Abstract
Targeting dendritic cell mannose receptors by mannosylating antigens represents a promising vaccination strategy. Using the model antigen ovalbumin (OVA) expressed recombinantly in bacterial and yeast vectors, we have previously demonstrated fungal mannosylation enhances antigen immunogenicity in the context of CD4+ T cell responses. However, because protection against many tumors and pathogens is thought to require MHC class I-restricted T cell responses, the capacity of differentially mannosylated OVA antigens to induce antigen-specific CD8+ T cell proliferation was determined. We found that mannosylated yeast-derived OVA antigens were more potent than their unmannosylated counterparts at inducing antigen-specific T cell proliferation. However, the type of mannosylation was critical as addition of extensive O-linked mannosylation increased lymphoproliferative responses while the presence of N-linked mannosylation was associated with decreased responses. Mannosylated OVA failed to stimulate TNF-α and IL-12 production from dendritic cells. These data suggest that vaccines incorporating mannosylation must take into account how the mannose groups are linked to the core antigen and may need to include an adjuvant to stimulate cytokine production.
Keywords: cross-presentation, CD8+ T cell, mannosylation
1. Introduction
Numerous vaccines which protect by inducing antibody responses are currently licensed for use in humans [1, 2]. However, for some pathogens (e.g., human immunodeficiency virus and Mycobacterium tuberculosis) and tumors, antibody responses are thought to be insufficient for complete protection [3]. By comparison, few vaccines are available which protect by inducing T cell-mediated immune responses [4]. In an effort to develop vaccines which induce cell-mediated immune responses, antigen mannosylation represents a promising strategy for enhancing antigen immunogenicity by targeting mannose receptors (MRs) on antigen-presenting cells (APCs) [5]. Mannosylation of antigens can be accomplished by chemical conjugation or by exploiting the natural capacity of fungi to preferentially use mannose groups when adding O- and N-linkages [6]. Using the model antigen OVA expressed in the yeast Pichia pastoris, we have demonstrated that fungal mannosylation enhances antigen immunogenicity in the context of major histocompatibility complex (MHC) class II-restricted CD4+ T cell responses [7]. Moreover, the presence of either O-linked or N-linked mannosylation was sufficient to boost antigen-specific CD4+ T cell stimulation. Optimal protection against many tumors and pathogens is thought to require the induction of MHC class I-restricted CD8+ T cell responses [8, 9]. Recognizing the value of priming cytotoxic T lymphocyte responses, Stubbs et al. have shown that vaccination of mice with yeast engineered to express tumor antigens induced protective antigen-specific CD8+ T cell responses [10].
The purpose of this study was to evaluate the capacity of fungal mannosylation to: 1) enhance antigen immunogenicity in the context of MHC class I-restricted CD8+ T cell responses and, 2) stimulate DC proinflammatory cytokine production. Unmannosylated OVA antigens were produced in Escherichia coli; whereas mannosylated OVA antigens were produced in the yeast P. pastoris. To examine the relative contributions of the type of mannosylation, the degree of N- and O-linkages was manipulated by site-directed mutagenesis of the asparagine-X-serine/threonine sequences responsible for N-linkages and the addition of a serine/threonine-rich region to provide extensive O-linkages. We found that mannosylated OVA antigens were significantly more potent than their unmannosylated counterparts at inducing antigen-specific CD8+ lymphoproliferation. Additionally, N-linked mannosylation decreased the immunogenicity of OVA antigens, whereas extensive O-linked mannosylation was stimulatory. Surprisingly, despite their less potent capacity to stimulate antigen-specific lymphoproliferation, unmannosylated antigens stimulated higher levels of tumor necrosis factor alpha (TNF-α) and interleukin 12 (IL-12) p70 production than mannosylated antigens.
2. Methods
2.1. Materials and cell culture
All chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Native OVA was purchased from Worthington Biochemical Corporation (Lakewood, NJ). Heat-killed, formalin-fixed Staphylococcus aureus Cowan 1 (SAC) bacterial cells were purchased from Calbiochem (La Jolla, CA). Tissue culture media were obtained from Invitrogen Life Technologies. R10 medium consisted of RPMI 1640 supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, and 50 μM 2-mercaptoethanol. Cells were cultured at 37°C, 5% CO2 in a humidified incubator.
2.2. Recombinant OVA antigens
Recombinant OVA antigens were produced as previously described [7]. Briefly, mannosylated antigens (ppOVA, ppOVANQ, ppOVAST and ppOVASTNQ) were produced in P. pastoris. Cells were grown at 30°C for 72 h prior to induction of protein expression by methanol. Supernatants were collected, concentrated and purified over a Ni-NTA column (Qiagen) using fast performance liquid chromatography (FPLC). Unglycosylated antigens (ecOVA and ecOVAST) were produced in E. coli. Cells were grown overnight prior to induction of protein expression by isopropyl β-D-thiogalactoside. Supernatants were collected, concentrated and purified over an amylose column using FPLC. E. coli- and P. pastoris-derived samples were filter-sterilized, and protein concentrations were determined by the bicinchoninic acid assay (Pierce, Rockford, IL). Table 1 lists select characteristics of recombinant OVA antigens produced in P. pastoris and E. coli.
Table 1.
Select characteristics of the recombinant OVA antigens used in the study. All proteins contain the portion of OVA spanning MHC class I and II-restricted epitopes (amino acids 230-359). Additionally, all proteins contain one c-Myc epitope and six His tags for the purpose of protein identification and purification. Proteins engineered for expression in E. coli contain an N-terminal maltose binding protein (MBP). A more detailed characterization can be found in Lam et al. [7].
| Antigen | ecOVA | evOVAST | ppOVA | ppOVANQ | ppOVAST | ppOVASTNQ |
|---|---|---|---|---|---|---|
| Source | E. coli | E. coli |
P. pastoris |
P. pastoris |
P. pastoris |
P. pastoris |
|
N- linkages |
none | none | yes | none | yes | none |
|
O- linkages |
none | none | few | few | extensive | extensive |
| MBP | yes | yes | none | none | none | none |
| His tag | yes | yes | yes | yes | yes | yes |
|
c-Myc tag |
yes | yes | yes | yes | yes | yes |
2.3. Mice
OT-I mice were purchased from The Jackson Laboratory. OT-I TCR-transgenic mice express an α/β TCR specific for OVA aa 257-264 (SIINFEKL) in the context of MHC class I (H2Kb). Mice were maintained under specific-pathogen free conditions in the Laboratory Animal Science Center at Boston University School of Medicine (BUSM). All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of BUSM.
2.4. CD8+ T cells and Bone-marrow derived DCs (BMDCs)
Mouse splenocytes were isolated as in our previous studies [11]. Naïve CD8+ T cells were purified from the splenocytes by positive selection with CD8a magnetic beads (Miltenyi Biotec). Purity was typically greater than 90%, as determined by flow cytometry. BMDCs were generated according to the protocol of Lutz et al. [12]. Briefly, bone marrow cells were obtained from femurs and tibiae of 8- to 16-wk-old mice and cultured in R10 medium supplemented with 10% supernatant from the GM-CSF-secreting J558L cell line. Cells were fed with fresh supplemented R10 medium on days 3, 6 and 8. BMDCs were used on day 9.
2.5. T cell proliferation assays
Lymphoproliferation was assayed by [3H]-thymidine incorporation. All antigens were preincubated with 20 μg/ml polymyxin B to minimize potential effects of endotoxin. Mitomycin C (0.5 ng/ml)-treated BMDCs served as the source of APCs and were seeded in U-bottom tissue culture plates at 3×103 cells/well. Antigens were added at 1 μM to each well in triplicate per group. Purified CD8+ T cells (3 ×104) were added to each well and cocultured with APCs for 72 h, before pulsing with [3H]-thymidine (1 μCi/well) for an additional 18 h. Cells were harvested onto filters, and [3H]-thymidine incorporation was measured using a beta counter.
2.6. Cytokine measurements
TNF-α and IL-12 p70 production were measured by ELISA, using kits obtained from eBioscience (San Diego, CA). Briefly, all antigens were preincubated with 20 μg/ml polymyxin B prior to coculture. BMDCs were cultured with antigens at 0.1 μM per well in triplicate per group for 18 h. SAC was used as a positive control at 0.0075%. Tissue culture plates were centrifuged for 5 min at 360 × g, and cell-free supernatants were collected for analysis.
2.7. Statistical analysis
Means and SEMs were compared using the Student's t test. Values of p<0.05 were considered statistically significant.
3. Results
3.1. Effect of antigen mannosylation on OT-I lymphoproliferation
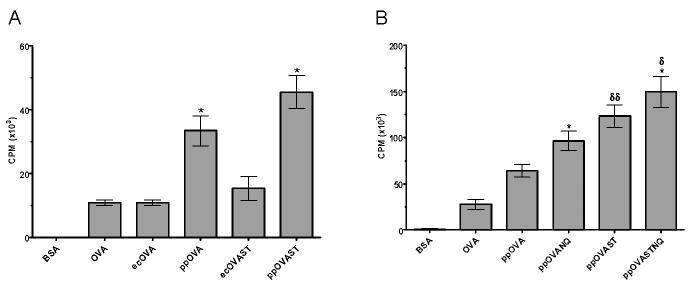
To determine the relative potency of unmannosylated versus mannosylated antigens at inducing antigen-specific T cell proliferation, BMDCs were cocultured with naïve OVA-specific CD8+ T cells in the presence of recombinant OVA antigens. BSA and native OVA served as negative and positive controls, respectively. Mannosylated ppOVA and ppOVAST were significantly more potent at inducing OT-I lymphoproliferation than unmannosylated ecOVA and ecOVAST (Fig. 1A). Because ppOVA and ppOVAST were engineered to express both N- and O-linkages, it was unclear from the data which form of mannosylation was primarily responsible for enhancing the antigens' immunogenicity. Therefore, we next compared four P. pastoris-derived OVA antigens that differed in the type and extent of mannosylation. Interestingly, these experiments revealed opposing effects of fungal N- and O-linked mannosylation on antigen immunogenicity (Fig. 1B). The OVA antigens lacking N-linked mannosylation, ppOVANQ and ppOVASTNQ, were more immunogenic than their fully mannosylated corresponding antigens, ppOVA and ppOVAST. In addition, OVA antigens bearing extensive O-linked mannosylation, ppOVAST and ppOVASTNQ, induced greater lymphoproliferation than their counterparts lacking extensive O-linkages, ppOVA and ppOVANQ.
Figure 1. Effect of mannosylation on CD8+ MHC I-restricted lymphoproliferation.
BMDCs were cocultured with CD8-purified T cells from OT-I mice in the presence of the indicated antigens for 72 h before pulsing with [3H]-thymidine for 18 h. A. Comparison of E. coli and P. pastoris-derived antigens. Data are the mean ± SEM (n=3) of a representative of three independent experiments. *, p<0.01 (comparing ppOVA with ecOVA and ppOVAST with ecOVAST). B, Comparison of antigens with varying degrees of N- and O-linked mannosylation. Data are the mean ± SEM (n=15) of five independent experiments, each of which was performed in triplicate. *, p<0.01 (comparing ppOVANQ with ppOVA and ppOVASTNQ with ppOVAST). δ, p<0.05 (comparing ppOVASTNQ with ppOVANQ). δδ, p<0.01 (comparing ppOVAST with ppOVA).
3.2. Effect of antigen mannosylation on TNF-α and IL-12 p70 production
Protection against many tumors and pathogens is thought to require induction of proinflammatory cytokine production, including TNF-α and IL-12 [13, 14]. Therefore, the levels of these immunological mediators were determined following stimulation of BMDCs with unmannosylated and mannosylated OVA antigens. Unstimulated BMDCs and BMDCs stimulated with SAC served as negative and positive controls, respectively. Unmannosylated ecOVA induced significantly higher levels of TNF-α and IL-12 p70 heterodimer than mannosylated ppOVA (Fig. 2). Moreover, ppOVA failed to induce cytokine release above that seen with unstimulated BMDCs. ppOVA did not inhibit cytokine production induced by ecOVA or Staphylococcus aureus Cowans strain (data not shown).
Figure 2. Stimulation of TNF-α and IL-12 p70 production by OVA antigens.
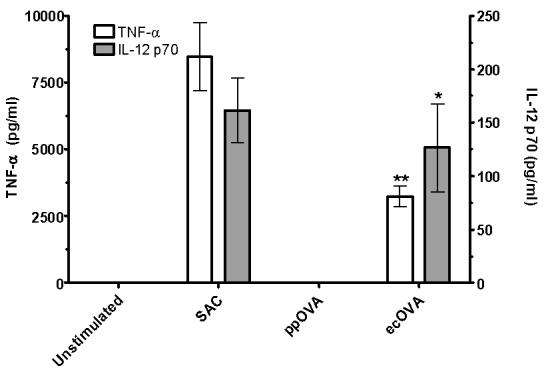
BMDCs were cocultured for 72 h in the presence of the indicated antigens. Cellfree supernatants were assayed for cytokine production by ELISA. Data are the mean ± SEM (n=12) of four independent experiments, each of which was performed in triplicate. White bars represent TNF-α production. **, p<0.0001 (comparing ppOVA with ecOVA). Grey bars represent IL-12 p70 production. *, p<0.01 (comparing ppOVA with ecOVA).
4. Discussion
We evaluated the capacity of fungal mannosylation to enhance antigen immunogenicity in the context of MHC class I-restricted CD8+ T cell responses. Unmannosylated and differentially mannosylated OVA antigens were used to stimulate antigen-specific T cell proliferation. Our results demonstrated a three-fold enhancement of OVA-specific CD8+ T cell proliferation induced by mannosylated ppOVA and ppOVAST versus unmannosylated ecOVA and ecOVAST, respectively (Fig. 1A). These data extend our previous findings that mannosylation augments the MHC class II-restricted CD4+ T cell response to OVA [7], and lends further support to the utility of antigen mannosylation in T cell-stimulating vaccines.
While both N-linked and O-linked glycans on P. pastoris are terminally mannosylated, there are significant differences in the chemical structure. The typical P. pastoris N-linked glycan structure, (GlcNAc)2 (Man)9-14, has a high-mannose configuration with (α-1,2), (α-1,3), and (α-1,6) linkages [15, 16]. In contrast, the predominant O-linked glycans attached to Ser/Thr are short (2-3 mannoses) and consist solely of (α-1,2)-linked mannose [16]. Because ppOVA contains both N- and O-linked mannosylation [7], it was unclear what the relative contributions of each form of mannosylation were to the CD8+ T cell response. The results of additional experiments provided evidence in support of an inhibitory effect of N-linked mannosylation. Antigen-specific OT-I lymphoproliferation was significantly higher in cocultures stimulated with ppOVANQ and ppOVASTNQ, which are devoid of N-linked glycosylation, when compared with ppOVA and ppOVAST, respectively, which contain N-linked mannosylation (Fig. 1B). This attenuating effect of N-linkages was not observed when the identical glycoproteins were utilized to stimulate CD4+ T cell responses [7], perhaps a reflection of the differences between cross-presentation and MHC class II processing and presentation pathways [7, 17]. In addition, disparate peptide fragments of OVA stimulate CD4+ and CD8+ responses [18, 19].
The mechanisms by which N-linked mannosylation decreases antigen immunogenicity remain to be determined. There are no N-linkages on SIINFEKL, the OVA peptide fragment which is presented by MHC Class I. Thus, glycosylation is not impairing the ability of SIINFEKL to fit into the MHC binding groove. However, glycosylation could selectively interfere with Class I MHC-restricted antigen-processing and presentation by impairing the ability of cellular proteases to break down OVA into SIINFEKL or by interfering with the chaperone functions of heat-shock proteins [20-22].
In contrast to N-linked glycans, extensive O-linked mannosylation enhanced the immunogenicity of recombinant OVA antigens. Significant increases in antigen-specific OT-I lymphoproliferation were observed when ppOVAST and ppOVASTNQ were compared to ppOVA and ppOVANQ, respectively (Fig. 1B). Despite bearing N-linked glycans, extensive O-linked mannosylation was sufficient for enhancing the immunogenicity of ppOVAST. Interestingly, ppOVASTNQ, the antigen engineered to have no N-linkages and extensive O-linked glycans, was the most immunogenic of the antigens tested. We postulate that extensive O-linked mannosylation enhances antigen immunogenicity by efficiently targeting MRs which results in enhanced antigen capture. The location of the ST-rich region (near the C-terminus far from SIINFEKL) may make it less likely to interfere with the antigen processing and presentation machinery involved in cross-presentation. Alternatively, due to their dissimilar structures, N-glycans and O-glycans may be preferentially taken up by different MRs. In this regard, DC-SIGN appears to preferentially recognize high-mannose structures, such as is found in P. pastoris N-glycans [23].
In addition to efficient antigen uptake, processing and presentation by DCs, optimal T cell responses require cytokine production. The demonstration that ppOVA did not induce TNF-α and IL-12 production suggest that in order to be effective, P. pastoris-derived vaccine candidates would need to be given with adjuvants that induced proinflammatory cytokine production [24]. Cross-linking the mannose receptor, CD206, has been shown to inhibit DC IL-12 release and induce IL-10 and IL-1R [25]. However, we have found that ppOVA did not inhibit DC secretion of TNF-α and IL-12.
Unglycosylated ecOVA potently induced TNF-α and IL-12 p70 production in BMDCs. These results cannot be attributed to endotoxin contamination, as polymyxin B was included in all incubations. However, we cannot eliminate the possibility that the ecOVA preparation contained other stimulatory bacterial products, such as the TLR2 ligands, lipopeptides [26]. In addition, ecOVA and ecOVAST were produced as fusion proteins containing MBP. Recently, it was demonstrated that MBP stimulates DC to produce proinflammatory cytokines, including TNF-α and IL-12 p70, apparently by acting as a ligand for TLR4 [27].
In summary, fungal mannosylation enhanced antigen immunogenicity in the context of MHC class I-restricted CD8+ T cell responses. Whereas both N-linked and extensive O-linked fungal mannosylation augmented CD4+ T cell responses, only extensive O-linked mannosylation proved effective at boosting cross-primed CD8+ T cell responses [7]. Future studies need to explore the in vivo correlates of these findings, particularly whether vaccines incorporating fungal mannosylation induce protective immunity against microbial pathogens and tumors.
Acknowledgements
This work was supported in part by National Institutes of Health grants RO1 AI066087 and RO1 AI25780. We thank Jennifer Dan and Dr. Karen Wozniak for help with experimental design and interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ben-Yedidia T, Arnon R. Towards an epitope-based human vaccine for influenza. Hum Vaccin. 2005;1(3):95–101. doi: 10.4161/hv.1.3.1851. [DOI] [PubMed] [Google Scholar]
- 2.Ljubojevic S. The human papillomavirus vaccines. Acta Dermatovenerol Croat. 2006;14(3):208. [PubMed] [Google Scholar]
- 3.Srivastava IK, Ulmer JB, Barnett SW. Neutralizing antibody responses to HIV: role in protective immunity and challenges for vaccine design. Expert Rev Vaccines. 2004;3(4 Suppl):S33–52. doi: 10.1586/14760584.3.4.s33. [DOI] [PubMed] [Google Scholar]
- 4.Esser MT, et al. Memory T cells and vaccines. Vaccine. 2003;21(56):419–30. doi: 10.1016/s0264-410x(02)00407-3. [DOI] [PubMed] [Google Scholar]
- 5.Keler T, Ramakrishna V, Fanger MW. Mannose receptor-targeted vaccines. Expert Opin Biol Ther. 2004;4(12):1953–62. doi: 10.1517/14712598.4.12.1953. [DOI] [PubMed] [Google Scholar]
- 6.Levitz SM, Specht CA. The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS Yeast Res. 2006;6(4):513–24. doi: 10.1111/j.1567-1364.2006.00071.x. [DOI] [PubMed] [Google Scholar]
- 7.Lam JS, et al. A model vaccine exploiting fungal mannosylation to increase antigen immunogenicity. J Immunol. 2005;175(11):7496–503. doi: 10.4049/jimmunol.175.11.7496. [DOI] [PubMed] [Google Scholar]
- 8.Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 9.Klebanoff CA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102(27):9571–6. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stubbs AC, et al. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat Med. 2001;7(5):625–9. doi: 10.1038/87974. [DOI] [PubMed] [Google Scholar]
- 11.Levitz SM, et al. Molecular characterization of a mannoprotein with homology to chitin deacetylases that stimulates T cell responses to Cryptococcus neoformans. Proc Natl Acad Sci U S A. 2001;98(18):10422–7. doi: 10.1073/pnas.181331398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutz MB, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223(1):77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 13.Lundberg P, et al. TNF Protects Resistant C57BL/6 mice Against Herpes Simplex Virus Induced Encephalitis Independently of signaling via TNFR1 or TNFR2. J Virol. 2006 doi: 10.1128/JVI.02243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pannellini T, et al. Timely DNA vaccine combined with systemic IL-12 prevents parotid carcinomas before a dominant-negative p53 makes their growth independent of HER-2/neu expression. J Immunol. 2006;176(12):7695–703. doi: 10.4049/jimmunol.176.12.7695. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton SR, et al. Humanization of yeast to produce complex terminally sialylated glycoproteins. Science. 2006;313(5792):1441–3. doi: 10.1126/science.1130256. [DOI] [PubMed] [Google Scholar]
- 16.Trimble RB, et al. Characterization of N- and O-linked glycosylation of recombinant human bile salt-stimulated lipase secreted by Pichia pastoris. Glycobiology. 2004;14(3):265–74. doi: 10.1093/glycob/cwh036. [DOI] [PubMed] [Google Scholar]
- 17.Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev. 2005;207:166–83. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 18.Braaten DC, et al. An optimized CD8+ T-cell response controls productive and latent gammaherpesvirus infection. J Virol. 2005;79(4):2573–83. doi: 10.1128/JVI.79.4.2573-2583.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robertson JM, Jensen PE, Evavold BD. DO11.10 and OT-II T cells recognize a C-terminal ovalbumin 323-339 epitope. J Immunol. 2000;164(9):4706–12. doi: 10.4049/jimmunol.164.9.4706. [DOI] [PubMed] [Google Scholar]
- 20.Bacik I, et al. Introduction of a glycosylation site into a secreted protein provides evidence for an alternative antigen processing pathway: transport of precursors of major histocompatibility complex class I-restricted peptides from the endoplasmic reticulum to the cytosol. J Exp Med. 1997;186(4):479–87. doi: 10.1084/jem.186.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cutler JE. N-glycosylation of yeast, with emphasis on Candida albicans. Med Mycol. 2001;39(Suppl 1):75–86. [PubMed] [Google Scholar]
- 22.Doody AD, et al. Glycoprotein 96 can chaperone both MHC class I- and class II-restricted epitopes for in vivo presentation, but selectively primes CD8+ T cell effector function. J Immunol. 2004;172(10):6087–92. doi: 10.4049/jimmunol.172.10.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Y, et al. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat Struct Mol Biol. 2004;11(7):591–8. doi: 10.1038/nsmb784. [DOI] [PubMed] [Google Scholar]
- 24.Ahlers JD, Belyakov IM, Berzofsky JA. Cytokine, chemokine, and costimulatory molecule modulation to enhance efficacy of HIV vaccines. Curr Mol Med. 2003;3(3):285–301. doi: 10.2174/1566524033479843. [DOI] [PubMed] [Google Scholar]
- 25.Chieppa M, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171(9):4552–60. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- 26.Schroder NW, et al. Lipopolysaccharide binding protein binds to triacylated and diacylated lipopeptides and mediates innate immune responses. J Immunol. 2004;173(4):2683–91. doi: 10.4049/jimmunol.173.4.2683. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez S, et al. A potential role for TLR4 in mediating Escherichia coli maltose-binding protein activation of dendritic cells. Infect Immun. 2007 doi: 10.1128/IAI.00486-06. [DOI] [PMC free article] [PubMed] [Google Scholar]