector, Frederikssund, Denmark) in saline (154 mM sodium chloride, Baxter Healthcare Corp., Deerfield, IL). In this report, aluminum hydroxide concentrations are reported as the Al2O3 content. The standard formulations used contain 1.6 mg/ml of Al2O3. In one experiment the concentration of Alhydrogel was increased to 6.4 mg/ml of Al2O3. Formulations were mixed at room temperature on a rotator at approximately 20 rpm for 1 hour. Vaccines were freshly prepared or stored at 4°C for up to 3 years (aged).
In some experiments, vaccines were formulated in the following buffers: 0.1 M sodium acetate (Quality Biological, Gaithersburg, MD); 1× phosphate-buffered saline, pH 7.4 (PBS, 1.06 mM monobasic potassium phosphate, 2.97 mM dibasic sodium phosphate, 155 mM sodium chloride, Life Technologies, Rockville, MD); 100 mM phosphate, pH 7.4 (17.36 mM monobasic potassium phosphate, 82.64 mM dibasic sodium phosphate); 0.2% Tween 80 (polyoxyethylenesorbitan monooleate, Sigma, St. Louis, MO) in saline, pH 7.5; 1× PBS/0.2% Tween 80; 10 mM Tris base, 1 mM disodium EDTA dihydrate, pH 8 (TE, Quality Biological). For one experiment, 1 ml of clinical grade CPG 7909 was dialyzed against purified water. For other experiments, calcium phosphate (1.25 mg/ml, Brenntag Biosector) or Adjuphos® (1.6 mg/ml aluminum phosphate, Brenntag Biosector) replaced Alhydrogel.
2.3. CPG 7909 binding
CPG 7909 was added to dilute Alhydrogel or vaccine using aseptic technique to a concentration of 1 mg/ml (unless otherwise indicated) in one of the above solutions, mixed by inverting the tubes end-over-end 10 times, and stored on ice at 0°C, at room temperature, or at 37°C for up to 6 h. Samples were centrifuged at 1700 × g for 4 min to pellet the alum, and supernatants were analyzed by spectrophotometry or electrophoresis to assess CpG and antigen binding.
2.4. CPG 7909 analysis by spectrophotometry
CpG was quantified by measuring absorbance at 260 nm (A260) with an Ultrospec 3300 Pro UV/Visible Spectrophotometer (Amersham Pharmacia Biotech, Piscataway, NJ). Samples were diluted 1:25 in saline and the readings compared with those of a 40 μg/ml reference solution of CpG. Percent CpG bound to Alhydrogel was calculated as 100 × (1- A260 of supernatant / A260 of reference CpG).
2.5. CPG 7909 and protein analysis by SDS-PAGE
SDS-PAGE was performed on 4 to 20% gradient polyacrylamide Tris-glycine gels using an X-cell II Mini Cell apparatus (Invitrogen Corp., Carlsbad, CA). For reducing conditions, 50 mM dithiothreitol (final concentration) was added. Gels were silver stained to visualize proteins and CpG, scanned with a laser densitometer (Molecular Dynamics Personal Densitometer SI, Molecular Dynamics, Sunnyvale, CA) and analyzed using ImageQuant version 5.2 (Molecular Dynamics) as previously described (Miles and Saul, 2005). Since heavily loaded protein and CpG samples cannot be uniformly silver stained (making densitometry problematic), loads in the range of approximately 25-200 ng of protein and 100-250 ng of CpG were used. Briefly, peak areas of protein or CpG were determined by integration, and standard curves were plotted as peak area versus known amounts of protein or CpG standards, with the data points fitted by linear regression. The amount of protein or CpG in a vaccine supernatant was determined from equations for the lines. The percentage of protein or CpG bound was then 100 minus the percentage in the supernatant.
2.6. Comparison of IP-10 induction with bound vs. unbound CPG 7909
All animal care and handling was performed in accordance with National Institutes of Health guidelines and with Animal Care and Use Committee-approved protocols. BALB/c mice (Taconic, Germantown, NY) received 50 μl intramuscular injections (anterior tibialis) of 3, 12.5 or 50 μg of CPG 7909 alone in hypertonic phosphate buffer or CPG 7909 formulated on Alhydrogel (1.6 mg/ml Al2O3) or Alhydrogel alone. Sera were obtained for IP-10 determinations by Quantikine Mouse IP-10/CRG-2/CXCL10 ELISA (R&D Systems, Inc., Minneapolis, MN) at 3, 6 and 24 h post injection.
A Mann-Whitney U test was performed to test for differences in mouse IP-10 responses with p values < 0.05 considered significant. The effect of CpG dose on IP-10 response was tested by Spearman rank correlation (SRC) for 3, 6 and 24 h time points with SRC values > 0 and p values < 0.05 considered significant.
3. Results
3.1. Mass spectrometry
Electrospray mass spectrometry was performed to ensure comparability between the clinical and preclinical lots of CPG 7909. Both matched the predicted size of 7698 Da (data not shown).
3.2. Phosphate inhibits CPG 7909 binding to Alhydrogel
Studies were performed to determine the percentage of CPG 7909 that bound to Alhydrogel in the absence of protein. Clinical grade CpG was added (final concentration 1 mg/ml and 10 mM phosphate) to 1.6 mg/ml of Alhydrogel in 154 mM saline, and samples were either centrifuged immediately or stored for 6 h at 0°C (on ice) or room temperature and then centrifuged. CpG in the supernatants was quantified by spectrophotometry and gel densitometry. Binding results obtained using the two methods agreed (Table 1). Spectrophotometry was used in subsequent CpG binding experiments.
Table 1.
Percentage of clinical grade CpG at 1 mg/ml that bound to Alhydrogela determined by two methods
| Method | t=0 | t=6 h on ice at 0°C | t=6 h room temp |
|---|---|---|---|
| Spectrophotometry (A260) | 25.5 | 26.1 | 21.4 |
| SDS-PAGE/laser densitometry | 24.4 | 26.8 | 29.6 |
Final phosphate concentration = 10 mM.
While the preclinical lots of CPG 7909 from Coley Pharmaceutical Group were in TE buffer, clinical lots were in 100 mM phosphate. The binding of the lots of CPG 7909 to Alhydrogel was compared. Table 2 part A shows that preclinical CpG gave twice the binding as clinical CpG. The stated concentrations of both lots were confirmed by A260 readings and densitometry (not shown). We found that low levels of phosphate added to preclinical lots of CpG markedly decreased binding (Table 2, A). When phosphate in clinical CpG was replaced with water by dialysis, the binding nearly doubled (Table 2, A).
Table 2.
CPG 7909 binding to Alhydrogel with and without bound antigen, aluminum phosphate, and calcium phosphate in various buffers (percents are given plus or minus values that varied depending on formulation and storage conditions)
| Formulation and buffer | Clinical CpG (supplied in 100 mM phosphate, 154 mM saline) a | Preclinical CpG (supplied in 10 mM Tris, 1 mM EDTA)a | |
|---|---|---|---|
| A | Alhydrogel in salineb | 24±3c | 60±3d |
| Alhydrogel in 0.078 mM phosphate | 55 | ||
| Alhydrogel in 0.313 mM phosphate | 47 | ||
| Alhydrogel in 1.25 mM phosphate | 38 | ||
| Alhydrogel in 9 mM phosphate | 20 | ||
| Alhydrogel in saline | 46±1 (after buffer exchange into water)d | ||
|
| |||
| B | Alhydrogel in saline with altered CpG/Alhydrogel ratio | 97 (final [CpG] = 0.25 mg/mL)e | |
| AMA1-C1/Alhydrogel in saline with altered CpG/Alhydrogel ratio | 89±5 ([Alhydrogel] = 6.4 mg/mL)c | ||
|
| |||
| C | Alhydrogel in 0.1 M sodium acetate, pH 6.0, 7.0, or 8.0 | 20±7c,f | |
| Alhydrogel in 0.1 M sodium acetate, pH 7.0 | 51±3d | ||
| Aluminum phosphate in salineg | 14±3 | ||
| Aluminum phosphate in 1× PBS, pH 7.4g | 7±2 | ||
| Aluminum phosphate in TE, pH 8.0g | 6±1 | ||
| Calcium phosphateh | 36 | ||
|
| |||
| D | AMA1-C1/Alhydrogel in saline (all protein concentrations) | 23±5c | 57±2d |
| Pvs25H/Alhydrogel in saline (all protein concentrations) | 66±3d | ||
|
| |||
| E | MSP142-FVO/Alhydrogel, 10 μg/ml (0.078 mM phosphate) | 56±6 | |
| MSP142-FVO/Alhydrogel, 40 μg/ml (0.313 mM phosphate) | 39±7 | ||
| MSP142-FVO/Alhydrogel, 160 μg/ml (1.25 mM phosphate) | 26±4 | ||
|
| |||
| F | Alhydrogel in 0.078 mM phosphate + 0.004% Tween 80 | 53±1 | |
| Alhydrogel in 0.313 mM phosphate + 0.016% Tween 80 | 44±1 | ||
| Alhydrogel in 1.25 mM phosphate + 0.063% Tween 80 | 31 | ||
| Alhydrogel in saline + 0.004% Tween 80 | 57d | ||
| Alhydrogel in saline + 0.016% Tween 80 | 57d | ||
| Alhydrogel in saline + 0.063% Tween 80 | 54d | ||
Final [CPG 7909] in the vaccine = 1 mg/mL except where indicated.
[Alhydrogel] = 1.6 mg/mL except where indicated.
Final [phosphate] = 10 mM.
Contained no phosphate.
Final [phosphate] = 2.5 mM.
Binding was lowest at t = 0 and highest after 6 h at 0°C; varying pH had little effect.
1.6 mg/mL.
Supplied as 1.25 mg/mL.
When 4-fold less clinical CpG was added to 1.6 mg/ml Alhydrogel (final [phosphate] = 2.5 mM), essentially all of the CpG bound (Table 2, B). Similarly, when the ratio of CpG to Alhydrogel was altered by increasing the amount of Alhydrogel to 4-fold in an AMA1-C1/Alhydrogel vaccine, 90% of the CpG bound. Thus, a ratio of 1:6.4 CpG:Alhydrogel was required for maximal binding of CpG.
We tested the binding of clinical and preclinical lots of CPG 7909 to Alhydrogel in the presence of 0.1 M sodium acetate. Binding to Alhydrogel at pH 6.0, 7.0 or 8.0 in the presence of 10 mM phosphate was indistinguishable from binding in saline in the presence of 10 mM phosphate. The binding in 0.1 M sodium acetate at pH 7.0 in the absence of 10 mM phosphate was slightly lower than that seen in the parallel saline experiment (51% vs 60%) (Table 2, C).
In addition to Alhydrogel (aluminum hydroxide), aluminum phosphate and calcium phosphate were evaluated for CpG binding (Table 2, C). Binding to aluminum phosphate was low in any buffer and addition of phosphate had little effect. The binding to calcium phosphate was substantially higher than to aluminum phosphate (Table 2, A and C).
3.3. CPG 7909 binding to Alhydrogel is independent of bound protein concentration
Clinical CpG (containing phosphate) was added to 2-yr old AMA1-C1/Alhydrogel vaccines with 3 different concentrations of AMA1-C1 (10, 40 and 160 μg/ml), and the percentage of bound CpG was determined to be 23±5% (Table 2, D). Similarly, with preclinical CpG (no phosphate), binding was 57±2% and did not vary with the protein concentration in each of the three AMA1-C1/Alhydrogel vaccines. Binding occurred within the first few minutes and increased 5-7% upon storage for 6 h at 0°C and 1-3% upon storage for 6 h at room temperature. Similar results were obtained with Pvs25H/Alhydrogel aged for 3 yr using preclinical CpG. Binding was uniformly high (66±3%), was unaffected by protein concentration (Table 2, D), and increased by only 2-5% upon storage for 6 h at either temperature.
3.4. CPG 7909 binding to MSP142/Alhydrogel vaccines
MSP142 bulk protein is dissolved in 1× PBS/0.2% Tween 80, pH 7.4 at manufacture to reduce aggregation. Therefore, vaccines containing 10, 40, or 160 μg/ml of MSP142 also contain 0.078, 0.313, or 1.25 mM phosphate, respectively, and 0.004, 0.016, or 0.063% Tween 80, respectively. Preclinical CpG (in TE) was added to MSP142-FVO/Alhydrogel and MSP142- 3D7/Alhydrogel vaccines. As shown in Table 2 part E for MSP142-FVO/Alhydrogel vaccines, the amount of CpG bound was dependent upon the formulation, with more CpG binding at lower concentrations of MSP142. Similar results were obtained with MSP142-3D7/Alhydrogel (data not shown). In the absence of MSP142, similar CpG binding occurred in the presence of corresponding amounts of phosphate/Tween 80 in saline (Table 2, F). Similar results were also obtained with corresponding amounts of phosphate with no Tween 80. However, all concentrations of Tween 80 in saline without phosphate resulted in consistently high levels of CpG binding (54-57%). Thus, the inhibition of CpG binding in formulations with higher MSP142 concentrations is due to increasing concentrations of phosphate.
3.5. CPG 7909 selectively displaces some Alhydrogel-bound antigens
When clinical grade CpG (in hypertonic phosphate buffer) was added to AMA1-C1/Alhydrogel formulations, dissociated AMA1-C1 was detectable in the vaccine supernatants within minutes (Fig. 1). The amount increased over time and was quantified in a separate experiment (see below). Table 3 illustrates the effects of storage temperature and vaccine age on antigen dissociation. Increased temperature caused greater AMA1-C1 dissociation with more dissociation occurring with freshly formulated vaccines than aged ones. Importantly, dissociation was not observed at 0°C (also see Fig. 1A and B). At ambient temperature, AMA1-C1 dissociation only occurred in the presence of both CPG 7909 and phosphate. It did not occur in the presence of CpG in TE buffer or phosphate alone (Table 3).
Fig. 1.
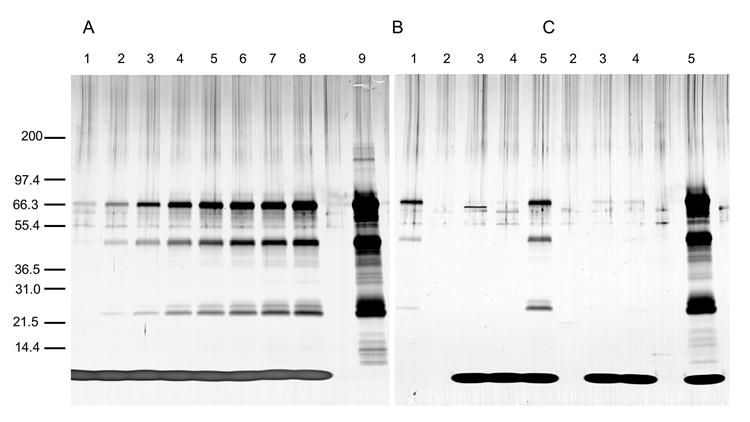
Stability of an AMA1-C1/Alhydrogel vaccine following CPG 7909 addition. Reducing silver stained SDS-PAGE gels are shown. Prior to CpG addition, a sample of vaccine was centrifuged and the supernatant loaded onto the gel (B and C, lanes 2). Clinical grade CpG containing phosphate was added (final concentration 1 mg/ml) to 2-yr old (A and B) or freshly prepared (C) 160 μg/ml AMA1-C1/Alhydrogel vaccine. A: Samples were analyzed at t=0 (lane 1) and after incubation at room temperature for 15 min, 30 min, 1 h, 1.5 h, 2 h, 3 h, and 4 h (lanes 2-8). B and C: Samples were taken at t=0 (lanes 3) and after storage for 6 h on ice at 0°C (lanes 4) and room temperature (lanes 5). For comparison, 1.8 μg (the amount of protein that would be present had all of it dissociated from Alhydrogel) of reference AMA1-C1 is in A lane 9, and 0.1 μg of reference AMA1-C1 is in B lane 1. Molecular weight markers are indicated in kDa.
Table 3.
Antigen dissociation following CPG 7909 addition to alum-based vaccines. Percent protein dissociation at the indicated storage temperature for 6 h (except where footnoted)
| Formulation with antigen concentration | CpGa and buffer added | Percent protein dissociation at | |
|---|---|---|---|
| 0°C on ice | Room temp | ||
| Fresh AMA1- C1/Alhydrogel in saline, 160 μg/mL | Clinical grade in hypertonic phosphate buffer | <1b | 53b |
|
| |||
| 2-yr old AMA1- C1/Alhydrogel in saline, 160 μg/mL | Clinical grade in hypertonic phosphate buffer | <1b | 33b |
|
| |||
| 2-yr old AMA1- C1/Alhydrogel in saline, 160 μg/mL | Preclinical grade in TE buffer | <1c | <1c |
|
| |||
| 2-yr old AMA1- C1/Alhydrogel in saline, 160 μg/mL | 0.4 mM phosphate (no CpG) | <1d | <1d |
|
| |||
| 2-yr old AMA1- C1/Alhydrogel in saline, 160 μg/mL | 10 mM phosphate (no CpG) | <1d | <4d |
|
| |||
| Fresh MSP142- FVO/Alhydrogel, 160 μg/ml (1.2 mM phosphate) | Preclinical grade in TE buffer | <1e | <1e |
|
| |||
| 9-mo old MSP142- FVO/Alhydrogel, 160 μg/ml (1.2 mM phosphate) | Preclinical grade in TE buffer | <1e | <3e |
|
| |||
| 3-yr old Pvs25H/Alhydrogel in saline 160 μg/ml | Preclinical grade in TE buffer | 32c | 51c |
|
| |||
| Engerix-B® in saline + 10 mM phosphate | Clinical grade in hypertonic phosphate buffer | <1f | <1f |
Final [CPG 7909] = 1 mg/mL.
Final [phosphate] = 10 mM.
Contained no phosphate.
Stored for up to 4 h.
Final [phosphate] = 1.2 mM.
Final [phosphate] = 19 mM.
MSP142 did not dissociate from the alum when CpG in TE buffer was added (Table 3). As the MSP142/Alhydrogel formulation already contains phosphate, dissociation in the presence of additional phosphate was not tested.
Pvs25H, however, showed marked dissociation at both 0°C and room temperature even in the absence of phosphate (Fig. 2).
Fig. 2.
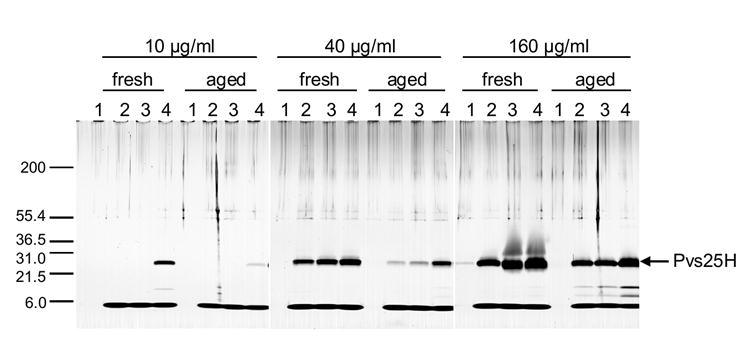
Analysis of the stability of freshly formulated and 3-yr old (aged) Pvs25H/Alhydrogel vaccines containing CPG 7909 by SDS-PAGE with silver staining on three reducing gels. Before CpG was added, a sample of each vaccine was centrifuged and the supernatant loaded onto the gel (lanes 1). Preclinical grade CpG was added (final concentration 1 mg/ml) to freshly formulated or aged vaccines containing either 10 μg/ml, 40 μg/ml or 160 μg/ml of protein. Samples were taken at t=0 (lanes 2), after a 6-hr incubation on ice at 0°C (lanes 3), and after a 6-hr incubation at room temperature.
3.6. Comparison of the induction of IP-10 with bound vs. unbound CPG 7909
As expected, free CPG 7909 gave a rapid and clear dose response. The peak response was measured at 3 hours for the 50 μg dose and 6 hours for the 3 μg and 12.5 μg doses. By contrast, no response was seen at any time point for the 3 μg dose of bound CPG 7909 and only seen at the 24 hour point for the 12.5 μg bound dose when it was substantially lower than the 12.5 μg free CPG 7909 dose. A strong response was seen with the 50 μg CPG 7909 dose adsorbed to Alhydrogel at each time point. However as this contains approximately 37.5 μg of free CPG 7909, and as the response was significantly lower than with 50 μg of free CPG 7909, the IP-10 response seen is consistent with the free CPG 7909 (Table 4).
Table 4.
IP-10 concentrations (pg/ml) in sera from mice receiving Alhydrogel (0 μg CpG), CPG 7909 without Alhydrogel (unbound) CPG 7909 with Alhydrogel (bound)a
| Hours following vaccination | ||||||
|---|---|---|---|---|---|---|
| μg CpG | 3 | 6 | 24 | |||
| CpG | CpG +
Alhydrogel |
CpG | CpG +
Alhydrogel |
CpG | CpG +
Alhydrogel |
|
| 0 | 6 | 16 | 10 | |||
| 3 | 11 | 9 | 94 | 15 | 43 | 14 |
| 12.5 | 83 | 12 | 155 | 22 | 120 | 48 |
| 50b | 873 | 523 | 457 | 352 | 188 | 82 |
| SRCc | 0.7857 | 0.7316 | 0.9148 | 0.7887 | 0.9476 | 0.8158 |
Arithmetic mean of values from 10 mice.
The CpG in 50 μg CpG + Alhydrogel is partially bound.
Spearman Rank Correlation for significant dose response to 0, 3, 12.5, and 50 μg CpG with p values < 0.0001.
4. Discussion
It is generally found that to form an effective antigen/adjuvant mixture, the antigen component in alum-based vaccines should be adsorbed to the aluminum compound (WHO, 1976). Atypical hypersensitivity reactions, reduced vaccine depot and decreased immunogenicity have been attributed to unbound antigen (Kaslow, 2002; Edelman et al., 2002). Recently, chemical or physical association of CpG ODN with the antigen has also been shown to be important for the ability of the CpG ODN to enhance immunogenicity. Therefore the optimal formulation of alum adjuvanted vaccines containing CpG ODNs is likely to require both the antigen and the CpG ODN to be fully bound to the alum since this would optimize co-presentation of both antigen and CpG (Morefield et al., 2005).
Ligand exchange of phosphate in solution with aluminum hydroxide results in conversion of the aluminum hydroxide to aluminum hydroxyphosphate and a similar reaction with phosphate covalently bound to protein is one mechanism that results in tight binding of phosphoproteins to aluminum hydroxide (Iyer et al., 2003). The chemistry of binding of CPG 7909 with its phosphorothioate backbone to aluminum hydroxide is not known, but the poor binding of CPG 7909 to aluminum phosphate would be consistent with a similar ligand exchange mechanism. It would also be consistent with the lack of IP-10 production at 3 and 6 hours from mice injected with 12.5 μg of bound CPG 7909 suggesting that even when exposed to interstitial fluid, the CPG 7909 remains bound to Alhydrogel. Since the binding of CPG 7909 was diminished even in the presence of 0.3 mM phosphate, it is important to take into account even minor traces of phosphate, e.g., as a component of the stock antigen buffer, when preparing these formulations.
None of the other limited range of additives we tested had a major impact on CPG 7909 binding, including previously bound antigen when tested at the maximum concentration used to date in human vaccine trials (Malkin et al., 2005a; Malkin et al., 2005b).
By contrast, the binding of CPG 7909 had a marked impact on the binding of some antigens to Alhydrogel. This was protein specific. AMA1-C1 dissociated at room temperature but not at 0°C in the presence of CpG and phosphate (but not CpG without phosphate), with 33- 53% dissociating after 6 h at room temperature depending on vaccine age. More dissociation occurred with fresh than with aged formulations. Immediately storing AMA1-C1/Alhydrogel/CpG vaccines at 0°C on ice, or formulating without phosphate, prevented antigen dissociation. The latter also increased CpG binding. CpG addition caused very little dissociation of MSP142, even in the presence of 1.25 mM phosphate. With Pvs25H, adding CpG caused rapid dissociation even in the absence of phosphate. At 0°C, approximately 32% dissociated after 6 h. Fresh formulations or room temperature storage allowed even more dissociation.
Induction of IP-10 following injection of CPG 7909 and CPG 7909 containing vaccines was investigated as a potency assay for clinical formulations. However, as the IP-10 response is sensitive to the amount of free CPG 7909 and as this is more readily and precisely measured by physical methods, our results suggest that IP-10 assays are not useful as the basis for potency assays of the CpG component of an alum based vaccine.
These results demonstrate the importance of careful control of formulation, storage conditions post formulation and the time interval between formulation and use. Some antigens, e.g., AMA1, may require that CpG addition always occurs as a point of injection formulation. For others, e.g. Pvs25H, the rate at which the antigen dissociates suggests that CpG may not be an effective adjuvant for these alum based vaccines. This may explain the lack of substantial effect when CPG 10105 was added to Pvs25H/Alhydrogel and used to vaccinate rhesus monkeys (Miura et al, submitted).
These results also highlight the need for assays to measure CpG and protein binding and dissociation in alum based formulations. The combination of the two assays used in this paper: spectroscopic assays of free CpG and the use of scanning laser densitometry with SDS-PAGE for measuring both free protein and CpG provided simple and practical means of monitoring association and dissociation in the concentration ranges of both CpG and the proteins of interest.
Acknowledgments
We are grateful to Cheryl Kothe and Brian Keegan for their excellent technical assistance, to Mark Garfield and Carl Hammer (Research Technologies Branch, Structural Biology Section, NIAID/NIH) for the mass spectrometry data, and to Heather L. Davis, Coley Pharmaceutical Group, for providing clinical grade CPG 7909.
This research was supported by the Intramural Research Program of the NIH, NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Blackwell SE, Krieg AM. CpG-A-induced monocyte IFN-gamma-inducible protein-10 production is regulated by plasmacytoid dendritic cell-derived IFN-alpha. J Immunol. 2003;170:4061. doi: 10.4049/jimmunol.170.8.4061. [DOI] [PubMed] [Google Scholar]
- Edelman R, Wasserman SS, Kublin JG, Bodison SA, Nardin EH, Oliveira GA, Ansari S, Diggs CL, Kashala OL, Schmeckpeper BJ, Hamilton RG. Immediate-type hypersensitivity and other clinical reactions in volunteers immunized with a synthetic multi-antigen peptide vaccine (PfCS-MAP1NYU) against Plasmodium falciparum sporozoites. Vaccine. 2002;21:269. doi: 10.1016/s0264-410x(02)00468-1. [DOI] [PubMed] [Google Scholar]
- Hartmann G, Weeratna RD, Ballas ZK, Payette P, Blackwell S, Suparto I, Rasmussen WL, Waldschmidt M, Sajuthi D, Purcell RH, Davis HL, Krieg AM. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J Immunol. 2000;164:1617. doi: 10.4049/jimmunol.164.3.1617. [DOI] [PubMed] [Google Scholar]
- Iyer S, HogenEsch H, Hem SL. Effect of the degree of phosphate substitution in aluminum hydroxide adjuvant on the adsorption of phosphorylated proteins. Pharm Dev Technol. 2003;8:81. doi: 10.1081/pdt-120017526. [DOI] [PubMed] [Google Scholar]
- Kaslow DC. Transmission-blocking vaccines. Chem Immunol. 2002;80:287. doi: 10.1159/000058850. [DOI] [PubMed] [Google Scholar]
- Kennedy MC, Wang J, Zhang Y, Miles AP, Chitsaz F, Saul A, Long CA, Miller LH, Stowers AW. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect Immun. 2002;70:6948. doi: 10.1128/IAI.70.12.6948-6960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman DM, Currie D, Gursel I, Verthelyi D. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol Rev. 2004;199:201. doi: 10.1111/j.0105-2896.2004.00148.x. [DOI] [PubMed] [Google Scholar]
- Krieg AM, Efler SM, Wittpoth M, Al Adhami MJ, Davis HL. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J Immunother. 2004;27:460. doi: 10.1097/00002371-200411000-00006. [DOI] [PubMed] [Google Scholar]
- Malkin EM, Diemert DJ, McArthur JH, Perreault JR, Miles AP, Giersing BK, Mullen GE, Orcutt A, Muratova O, Awkal M, Zhou H, Wang J, Stowers A, Long CA, Mahanty S, Miller LH, Saul A, Durbin AP. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect Immun. 2005a;73:3677. doi: 10.1128/IAI.73.6.3677-3685.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin EM, Durbin AP, Diemert DJ, Sattabongkot J, Wu Y, Miura K, Long CA, Lambert L, Miles AP, Wang J, Stowers A, Miller LH, Saul A. Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria. Vaccine. 2005b;23:3131. doi: 10.1016/j.vaccine.2004.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles AP, Saul A. Quantifying Recombinant Proteins and Their Degradation Products Using SDS-PAGE and Scanning Laser Densitometry. In: Smales CM, James DC, editors. Methods in Molecular Biology, Therapeutic Proteins: Methods and Protocols. Vol. 308. Copyright Humana Press; Totowa, NJ: 2005. pp. 349–56. [DOI] [PubMed] [Google Scholar]
- Miles AP, Zhang Y, Saul A, Stowers AW. Large-Scale Purification and Characterization of Malaria Vaccine Candidate Antigen Pvs25H for Use in Clinical Trials. Protein Expr Purif. 2002;25:87. doi: 10.1006/prep.2001.1613. [DOI] [PubMed] [Google Scholar]
- Morefield GL, Sokolovska A, Jiang D, HogenEsch H, Robinson JP, Hem SL. Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine. 2005;23:1588. doi: 10.1016/j.vaccine.2004.07.050. [DOI] [PubMed] [Google Scholar]
- Mullen GE, Giersing BK, Ajose-Popoola O, Davis HL, Kothe C, Zhou H, Aebig J, Dobrescu G, Saul A, Long CA. Enhancement of functional antibody responses to AMA1-C1/Alhydrogel, a Plasmodium falciparum malaria vaccine, with CpG oligodeoxynucleotide. Vaccine. 2006;24:2497. doi: 10.1016/j.vaccine.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Singh S, Kennedy MC, Long CA, Saul AJ, Miller LH, Stowers AW. Biochemical and immunological characterization of bacterially expressed and refolded Plasmodium falciparum 42-kilodalton C-terminal merozoite surface protein 1. Infect Immun. 2003;71:6766. doi: 10.1128/IAI.71.12.6766-6774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe H, Takabayashi K, Schwartz D, Marsden R, Beck L, Corbeil J, Richman DD, Eiden JJ, Jr, Spiegelberg HL, Raz E. Conjugation of protein to immunostimulatory DNA results in a rapid, long-lasting and potent induction of cell-mediated and humoral immunity. Eur J Immunol. 2000;30:1939. doi: 10.1002/1521-4141(200007)30:7<1939::AID-IMMU1939>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- WHO. Immunological adjuvants. Report of a WHO scientific group; World Health Organ Tech Rep Ser 1; 1976. [PubMed] [Google Scholar]


