Abstract
The BIG 1-98 trial is a large, randomized, independently conducted clinical trial designed to compare the efficacy of upfront letrozole versus tamoxifen monotherapy and to compare sequential or up-front use of letrozole and/or tamoxifen as an early adjuvant therapy for patients with early breast cancer. We report on the results from the primary core analysis of the BIG 1-98 trial of 8,010 patients, which compares monotherapy with letrozole versus tamoxifen. This pre-planned core analysis allowed the use of patient data from the monotherapy arms of letrozole and tamoxifen and from the sequential arms prior to the drug switch point. Patients randomized to letrozole had a 19% improved disease-free survival (hazard ratio [HR] = 0.81; P = 0.003), due especially to reduced distant metastases (HR = 0.73; P = 0.001). A 14% risk reduction of fatal events in favor of letrozole was also observed (P = NS). The results from the monotherapy arms alone confirmed the findings from the primary core analysis. Based on the results from this trial, the aromatase inhibitor letrozole (Femara®) is currently recommended as a part of standard adjuvant therapy for postmenopausal women with endocrine-responsive breast cancer and has recently been approved in the early adjuvant setting in both Europe and the United States. A subsequent analysis after additional follow-up will address the question of monotherapy versus sequential therapy.
Keywords: Adjuvant therapy, Aromatase inhibitor, Breast cancer, BIG 1-98, Letrozole, Tamoxifen
Introduction and rationale
During the last century the management of primary breast cancer has evolved from gross surgical intervention to a sophisticated approach involving surgical, radiotherapeutic, hormonal, chemotherapeutic, and targeted biologic strategies. Currently, pharmacologic treatment is tailored according to disease and patient characteristics, including tumor size, histologic grade, lymph-node involvement, hormone receptor (HR) status, and human epidermal growth factor receptor-2 (HER2) overexpression. The benefits from adjuvant chemotherapy, adjuvant hormonal therapy, and adjuvant therapy with trastuzumab have been well-established [1, 2].
Patients with early breast cancer presenting with estrogen receptor (ER)-positive and/or progesterone receptor (PgR)-positive tumors typically receive adjuvant hormonal therapy. Adjuvant therapy with tamoxifen, a selective estrogen receptor modulator (SERM), significantly reduces the risk of breast cancer recurrence [1]; the Early Breast Cancer Trialists Collaborative Group reported that 5 years of tamoxifen reduces the annual breast cancer death rate by 31% and is significantly more effective than just 1–2 years of tamoxifen in ER-positive breast cancer [1]. However, intrinsic estrogenic activity [3–5] and increased risk of endometrial cancer [6] and thromboembolism [7, 8] have emerged as disadvantages when using tamoxifen. Based on current published data, extending the course of adjuvant tamoxifen beyond 5 years is not beneficial [9] despite the persistent risk of relapse in patients with HR+ breast cancer [10].
In the first-line treatment of advanced breast cancer, the third-generation aromatase inhibitors (AIs) have shown superior or equivalent efficacy compared with tamoxifen [11–15]. Letrozole demonstrated significant superiority in time to progression and overall response rate [12] and, in addition, an early survival benefit [11]. This indicates that a subset of the breast tumors is inherently less sensitive to tamoxifen [16] and that resistance to tamoxifen is acquired more quickly [17]. This superiority of letrozole over tamoxifen in the advanced setting led to the hypothesis that this AI may also be superior to tamoxifen when administered in the adjuvant setting.
The Breast International Group (BIG) 1-98 was designed, coordinated, analyzed, and reported by an independent academic group and currently is the largest ongoing adjuvant trial in breast cancer investigating the role of an AI. This review summarizes the design and results from the primary core analysis of the BIG 1-98 trial, which compared monotherapy with letrozole to monotherapy with tamoxifen and identified letrozole as a better alternative to tamoxifen in this setting. It also summarizes some of the additional published research using the BIG 1-98 database.
Trial design and patients
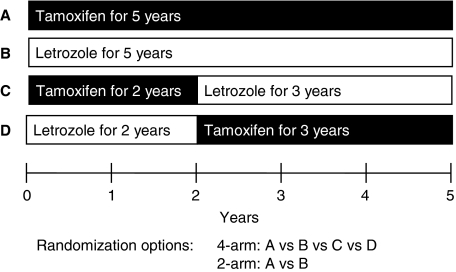
BIG 1-98 was a randomized, phase III, double-blind trial with two randomization options: two-arms (A or B) and four-arms (A, B, C or D) (see Fig. 1) for women with operable invasive HR+ (ER+ and/or PgR+) breast cancer. The treatment arms were: (A) initial therapy with tamoxifen for 5 years, (B) initial therapy with letrozole for 5 years, (C) initial therapy with tamoxifen for 2 years followed by letrozole for 3 years, or (D) initial therapy with letrozole for 2 years followed by tamoxifen for 3 years. The doses administered were 2.5 mg/day of letrozole and 20 mg/day of tamoxifen.
Fig. 1.
BIG-98 trial design
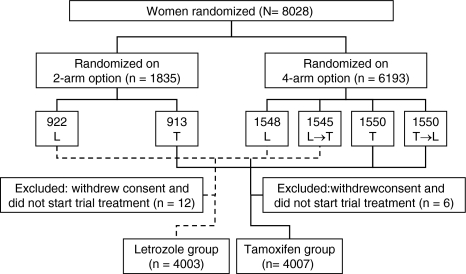
Between March 1998 and March 2000, 1,835 women were randomly assigned to arms A or B, and between April 1999 and May 2003 a further 6,193 were assigned to arms A, B, C, or D. The primary core analysis compared treatment effects of patients randomized to receive letrozole initially (arms B and D) and those assigned to receive tamoxifen initially (arm A and C). In the sequential treatment groups (arms C and D), only events that occurred up to 30 days after switching treatments were included in the analysis. In total, 8,010 patients were included in the primary core analysis: 4,003 in the initial letrozole group (arms B and D) and 4,007 in the initial tamoxifen group (arms A and C) (see Fig. 2). Patient characteristics were similar in the two treatment groups. Overall, the trial included 41.3% node-positive and 57.3% node-negative women, while 1.4% had unknown nodal status. Positive HR status was an eligibility criterion. The majority of patients (63.1%) had both ER+ and PgR+ tumors. A total of 25.3% of women received adjuvant or neoadjuvant chemotherapy.
Fig. 2.
CONSORT (Consolidated Standards of Reporting Trials) flowchart of the BIG 1-98 trial. The primary core analysis includes all 8,010 assessable patients, but events and follow-up in the sequential treatment groups (L → T and T → L) are truncated at 30 days after switching to the other treatment. L denotes letrozole and T tamoxifen. Reprinted from [18, Supplementary Appendix]
End points
The primary end point of the trial was disease-free survival (DFS), defined as the time from randomization to first occurrence of: invasive recurrence in ipsilateral breast, chest wall, regional site (internal mammary/axilla), or distant site (including ipsilateral supraclavicular); contralateral breast cancer (invasive); second malignancy (non-breast); or death without prior cancer event. Protocol-specified secondary end points in the BIG 1-98 trial were: overall survival (OS), defined as time from randomization to death from any cause; systemic DFS, defined as time from randomization to distant recurrence; second non-breast malignancy or death from any cause (ignoring local and contralateral-breast events); and safety. Three additional end points were defined in the statistical analysis plan: (1) DFS excluding second, non-breast malignancies; (2) time to recurrence, defined as time from randomization to first breast cancer recurrence (excluding second, non-breast cancers and censoring data on patients who died without a prior cancer event); and (3) time to distant recurrence, defined as the time from randomization to the first breast cancer recurrence at a distant site.
Efficacy analyses
Primary, secondary, and additional end points
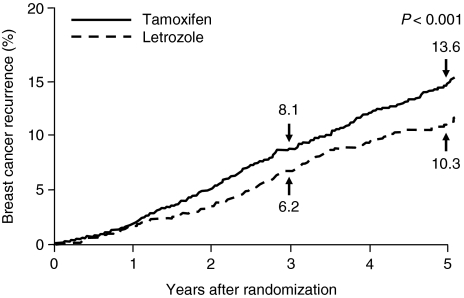
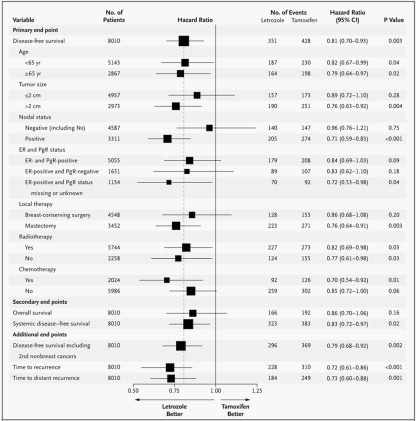
At 25.8 months of follow-up, letrozole improved DFS by 19% (P = 0.003). The cumulative incidence of breast cancer relapse was significantly reduced with letrozole compared with tamoxifen (see Fig. 3). The difference was evident from 1 year after randomization and at 5 years was 10.3% in the letrozole group compared with 13.6% in the tamoxifen group (P < 0.001). In addition, letrozole significantly improved systemic DFS compared with tamoxifen (hazard ratio 0.83; 95% CI 0.72–0.97). There was significant improvement with letrozole compared with tamoxifen for the additional end point of DFS excluding second non-breast cancers (hazard ratio 0.79; 95% CI 0.68–0.92). Letrozole was particularly effective in decreasing the risk of distant recurrence by 27% compared with tamoxifen (hazard ratio 0.73; 95% CI 0.60–0.88; P = 0.001) (see Fig. 4). A non-significant 14% improvement in OS was observed in patients receiving letrozole. Thus, 166 deaths (4.1%) were observed in the letrozole group compared with 192 deaths (4.8%) in the tamoxifen group.
Fig. 3.
Cumulative incidence of a breast cancer relapse in the BIG 1-98 trial. Reprinted from [18] with permission from the Massachusetts Medical Society
Fig. 4.
Cox proportional-hazards model of data from the BIG 1-98 trial. The size of the boxes is inversely proportional to the standard error of the hazard ratio. The dashed vertical shows the hazard-ratio estimate for the overall analysis of the primary study end point (disease-free survival). Reprinted from [18] with permission from the Massachusetts Medical Society
Subgroup analyses
Prospectively planned subgroup analyses, using the Cox proportional-hazards model, for primary, secondary, and additional end points are summarized in Fig. 4. Subgroup analysis showed that the beneficial effect of letrozole on DFS was seen in ER+ tumors irrespective of PgR receptor status or patient age [18]. The role of ER and PgR in trials comparing AIs with tamoxifen remains an area of continued research [19, 20]. To explore this further, a central assessment of ER, PgR, and HER2 was recently completed for 6,500 patients in BIG 1-98. Results from the first 3,533 patients on the two monotherapy arms with ER+ tumors (by central assessment) and centrally assessed PgR and HER2 confirmed the results reported above from the local assessment of receptor status [18, 21], indicating that PgR status in ER+ tumors does not predict responsiveness to letrozole when compared with tamoxifen. The small group of patients with HER2 overexpression/amplification in the tumor had a higher rate of recurrence with both treatments, but the superiority of letrozole over tamoxifen was similar irrespective of HER2 status [21].
For the primary end point of DFS, the relative risk reductions for letrozole compared with tamoxifen were 29% in patients with node-positive tumors (hazard ratio 0.71, 95% CI 0.59–0.85), 24% in patients with tumors >2 cm (hazard ratio 0.76, 95% CI 0.63–0.92), and 30% in patients with prior chemotherapy (hazard ratio 0.70, 95% CI 0.54–0.92). An additional logistic regression analysis of BIG 1-98 was performed to retrospectively identify clinical and pathological factors predictive of early breast cancer recurrence [22]. The final model, based on 5,980 patients from the four-arm option and 212 events, identified the following significant factors: tumor size (P < 0.001), ER/PgR status (P < 0.001), node positivity (P < 0.001), and tumor grade (P < 0.001). There was a significant interaction between node positivity and treatment (P = 0.003). Patients with the greatest risk of recurrence had ≥4 positive nodes, tumors ≥5 cm, ER+/PgR− tumors, and grade 3 tumors. The increase in risk associated with increased node positivity was greater for patients randomized to tamoxifen than to letrozole [22].
Letrozole-only versus tamoxifen-only arms
The superiority of letrozole was confirmed in a protocol-defined supplementary analysis, which was restricted to the 4,922 patients randomized to the monotherapy tamoxifen or letrozole arms. At a median follow-up of 51 months, letrozole provided a significant benefit for the end points DFS (P = 0.007), DFS excluding secondary malignancy (P = 0.01), time to recurrence (P = 0.004), and time to distant recurrence (P = 0.03) (see Table 1) [23].
Table 1.
Efficacy end points in patients randomized to treatment with letrozole (n = 2,463) or tamoxifen (n = 2,459) for 5 years in the BIG 1-98 trial [23]
| End point | Events | Hazard ratio | 95% CI | P-value | |
|---|---|---|---|---|---|
| Let | Tam | ||||
| DFS (primary protocol definition) | 352 | 418 | 0.82 | 0.71–0.95 | 0.007 |
| Overall survival | 194 | 211 | 0.91 | 0.75–1.11 | 0.35 |
| Systemic DFS | 331 | 374 | 0.87 | 0.75–1.01 | 0.07 |
| DFS (ignoring second non-breast cancer) | 307 | 364 | 0.83 | 0.71–0.96 | 0.01 |
| Time to recurrence | 231 | 291 | 0.78 | 0.65–0.92 | 0.004 |
| Time to distant recurrence | 193 | 234 | 0.81 | 0.67–0.98 | 0.03 |
DFS disease-free survival, Let letrozole, Tam tamoxifen, CI confidence interval
Safety
All adverse events were graded according to the Common Toxicity Criteria of the National Cancer Institute (version 2). Predefined adverse events were specifically asked and documented at each study visit. Furthermore, the IBCSG Coordinating Center conducted a medical review (reviewers were blinded to randomization) of all grade 3–5 cardiovascular events and other grade 3–5 adverse events that were considered clinically relevant but whose cause was unclear, and all deaths of women in whom there was no prior cancer-related event. The results of BIG 1-98 showed that letrozole was well-tolerated and had a safety profile different from tamoxifen (see Table 2) [18]. A more detailed analysis of cardiovascular side effects, including baseline risk factors and cholesterol values over time, has recently been presented [24].
Table 2.
Cardiovascular adverse events and significant other adverse events among patients included in the BIG 1-98 safety analysis [18]
| Adverse event | Incidence of any grade (%) | P-value | |
|---|---|---|---|
| Letrozole (n = 3,975) | Tamoxifen (n = 3,988) | ||
| Cerebrovascular accident or transient ischemic attack | 1.0 | 1.0 | 0.91 |
| Thromboembolic event | 1.5 | 3.5 | <0.001 |
| Cardiac event | 4.1 | 3.8 | 0.61 |
| Other cardiovascular event | 0.5 | 0.2 | 0.04 |
| Vaginal bleeding | 3.3 | 6.6 | <0.001 |
| Hot flashes | 33.5 | 38.0 | <0.001 |
| Night sweats | 13.9 | 16.2 | 0.004 |
| Fracture | 5.7 | 4.0 | <0.001 |
| Arthralgia | 20.3 | 12.3 | <0.001 |
Tamoxifen compared with letrozole was associated with an increased risk of thromboembolism, vaginal bleeding, and more endometrial biopsies (9.1% vs. 2.3%, respectively; P < 0.001), with a higher incidence of invasive endometrial cancers (0.3% vs. 0.1%, respectively; P = 0.18). In addition, tamoxifen was associated with a higher incidence of hot flushes (38.0% vs. 33.5%, respectively; P < 0.001) and night sweats (16.2% vs. 13.9%; respectively; P = 0.004).
There was a higher incidence of arthralgia and skeletal events with letrozole compared with tamoxifen, including a higher rate of fractures (5.7% vs. 4.0%, respectively; P < 0.001) and a shorter time to first fracture.
Hypercholesterolemia was among the adverse events listed on the case-report forms and was graded at each study visit during treatment [18]. A total of 43.6% of letrozole-treated and 19.2% of patients in the tamoxifen group had hypercholesterolemia, reported at least once during treatment [18]. Nevertheless, more than 80% of reported hypercholesterolemia was grade 1, and thus of uncertain clinical significance. In addition, serum total cholesterol values remained stable throughout the trial in the letrozole arm but decreased in the tamoxifen arm by approximately 13%, which is consistent with the known lipid-lowering effect of tamoxifen [25]. Thus, median changes in cholesterol values from baseline were 0, 0, and −1.8% at 6, 12, and 24 months in the letrozole group and −12.0, −13.5, and −14.1% in the tamoxifen group.
The overall incidence of cardiovascular events was similar for the letrozole and tamoxifen groups. Although there were higher incidences of grade 3–5 cardiac events and cardiac failure in the letrozole arm, the incidences were low in both groups (2.1% vs. 1.1%; P < 0.001 and 0.8% vs. 0.4%, P = 0.01, respectively) in this older patient population at competing risk for cardiovascular events [18].
Discussion
Clinical implications of BIG 1-98
The results of the primary core analysis [18], which included all available data from patients randomly assigned to the monotherapy arms and data from the sequential therapy arms censored at the time of the therapy switch, as well as the results from a recently published analysis limited to patients randomly assigned to the continuous therapy arms [23], demonstrate a significant benefit of letrozole over tamoxifen. Letrozole is at least as well-tolerated as tamoxifen, offering patients and physicians a true alternative. However, bone metabolism is differently affected by letrozole and tamoxifen. Patients receiving AIs are at increased risk for bone loss and osteoporosis and should therefore receive appropriate monitoring and medical intervention as part of daily practice.
As a result of the findings from BIG 1-98, letrozole was approved both in Europe and the United States as an early adjuvant treatment for postmenopausal women with HR+ breast cancer. The 2007 St. Gallen international consensus guidelines [26] and updated National Comprehensive Cancer Network (NCCN) guidelines [27] recommend letrozole as an option for adjuvant treatment of early breast cancer. In the 2007 St. Gallen Guidelines, use of an AI is considered as an alternative to tamoxifen for postmenopausal women with low-, intermediate-, or high-risk tumors that are classified as endocrine-responsive or endocrine response uncertain [26]. Similarly, in the NCCN guidelines, letrozole is a recommended adjuvant hormonal therapy for all postmenopausal women with hormone-responsive tumors, regardless of HER2 status [27]. The current guidelines do not recommend one AI over another but emphasize that treatment should be selected on the basis of clinical trial evidence in specific settings [27], nor do the guidelines provide recommendations as concerns the optimal use of the AIs as upfront monotherapy or sequenced with tamoxifen.
Results from the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial provided evidence for superior DFS with anastrozole versus tamoxifen used as initial adjuvant hormonal therapy in postmenopausal women with HR+ breast cancer [28, 29]. At a median follow-up of 68 months, no survival advantage has been observed in the ATAC trial, and it remains an open question whether the DFS advantage observed in AI trials will translate into an OS advantage.
Both letrozole and anastrozole have demonstrated superiority over tamoxifen as initial adjuvant therapy [18, 28], but a direct comparison of letrozole with anastrozole awaits the results of a randomized head-to-head trial (Femara Anastrozole Clinical Evaluation [FACE]) [30, 31]. The adjuvant FACE trial compares upfront therapy with letrozole 2.5 mg with anastrozole 1 mg daily for up to 5 years in postmenopausal, HR+, node-positive breast cancer patients. In addition, recruitment of a direct comparison of anastrozole and exemestane (MA.27) has been completed recently.
Other trials demonstrated better disease control when an AI was given after 2–3 years of adjuvant tamoxifen [32], but so far, no trial has reported on a regimen of an AI given 2–3 years before tamoxifen. Initial treatment with the “gold standard” tamoxifen followed by an AI may be a logical long-term strategy because of the lack of complete cross-resistance between these hormonal strategies. On the other hand, the greater anti-estrogenic potency and higher anti-tumor activity of AIs over tamoxifen, as demonstrated in preclinical models and randomized clinical trials [12, 17, 33, 34], may suggest that it is preferable to use an AI upfront to avoid early relapses that may occur while on tamoxifen therapy. Thus, the key question, “should AIs be given as initial therapy or used sequentially after tamoxifen?” is as yet unanswered. Physicians often extrapolate data from switch trials, e.g., the Intergroup Exemestane Study [35, 36] or the MA.17 trial [33, 37] to sequential trials (e.g., BIG 1-98, Austrian Breast and Colorectal Cancer Study Group [ABCSG] 8 [38]). In these sequential trials, events are included in the analysis from treatment start and not from point of switch after 2–3 years of tamoxifen. Sequential and switch trials investigate obviously the same intervention but are conducted in different patient groups, thus, results are expected to be different and are different indeed.
Best use of AIs remains an open question, at least until results of BIG 1-98 from the sequential use of letrozole and tamoxifen, in comparison with continuous monotherapy, as well as from the Tamoxifen and Exemestane Adjuvant Multicenter trial investigating exemestane monotherapy versus tamoxifen followed by exemestane [39] and from an updated analysis of ABCSG-8, become available.
Conclusions
BIG 1-98 has shown that the AI letrozole results in better disease control than tamoxifen when given as initial endocrine therapy for postmenopausal women with hormone-responsive early breast cancer. Letrozole significantly reduced the risk of recurrence and of distant recurrence and has a reasonable safety profile [18]. The comparison between the monotherapy and the sequential treatment arms within the BIG 1-98 trial are eagerly awaited and are expected to have an important impact on the management of breast cancer.
Acknowledgement
We greatly appreciate the help of Miss Sharon Thomas (sthomas@ghgroup.com) in preparing the manuscript.
BIG 1-98 Collaborative Group Participants
Steering Committee: B. Thürlimann (Chair), L. Blacher, M. Castiglione, A. S. Coates, T. Cufer, J. F. Forbes, R. D. Gelber, A. Goldhirsch, A. Hiltbrunner, S. B. Holmberg, A. Keshaviah, R. Maibach, A. Martoni, L. Mauriac, H. T. Mouridsen, K. N. Price, M. Rabaglio, A. Santoro, I. E. Smith, C. Straehle, G. Viale.
Novartis: H. A. Chaudri-Ross, A. Covelli, D. B. Evans, W. Hackl, E. Raman, M.G. Porro.
IBCSG Scientific Committee: A. Goldhirsch, A. S. Coates (Co-Chairs), L. Blacher, M. Castiglione, J. F. Forbes, R. D. Gelber, B. A. Gusterson, A. Hiltbrunner, C. Hürny, E. Murray, K. N. Price, M. Rabaglio, R. Studer, G. Viale, A. Wallgren.
IBCSG Foundation Council: B. Thürlimann (President), M. Castiglione, A. S. Coates, J. P. Collins, H. Cortés Funes, R. D. Gelber, A. Goldhirsch, M. Green, A. Hiltbrunner, S. B. Holmberg, D. K. Hossfeld, I. Láng, J. Lindtner, F. Paganetti, C.-M. Rudenstam, R. Stahel, H.-J. Senn, A. Veronesi.
Coordinating Center (Berne, Switzerland): M. Castiglione (CEO), A. Hiltbrunner (Director), M. Rabaglio, G. Egli, B. Cliffe, S. Ribeli-Hofmann, F. Munarini, R. Kammler, R. Studer, B. Ruepp, R. Maibach, N. Munarini.
Statistical Center (Dana-Farber Cancer Institute, Boston, MA, USA): R. D. Gelber (Group Statistician), K. N. Price (Director of Scientific Administration), A. Keshaviah (Trial Statistician), H. Litman, H. Huang, L. J. Somos, B. Timmers, L. Nickerson.
Data Management Center (Frontier Science & Technology Research Foundation, Amherst, NY, USA): L. Blacher (Director of Data Management), T. Heckman Scolese (Coordinating Data Manager), M. Belisle, M. Caporale, J. Celano, L. Dalfonso, L. Dooley, S. Fischer, K. Galloway, J. Gould, R. Hinkle, M. Holody, G. Jones, R. Krall, S. Lippert, J. Meshulam, L. Mundy, A. Pavlov-Shapiro, K. Scott, M. Scott, S. Shepard, J. Swick, L. Uhteg, D. Weinbaum, C. Westby, T. Zielinski.
Central Pathology Review Office (University of Glasgow, Glasgow, UK): B. A. Gusterson, E. Mallon; (European Institute of Oncology, Division of Pathology, Milano, Italy): G. Viale, P. Dell’Orto, M. Mastropasqua, B. Del Curto.
Data and Safety Monitoring Committee: D.F. Hayes, J.E. Garber, S.W. Lagakos, I. Lindgren.
Study Support (Novartis Corp. Basel, Switzerland): E. Waldie, I. van Hoomissen, M. De Smet, W. Schmidt, A. Bolton, W. Hackl.
Breast International Group (BIG)
International Breast Cancer Study Group (IBCSG)
Australian New Zealand Breast Cancer Trials Group (ANZ BCTG): Board Chair: R. D. Snyder, Group Co-ordinator: J. F. Forbes, Chair Scientific Advisory Committee: A. S. Coates; ANZ BCTG Operations Office (Newcastle, Australia): D. Lindsay (Head Data Management), D. Preece (Senior Study Coordinator), J. Cowell, D. Talbot, A. Whipp.
Australia: The Cancer Council Victoria, Melbourne, VIC: F. Abell, R. Basser, R. Bell, B. Brady, D. Blakey, P. Briggs, I. Burns, P. Campbell, M. Chao, J. Chirgwin, B. Chua, K. Clarke, J. Collins, R. De Boer, J. C. Din, R. Doig, A. Dowling, R. Drummond, N. Efe, S. T. Fan, M. Francis, P. Francis, V. Ganju, P. Gibbs, G. Goss, M. Green, P. Gregory, J. Griffiths, I. Haines, M. Henderson, R. Holmes, P. James, J. Kiffler, M. Lehman, M. Leyden, L. Lim, G. Lindeman, R. Lynch, B. Mann, J. McKendrick, S. McLachlan, R. McLennan, G. Mitchell, S. Mitra, C. Murphy, I. Parker, K. Phillips, I. Porter, G. Richardson, J. Scarlet, S. Sewak, J. Shapiro, R. Snyder, R. Stanley, C. Steer, D. Stoney, A. Strickland, G. Toner, C. Underhill, K. White, M. White, A. Wirth, S. Wong; W P Holman Clinic; Launceston General Hospital, Launceston, Tasmania: D. Byram, I. Byard; Liverpool Hospital, Sydney, NSW: S. Della-Fiorentina, A. Goldrick, E. Hovey, E. Moylan, E. Segelov; Mount Hospital, Perth, WA: A. Chan, M. Buck, D. Hastrich, D. Ingram, G. Van Hazel, P. Willsher; Nepean Cancer Care Centre, Sydney, NSW: N. Wilcken, C. Crombie; Newcastle Mater Hospital, Newcastle, NSW: J. F. Forbes, F. Abell, S. Ackland, A. Bonaventura, S. Cox, J. Denham, R. Gourlay, D. Jackson, R. Sillar, J. Stewart; Prince of Wales Hospital, Sydney, NSW: C. Lewis, B. Brigham, D. Goldstein, M. Friedlander; Princess Alexandra Hospital, Woollongabba, QLD: E. Walpole, D. Thompson; Royal Adelaide Hospital, Adelaide, SA: P. G. Gill, M. Bochner, J. Coventry, J. Kollias, P. Malycha, I. Olver; Royal Brisbane and Women’s Hospital, Brisbane, QLD: M. Colosimo, R. Cheuk, L. Kenny, N. McCarthy, D. Wyld; Royal Hobart Hospital, Hobart, Tasmania: R. Young, R. Harrup, R. Kimber, R. Lowenthal; Royal Perth Hospital, Perth, WA: J. Trotter, E. Bayliss, A. Chan, D. Ransom; Sir Charles Gairdner Hospital, Perth, WA: M. Byrne, M. Buck, J. Dewar, A. Nowak, A. Powell, G. Van Hazel; Toowoomba Hospital, Toowoomba, QLD: E. A. Abdi, R. Brodribb, Z. Volobueva; Westmead Hospital, Sydney, NSW: P. Harnett, V. Ahern, H. Gurney, N. Wilcken.
New Zealand:Auckland Hospital, Auckland: V. J. Harvey, B. Evans, W. Jones, M. McCrystal, D. Porter, P. Thompson, M. Vaughan; Christchurch Hospital, Christchurch: D. Gibbs, C. Atkinson, R. Burcombe, B. Fitzharris, B. Hickey, M. Jeffery, B. Robinson; Dunedin Hospital, Dunedin: B. McLaren, S. Costello, J. North, D. Perez; Waikato Hospital, Hamilton: I. D. Campbell, L. Gilbert, R. Gannaway, M. Jameson, I. Kennedy, J. Long, G. Round, L. Spellman, D. Whittle, D. Woolerton.
Brazil: Hospital de Clinicas de Porto Alegre, Porto Alegre: C. Menke, J. Biazús, R. Cericatto, J. Cavalheiro, N. Xavier, A. Bittelbrunn, E. Rabin.
Chile: Chilean Cooperative Group for Oncologic Research, GOCCHI: J. Gutiérrez (Chairman), R. Arriagada (Scientific Adviser), L. Bronfman (Principal Investigator), M. Zuñiga (Data Manager); Clinica Las Condes, Santiago: J. Gutiérrez, J. C. Acevedo, S. Torres, A. León, E. Salazar; Hospital DIPRECA, Las Condes, Santiago: L. Soto Diaz, R. Duval, N. Oddeshede, M. C. Venti; Hospital San Juan de Dios, Santiago: K. Peña, L. Puente, V. Maidana; IRAM/Instituto de Radiomedicina, Vitacura, Santiago: R. Baeza, R. Arriagada, P. Olfos, J. Solé, E. Vinés, C. Mariani.
Hungary: National Institute of Oncology, Budapest: I. Láng, E. Hitre, E. Szabó, Z. Horváth, E. Ganofszky, E. Juhos.
Italy: Centro di Riferimento Oncologico, Aviano: A. Veronesi, D. Crivellari, M. D. Magri, A. Buonadonna, F. Coran, E. Borsatti, E. Candiani, S. Massarut, M. Roncadin, M. Arcicasa, A. Carbone, T. Perin, A. Gloghini; Ospedali Riuniti di Bergamo, Bergamo: C. Tondini, R. Labianca, P. Poletti, A. Bettini; Ospedale degli Infermi, Biella: M. Clerico, M. Vincenti, A. Malossi, E. Seles, E. Perfetti, B. Sartorello; Spedali Civili, Brescia: E. Simoncini, G. Marini, P. Marpicati, R. Farfaglia, A. M. Bianchi, P. Grigolato, L. Lucini, P. Frata, A. Huscher, E. Micheletti, C. Fogazzi; U. O. Medicina Oncologica, Ospedale Capri, Ospedale Mirandola: F. Artioli, K. Cagossi, L. Scaltriti, E. Bandieri, L. Botticelli, G. Giovanardi; Ospedale di Cattolica “Cervesi”, Cattolica: A. Ravaioli, E. Pasquini, B. Rudnas; Ospedale Civile, Gorizia: L. Foghin; Ospedale “A. Manzoni” Lecco, Lecco: M. Visini, L. Zavallone, G. Ucci; Istituto Europeo di Oncologia, Milano: M. Colleoni, G. Viale, P. Veronesi, G. Peruzzotti, L. Corsetto, R. Ghisini, G. Renne, A. Luini, L. Orlando, R. Torrisi, A. Rocca, T. De Pas, E. Munzone, V. Galimberti, S. Zurrida, M. Intra, F. Nolé, R. Orecchia, G. Martinelli, F. de Braud, A. Goldhirsch; Ospedale Infermi, Rimini: A. Ravaioli, L. Gianni.
Peru: Instituto de Enfermedades Neoplásicas, Lima: H. Gome.
Slovenia: Institute of Oncology, Ljubljana: T. Cufer, B. Pajk, J. Cervek.
South Africa: Groote Schuur Hospital and University of Cape Town, Cape Town: I. D. Werner, E. Murray, D. Govender, S. Dalvie, T. Erasmus, B. Robertson, B. Read, E. Nel, J. Toop, N. Nedeva, E. Panieri; Sandton Oncology Centre, Johannesburg: D. Vorobiof, M. Chasen, G. McMichael, C. Mohammed. Local funding provided by the Cancer Association of South Africa.
Sweden: West Swedish Breast Cancer Study Group: S. B. Holmberg; Sahlgrenska U Hospital, Moelndal: S. B. Holmberg, J. Mattsson; Boras Hospital, Boras; Karlstads Hospital, Karlstads: H. Sellström; Kungalvs Hospital, Kungalvs: B. Lindberg.
Switzerland: Swiss Group for Clinical Cancer Research (SAKK): A. Goldhirsch (up to January 2004), R. Herrmann (from June 2004): Kantonsspital Aarau, Zentrum f. Onkologie, Aarau: A. Schönenberger, W. Mingrone, Ch. Honegger, E. Bärtschi, M. Neter, M. Rederer, G. Schär; University Hospital Basel, Basel: C. Rochlitz, R. Herrmann, D. Oertli, E. Wight, H. Moch; Institute of Oncology of Southern Switzerland: Ospedale San Giovanni, Bellinzona: J. Bernier, L. Bronz, F. Cavalli, E. Gallerani, A. Richetti, A. Franzetti; Ospedale Regionale di Lugano (Civico & Italiano), Lugano: M. Conti-Beltraminelli, M. Ghielmini, T. Gyr, S. Mauri, P. C. Saletti; Ospedale Regionale Beata Vergine, Mendrisio: A. Goldhirsch, O. Pagani, R. Graffeo, M. Locatelli, S. Longhi, P.C. Rey, M. Ruggeri; Ospedale Regionale La Carità, Locarno: E. Zucca, D. Wyss; Istituto Cantonale di Patologia, Locarno: L. Mazzucchelli, E. Pedrinis, T. Rusca; Inselspital, Berne: S. Aebi, M. F. Fey, M. Castiglione, M. Rabaglio; Kantonsspital Olten, Olten: S. Aebi, M. F. Fey, M. Zuber, G. Beck; Bürgerspital, Solothurn: S. Aebi, M. F. Fey, R. Schönenberger; Spital Thun-Simmental AG Thun: J.M. Lüthi, D. Rauch; Hôpital Cantonal Universitaire HCUG, Geneva: H. Bonnefoi; Rätisches Kantons- und Regionalspital, Chur: F. Egli, R. Steiner, P. Fehr; Centre Pluridisciplinaire d’Oncologie, Lausanne: L. Perey, P. de Grandi, W. Jeanneret, S. Leyvraz, J.-F. Delaloye; Kantonsspital St. Gallen, St. Gallen: B. Thürlimann, D. Köberle, F. Weisser, S., Mattmann, A. Müller, T. Cerny, B. Späti, M. Höfliger, G. Fürstenberger, B. Bolliger, C. Öhlschlegel, U. Lorenz, M. Bamert, J. Kehl-Blank, E. Vogel; Kantonales Spital Herisau, Herisau: B. Thürlimann, D. Hess, I. Senn, D. Köberle, A. Ehrsam, C. Nauer, C. Öhlschlegel, J. Kehl-Blank, E. Vogel; Stadtspital Triemli, Zürich: L. Widmer, M. Häfner; Universitätsspital Zürich, Zürich: B. C. Pestalozzi, M. Fehr, R. Caduff, Z. Varga, R. Trüb, D. Fink.
Swiss Private MDs: Private Praxis, Zürich: B. A. Bättig; Sonnenhof-Klinik Engeried, Berne: K. Buser; Frauenklinik Limmattalspital, Schlieren: N. Bürki; Private Praxis, Birsfelden: A. Dieterle; Private Praxis, Biel: L. Hasler; Private Praxis, Baar: M. Mannhart-Harms; Brust-Zentrum, Zürich: C. Rageth; Private Praxis, Berne: J. Richner; Private Praxis, Bellinzona: V. Spataro; Private Praxis, Winterthur: M. Umbricht.
United Kingdom: King’s College Hospital/Breast Unit, London: P. Ellis, S. Harris, N. Akbar, H. McVicars, C. Lees, R. Raman, G. Crane.
Danish Group (DBCG)
H. T. Mouridsen; Rigshospitalet, Copenhagen: H. T. Mouridsen; Vejle Hospital, Vejle: E. Jakobsen; Odense University Hospital, Odense: S. Cold; KAS Herlev/Herlev University Hospital, Herlev: C. Kamby; Aalborg Sygehus Syd, Aalborg: M. Ewertz; Hilleroed Hospital, Hilleroed: P.M. Vestlev; Aarhus University Hospital, Aarhus: J. Andersen; Roskilde County Hospital, Roskilde: P. Grundtvig; Esbjerg Central Hospital, Esbjerg: E. Sandberg; Naestved Central Hospital, Naestved: P. Philip; Soenderborg Sygehus, Soenderborg: E. L. Madsen; Herning Central Hospital, Herning: K. A. Moeller; Viborg Sygehus, Viborg: V. Haahr; Landspitali University Hospital, Reykjavik, Iceland: J. Johansson.
French Group (FNCLCC)
Institut Bergonié, Bordeaux: L. Mauriac, M. Debled, P. Campo; Centre Hospitalier de la Côte Basque, Bayonne: D. Larregain-Fournier, S. Remy; Centre Jean Perrin, Clermont-Ferrand: H. Auvray; Centre Georges François Leclerc, Dijon: C. De Gislain, F. Delille, M.-C. Porteret; Centre Oscar Lambret, Lille: V. Servent, M. Chapoutier; CHRU, Limoges: N. Tubiana-Mathieu, S. Lavau-Denes, P. Bosc; Centre Léon Bérard, Lyon: J. P. Guastalla, Th. Bachelot, C. Arbault; Centre Hospitalier Meaux, Meaux: G. Netter-Pinon; C.H.G. André Boulloche, Montbéliard: V. Perrin, A. Monnier, Y. Hammoud; Centre Paul Lamarque, Montpellier: G. Romieu, L. Culine, V. Pinosa; Clinique Francheville, Périgueux: L. Cany, C. Maguire; Hôpital de la Milétrie, Poitiers: A. Daban, M. Le Saux, C. Grandon; Centre Eugène Marquis, Rennes: P. Kerbrat, C. Catheline; Centre Henri Becquerel, Rouen: C. Veyret, E. Jugieau, V. Talon; Centre René Gauducheau, Saint-Herblain: A. Le Mevel, S. Maury; Centre Claudius Régaud, Toulouse: L. Gladieff, N. Lignon.
North Yorkshire Group
D. Dodwell; Harrogate District Hospital, Harrogate, North Yorkshire: D. Dodwell; Huddersfield Royal Infirmary, Huddersfield: J. Joffe; Castlehill Hospital, Hull: P. Drew; Airedale General Hospital, Keighley, W. Yorkshire: A. Nejim; Leeds General Infirmary, Leeds: D. Dodwell, K. Horgan; St. James’s University Hospital, Leeds: M. Lansdown, T. Perren; Weston Park Hospital, Sheffield: R. E. Coleman.
Independent Centers/Groups
Argentina: Centro Oncológico Confidence, Buenos Aires: D. Campos; Hospital Allemán, Buenos Aires: F. Cóppola; Hospital Británico, Buenos Aires: J. Martinez; Hospital Evita, Buenos Aires: M. Freue; Hospital Posadas, Buenos Aires: C. Wainstein; Hospital Zubizarreta, Buenos Aires: A. Zori Comba; Instituto Dr. Estevez, Buenos Aires: E. Cazap; Instituto Oncológico Dr. Angel H. Roffo, Buenos Aires: E. Mickiewicz; Sanatorio Municipal Julio A. Mendez, Buenos Aires: L. Balbiani; Centro Privado de Ginecología, Córdoba: A. Osuna; Hospital Privado de Córdoba, Córdoba: E. Palazzo; Instituto Modelo de Ginecología y Obstetricia, Córdoba: M. de Romedis; Fundación Mainetti-Centro Oncológico de Excelencia, La Pllata: S. Cagnolati; Hospital Privado de la Comunidad, Mar del Plata: C. A. Delfino, G. Caccia; Escuela de Medicina Nuclear (COIR), Mendoza: R. L. de Angelis; Centro Oncológico de Rosario, Rosario: L. Fein, R. Sala; Hospital Provincial de Rosario, Rosario: C. Nassurdi, A. Colombo Berra; Clínica Especializada ISIS, Santa Fe: R. Viroglio, C. Blajman; Hospital Regional de Concepción, Tucumán: H. Requejo; Instituto de Maternidad y Ginecología Nuestra Señoras de las Mercedes, Tucumán: L. Silberman.
Australia: Flinders Medical Centre, Adelaide, SA: S. Birrell, M. Eaton, C. Hoffman; Queen Elizabeth Hospital, Adelaide, SA: V. Humeniuk; The Canberra Hospital, Canberra, ACT; P. Craft, R. Stuart-Harris, D. Yip; The Geelong Hospital, Geelong, VIC: R. Bell, F. Abell, M. Francis, J. Kiffer, R. Lynch, R. McLennan, K. White; Royal Melbourne Hospital, Melbourne, VIC: M. Green, R. Basser, J. Collins, R. De Boer, J. C. Din, N. Efe, S. T. Fan, G. Lindeman, S. Wong; Western General Hospital, Melbourne, VIC: M. Green, R. Basser, J. Collins, R. De Boer, J. C. Din, N. Efe, S. T. Fan, G. Lindeman, S. Wong; Newcastle Mater Hospital, Newcastle, NSW: J. Stewart, F. Abell, S. Ackland, A. Bonaventura; Royal Perth Hospital, Perth, WA: J. Trotter, E. Bayliss, A. Chan, D. Ransom, A. Redfern; St. George Hospital, Sydney, NSW: P. de Souza, M. Links; St. Vincent’s Hospital, Sydney, NSW: D. Dalley, J. Grygiel, R. Ward; Murray Valley Private Hospital, Wodonga, VIC: C. Underhill, K. Clarke, C. Steer; Princess Alexandra Hospital, Woolloongabba, QLD: E. Walpole, D. Thompson.
Belgium: Institut Jules Bordet, Bruxelles: J. M. Nogaret; University Hospitals Leuven, Leuven: M.R. Christiaens, P. Neven, R. Paridaens, A. Smeets, I. Vergote, C. Weltens, H. Wildiers; Les Cliniques Saint-Joseph ASBL, Liège: C. Focan; Clinique du Parc Léopold, Bruxelles: L. Marcelis; C. H. Etterbeek-Ixelles, Bruxelles: J. P. Kains; Service d’Oncologie Clinique Notre-Dame, Charleroi: J.-L. Canon; C. H. U. André Vèsale, Montigny-Le Tilleul: D. Brohèe.
Canada: Cambridge Memorial Hospital, Cambridge: J. Gowing; CHUM- Campus Notre-Dame, Montreal: L. Yelle; Hôpital Maisonneuve-Rosemont, Montreal: P. Dubé.
Chile: Fundacion Lopez Perez, Santiago: C. Vogel; Hospital Carlos Van Buren, Valparaiso: M. León Prieto.
Czech Republic: Institute of Oncology, Brno: K. Petrakova, M. Palacova, R. Demlova; Dept. of Clinical and Radiation Oncology, Ceske Budejovice: H. Siffnerova, J. Fischer, I. Bustova; Centre of Breast Diseases, Prague: H. Kankova, M. Pintova; Institute of Radiation Oncology, Prague: P. Vitek; University Hospital, Prague: J. Abrahamova, D. Kordikova; University Hospital Prague: L. Petruzelka, E. Sedlackova, H. Honova.
Germany: Onkologische Gemeinschaftspraxis, Augsburg: B. Heinrich; Zentralklinikum/Frauenklinik, Augsburg: A. Wischnik; Universitätsklinikum Essen, Essen: C. Oberhoff, A. E. Schindler; Universitäts-Frauenklinik d. JLU Giessen, Giessen: K. Münstedt; Onkologische Gemeinschaftspraxis, Göttingen: D. Meyer; Martin-Luther-Universität Halle-Wittenberg, Halle: R. Grosse, H. Kölbl; Universitätskliniken des Saarlandes, Homburg: W. Schmidt, D. Mink; Universitäts-Frauenklinik und Poliklinik Universitätskrankenhaus Eppendorf, Hamburg: F. Jänicke; Kliniken d. Med. Hochschule, Frauenklinik, Hannover: H. J. Lück; Krankenanstalt Mutterhaus der Borromäerinnen, Trier: W. Dornoff; Gynäkologische Abteilung des St. Josefshospital, Wiesbaden: G. Hoffmann; Gynäkologische Abteilung d. Marienhospitals, Universität Witten-Herdecke, Witten: J. Hackmann, W. Bader.
Hungary: SZOTE Onkoterápiás Klinika, Szeged: Z. Kahan; BM Központi Kórház, Budapest: G. Pajkos, K. Kristo; SOTE Radiológiai és Onkoterápiás Klinika, Budapest: M. Dank; Uzsoki Utcai Kórház, Budapest: T. Nagykalnai, L. Landherr; Almási Balogh Pál Kórház, Ózd: E. Kner; Területi Kórház Onkologia, Szentes: M. Kispál; Szent Borbála Kórház, Megyei Onkológiai Gondozó, Tatabánya: Á. Dani.
Italy: Policlinico S. Orsola-Malpighi, Bologna: A. Martoni, C. Zamagni, S. Giaquinta, E. Piana; Ospedale S. Croce, Fano: R. Mattioli, L. Imperatori; Istituto Clinica Humanitas, Milan/Rozzano: A. Santoro, C. Carnaghi, L. Rimassa; Azienda Ospedaliera San Filippo Neri, Rome: G. Gasparini, G. Sciarretta, A. Morabito; Az. Ospedaliera Treviglio-Caravaggio, Treviglio: S. Barni, M. Cazzaniga, M. Cabiddu; Policlinico Universitario (PUDG), Udine: F. Puglisi; Ospedale di Torrette, Ancona: R. Cellerino, S. Antognoli, F. Freddari; Universitiy of Cagliari, Policlinico Universitario, Cagliari: G. Mantovani, E. Massa, G. Astara; Ospedale Civile Feltre, Feltre: R. Segati; Istituto Nazionali Ricerca Cancro, Genova: R. Rosso, L. Del Mastro, M. Venturini, C. Bighin; Istituto Nazionale dei Tumori, Milano: E. Bajetta, N. Zilembo, D. Paleari, G. Procopio; Azienda Ospedaliera di Parma, Parma: S. Salvagni, M. A. Perrone , V. Franciosi; Azienda Ospedaliera “S. Salvatore”, Pesaro: G. Catalano, S. Luzi Fedeli; Azienda Ospedaliera “Ospedale di Circolo e Fondazione Macchi” Varese: G. Pinotti, G. Giardina, I. Vallini; Universitiy of Cagliari, Policlinico Universitario, Cagliari: B. Massidda, M. T. Ionta, M. C. Deidda; Ospedale Maggiore, Lodi: G. Nalli, G. Sita; Policlinico Universitario, Palermo: I. Carreca, S. Cucciarré, D. Burgio; Ospedale Civile dello Spirito Santo, Pescara: M. Lombardo, G. Pandoli, P. Di Stefano; Azienda Ospedaliera Santa Maria Nuova, Reggio Emilia: C. Boni, G. Bisagni, M. C. Banzi, P. Linarello; Azienda Ospedaliera Desenzano del Garda, Manerbio: G. Colosini, A. Spasiano, A. Caldonazzo; Ospedale Civile ASL 20, Tortona: M. G. Pacquola.
Netherlands: Ziekenhuis Leyenburg, Den Haag: H. P. Sleeboom; Catharina Ziekenhuis, Eindhoven: H. J. T. Rutten; St. Anna Ziekenhuis, Geldrop: E. J. T. Luiten; Tweesteden Ziekenhuis, Tilburg: H. Th. J. Roerdink; Maxima Medisch Centrum, Veldhoven: R. H. M. Roumen.
New Zealand: Dunedin Hospital, Dunedin: B. McLaren, S. Costello, J. North, D. Perez, K., Bayston, M. Pfieffer; Waikato Hospital, Hamilton: I. Kennedy, I. D. Campbell, L. Gilbert, R. Gannaway, M. Jameson, J. Long, G. Round, L. Spellman, D. Whittle, D. Woolerton.
Poland: Department of Oncology and Radiotherapy, Medical University of Gdansk, Gdansk: J. Jassem, M. Welnicka-Jaskiewicz, E. Senkus-Konefka, K. Matuszewska; Rydygier’s Memorial Hospital, Krakow-Nova Huta: P. Koralewski, J. Pernal; Klinika Nowotworów Piersi i, Chirurgii Rekonstrukcyjnej-Warszawa, Warszawa: T. Pienkowski, E. Brewczynska, B. Bauer-Kosinska, R. Sienkiewicz-Kozlowska, A. Jagiello-Gruszfeld, K. Sudol.; Centrum Onkologii w Bydgoszczy, Oddzial Onkologii Klinicznej, Bydgoszcz: J. Tujakowski, B. Zurawski; Collegium Medicum Jagiellonian University, Krakow: J. Pawlega, E. Jablonska, A. Zygulska; Oddzial Kliniczny Onkologiczny, Centralnego Szpitala Klinicznego Wojskowej, Akademii Medycznej-Warszawa, Warszawa: M. Górnasiowa; Dolnoslaskie Centrum Onkologii, Wroclaw: E. Filypczyk-Cisarz, K. Pajak.
Portugal: Hospital de S. João, Porto: M. Damasceno; Instituto Português de Oncologia de Coimbra, Coimbra: J. Q. Albano; Hospital de Santa Maria, Lisboa: B. da Costa, L. Costa; Instituto Português de Oncologia de Lisboa, Lisboa: A. Henriques, H. Amaral; Hospital Geral de Santo António, Porto: F. Marques.
Russia: Cancer Research Centre, Moscow: D. V. Komov, S. B. Polikarpova; Moscow Municipal Hospital No. 62, Moscow: A. N. Makhson, N. V. Zabaznyi; Moscow Research Institute of Diagnostics and Surgery, Moscow: E. K. Vozny, N. Y. Dobrovolskaya, S. Bolshakova, O. V. Yurgina; N. M. Emmanuel Institute of Biochemical Physics, Moscow: D. B. Korman, I. A. Maslova; N.N. Petrov Research Institute of Oncology, St. Petersburg: V. Semiglazov, V. Ivanov; Saint-Petersburg City Oncological Dispensary, St. Petersburg: G. Manikhas, G. Dolmatov.
South Africa: Mamma Clinic, Tygerberg Hospital, Cape Town: J. Apffelstaedt; Southern Cross Hospital, Cape Town: D. Eedes; Pretoria Academic Hospital, Pretoria: C. Slabber; Pretoria East Hospital, Pretoria: M. A. Coccia-Portugal; Eastern Cape Oncology Centre, Port Elizabeth: K. Maart.
Spain:Hospital Ruber Internacional, Madrid: J. E. Alés Martinez, P. Aramburo, R. Sánchez; Hospital Son Dureta, Palma del Mallorca: J. Rifa, J. Martin; Centro Oncológico Integral de Madrid (CONIM), Madrid: R. Pérez-Carrión, J. L. González Larriba, A. Cubillo; Hospital Universitario San Carlos, Madrid: M. M. Jiménez, A. Casado; Hospital Central de Asturias, Oviedo: J. Fra, J. M. Vieitez, E. Esteban, A. J. Lacave.
Switzerland: Universitätsfrauenklinik, Basel: E. Wight, S. Bartens, R. Decio, U. Güth; Klinik am Park, Zürich: U. Breitenstein.
Turkey: Ankara University Ibni Sina Hospital, Ankara: F. Icli, D. Dincol; Hacettepe University Oncology Institute, Ankara: E. Baltali, Y. Ozisik; Istanbul University Oncology Institute, Istanbul: E. Topuz, M. Basaran, A. Aydiner; Ege University Medical School, Izmir: E. Ozdedeli; 9 Eylul University Medical School, Izmir: O. Harmancioglu, A. U. Yilmaz.
United Kingdom: The Royal Marsden Hospital, London, Royal Marsden NHS Trust, Surrey: I. E. Smith; University of Dundee, Dundee: A. M. Thompson; Christie Hospital NHS Trust, South Manchester University Hospital Trust, Manchester: A. Wardley; Royal Bournemouth Hospital, Bournemouth: T. Hickish; North Middlesex Hospital, London: F. Neave.
Uruguay: Hospital de Clinicas Dr. Manuel Quintela, Montevideo, Uruguay: G. Sabini.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s10549-007-9858-3
Contributor Information
Dieter Koeberle, Phone: +41-71-4941111, FAX: +41-71-4946325, Email: dieter.koeberle@kssg.ch.
Beat Thuerlimann, Email: beat.thuerlimann@kssg.ch.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717 [DOI] [PubMed]
- 2.Demonty G, Bernard-Marty C, Puglisi F, Mancini I, Piccart M (2007) Progress and new standards of care in the management of HER-2 positive breast cancer. Eur J Cancer 43:497–509 [DOI] [PubMed]
- 3.Boccardo F, Bruzzi P, Rubagotti A, Nicolo GU, Rosso R (1981) Estrogen-like action of tamoxifen on vaginal epithelium in breast cancer patients. Oncology 38:281–285 [DOI] [PubMed]
- 4.Helgason S, Wilking N, Carlstrom K, Damber MG, von Schoultz B (1982) A comparative study of the estrogenic effects of tamoxifen and 17 beta-estradiol in postmenopausal women. J Clin Endocrinol Metab 54:404–408 [DOI] [PubMed]
- 5.Ellmen J, Hakulinen P, Partanen A, Hayes DF (2003) Estrogenic effects of toremifene and tamoxifen in postmenopausal breast cancer patients. Breast Cancer Res Treat 82:103–111 [DOI] [PubMed]
- 6.Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM (1994) Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst 86:527–537 [DOI] [PubMed]
- 7.Pritchard KI, Paterson AH, Paul NA, Zee B, Fine S, Pater J (1996) Increased thromboembolic complications with concurrent tamoxifen and chemotherapy in a randomized trial of adjuvant therapy for women with breast cancer. National Cancer Institute of Canada Clinical Trials Group Breast Cancer Site Group. J Clin Oncol 14:2731–2737 [DOI] [PubMed]
- 8.Bushnell CD, Goldstein LB (2004) Risk of ischemic stroke with tamoxifen treatment for breast cancer: a meta-analysis. Neurology 63:1230–1233 [DOI] [PubMed]
- 9.Fisher B, Dignam J, Bryant J, Wolmark N (2001) Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst 93:684–690 [DOI] [PubMed]
- 10.Anderson WF, Jatoi I, Devesa SS (2005) Distinct breast cancer incidence and prognostic patterns in the NCI’s SEER program: suggesting a possible link between etiology and outcome. Breast Cancer Res Treat 90:127–137 [DOI] [PubMed]
- 11.Mouridsen H, Sun Y, Gershanovich M, Perez-Carrion R, Becquart D, Chaudri-Ross HA, Lang R (2004) Superiority of letrozole to tamoxifen in the first-line treatment of advanced breast cancer: evidence from metastatic subgroups and a test of functional ability. Oncologist 9:489–496 [DOI] [PubMed]
- 12.Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, Apffelstaedt J, Smith R, Sleeboom HP, Jaenicke F, Pluzanska A, Dank M, Becquart D, Bapsy PP, Salminen E, Snyder R, Chaudri-Ross H, Lang R, Wyld P, Bhatnagar A (2003) Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 21:2101–2109 [DOI] [PubMed]
- 13.Nabholtz JM, Buzdar A, Pollak M, Harwin W, Burton G, Mangalik A, Steinberg M, Webster A, von Euler M (2000) Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group (2000). J Clin Oncol 18:3758–3767 [DOI] [PubMed]
- 14.Bonneterre J, Thürlimann B, Robertson JF, Krzakowski M, Mauriac L, Koralewski P, Vergote I, Webster A, Steinberg M, von Euler M (2000) Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol 18:3748–3757 [DOI] [PubMed]
- 15.Paridaens R, Dirix L, Lohrisch C, Beex L, Nooij M, Cameron D, Biganzoli L, Cufer T, Duchateau L, Hamilton A, Lobelle JP, Piccart M (2003) Mature results of a randomized phase II multicenter study of exemestane versus tamoxifen as first-line hormone therapy for postmenopausal women with metastatic breast cancer. Ann Oncol 14:1391–1398 [DOI] [PubMed]
- 16.Ellis MJ, Coop A, Singh B, Tao Y, Llombart-Cussac A, Janicke F, Mauriac L, Quebe-Fehling E, Chaudri-Ross HA, Evans DB, Miller WR (2003) Letrozole inhibits tumor proliferation more effectively than tamoxifen independent of HER1/2 expression status. Cancer Res 63:6523–6531 [PubMed]
- 17.Long BJ, Jelovac D, Handratta V, Thiantanawat A, MacPherson N, Ragaz J, Goloubeva OG, Brodie AM (2004) Therapeutic strategies using the aromatase inhibitor letrozole and tamoxifen in a breast cancer model. J Natl Cancer Inst 96:456–465 [DOI] [PubMed]
- 18.Breast International Group (BIG) 1-98 Collaborative Group (2005) A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353:2747–2757. Erratum in: N Engl J Med 2006;354:2200 [DOI] [PubMed]
- 19.Dowsett M, Cuzick J, Wale C, Howell T, Houghton J, Baum M (2005) Retrospective analysis of time to recurrence in the ATAC trial according to hormone receptor status: an hypothesis-generating study. J Clin Oncol 23:7512–7517 [DOI] [PubMed]
- 20.Rhodes A, Jasani B, Balaton AJ, Barnes DM, Anderson E, Bobrow LG, Miller KD (2001) Study of interlaboratory reliability and reproducibility of estrogen and progesterone receptor assays in Europe: documentation of poor reliability and identification of insufficient microwave antigen retrieval time as a major contributory element of unreliable assays. Am J Clin Pathol 115:44–58 [DOI] [PubMed]
- 21.Rasmussen BB, Regan MM, Lykkesfeldt AE, Dell’Orto P, Del Curto B, Henriksen KL, Mastropasqua MG, Thürlimann B, Viale G for the BIG 1-98 Collaborative Group, the IBCSG (2007) Central assessment of ER, PgR and HER2 in BIG 1-98 evaluating letrozole (L) vs. tamoxifen (T) as initial adjuvant endocrine therapy for postmenopausal women with hormone receptor-positive breast cancer. J Clin Oncol 25(18S):12S. Abstract 538
- 22.Mauriac L, Keshaviah A, Debled M, Mouridsen H, Forbes J, Thuerlimann B, Paridaens R, Gelber R, Castiglione-Gertsch M, Goldhirsch A (2007) Predictors of early recurrence in postmenopausal women with hormone receptor positive breast cancer in the BIG 1-98 trial. Ann Oncol 18:859–867 [DOI] [PubMed]
- 23.Coates AS, Keshaviah A, Thürlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Colleoni M, Láng I, Del Mastro L, Smith I, Chirgwin J, Nogaret JM, Pienkowski T, Wardley A, Jakobsen EH, Price KN, Goldhirsch A (2007) Five years of letrozole versus tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: further analyses and update of study BIG 1-98. J Clin Oncol 25:486–492 [DOI] [PubMed]
- 24.Coates AS, Mourisdsen H, Sun Z, Rabaglio M, Castiglione-Gertsch M, Thürlimann B, Mauriac L, Price KN, Colleoni M, Smith I (2007) Cardiovascular adverse events during adjuvant endocrine therapy for early breast cancer using letrozole or tamoxifen: updated safety analysis of trial BIG 1-98. J Clin Oncol 25(18S):8S. Abstract 521 [DOI] [PubMed]
- 25.Chang J, Powles TJ, Ashley SE, Gregory RK, Tidy VA, Treleaven JG, Singh R (1996) The effect of tamoxifen and hormone replacement therapy on serum cholesterol, bone mineral density and coagulation factors in healthy postmenopausal women participating in a randomised, controlled tamoxifen prevention study. Ann Oncol 7:671–675 [DOI] [PubMed]
- 26.Goldhirsch A, Wood W, Gelber R, Coates A, Thürlimann B, Senn HJ, and Panel members (2007) Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 18:1133–1144 [DOI] [PubMed]
- 27.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology – v.1.2007. http://www.nccn.org/ [DOI] [PubMed]
- 28.Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, Sahmoud T; ATAC Trialists’ Group (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359:2131–2139. Erratum in: Lancet 2002;360:1520 [DOI] [PubMed]
- 29.Baum M, Buzdar A, Cuzick J, Forbes J, Houghton J, Howell A, Sahmoud T; The ATAC (Arimidex, Tamoxifen Alone or in Combination) Trialists’ Group (2003) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer 98:1802–1810 [DOI] [PubMed]
- 30.De Boer R, Burris H, Monnier A, Mouridsen H, O’Shaughnessy J, McIntyre K, Pritchard K, Smith I, Yardley D, on behalf of the H2H trial steering committee (2006) The Head to Head trial: letrozole vs anastrozole as adjuvant treatment of postmenopausal patients with node positive breast cancer. J Clin Oncol 24(18S):582s. Abstract 10672
- 31.International Breast Cancer Study Group. http://www.ibcsg.org/index.shtm
- 32.Geisler J, Lønning PE (2006) Aromatase inhibitors as adjuvant treatment of breast cancer. Crit Rev Oncol Hematol 57:53–61 [DOI] [PubMed]
- 33.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Cameron DA, Palmer MJ, Pater JL (2005) Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 97:1262–1271 [DOI] [PubMed]
- 34.Geisler J, Haynes B, Anker G, Dowsett M, Lonning PE (2002) Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol 20:751–757 [DOI] [PubMed]
- 35.Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, Bertelli G, Ortmann O, Coates AS, Bajetta E, Dodwell D, Coleman RE, Fallowfield LJ, Mickiewicz E, Andersen J, Lonning PE, Cocconi G, Stewart A, Stuart N, Snowdon CF, Carpentieri M, Massimini G, Bliss JM, van de Velde C; Intergroup Exemestane Study (2004) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350:1081–1092. Erratum in: N Engl J Med 2004;351:2461 [DOI] [PubMed]
- 36.Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE, Jassem J, Van de Velde CJ, Delozier T, Alvarez I, Del Mastro L, Ortmann O, Diedrich K, Coates AS, Bajetta E, Holmberg SB, Dodwell D, Mickiewicz E, Andersen J, Lønning PE, Cocconi G, Forbes J, Castiglione M, Stuart N, Stewart A, Fallowfield LJ, Bertelli G, Hall E, Bogle RG, Carpentieri M, Colajori E, Subar M, Ireland E, Bliss JM; Intergroup Exemestane Study (2007) Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet 369:559–570. Erratum in: Lancet 2007;369:906 [DOI] [PubMed]
- 37.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Therasse P, Palmer MJ, Pater JL (2003) A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349:1793–1802 [DOI] [PubMed]
- 38.Jakesz R, Gnant M, Greil R, Tausch C, Samonigg H, Kwasny W, Kubista E, Stierer M, Luschin G, Mittlboeck M (2005) The benefits of sequencing adjuvant tamoxifen and anastrozole in postmenopausal women with hormone-responsive early breast cancer: 5 year-analysis of ABCSG Trial 8. Breast Cancer Res Treat 94(suppl 1):S10. Abstract 13
- 39.Phase III randomized study of adjuvant exemestane versus adjuvant tamoxifen in postmenopausal women with early breast cancer. Protocol CRC-TU-TEAM. Available at: http://clinicaltrials.gov/ct/show/NCT00032136. Accessed May 29, 2007