Abstract
Long-term estrogen deprivation treatment for breast cancer can, in some patients, lead to the activation of alternate cellular pathways, resulting in the re-emergence of the disease. This is a distressing scenario for oncologists and patients, but recent intensive molecular and biochemical studies are beginning to unravel these pathways, revealing opportunities for new targeted treatments. Far from making present therapies redundant, these new discoveries open the door to novel combination therapies that promise to provide enhanced efficacy or overcome treatment resistance. Letrozole, one of the most potent aromatase inhibitors, is the ideal candidate for combination therapy; indeed, it is one of the most intensively studied aromatase inhibitors in the evolving combinatorial setting. Complementary to the use of combination therapy is the development of molecular tools to identify patients who will benefit the most from these new treatments. Microarray gene profiling studies, designed to detect letrozole-responsive targets, are currently under way to understand how the use of the drug can be tailored more efficiently to specific patient needs.
Keywords: Adjuvant therapy, Aromatase inhibitors, Breast cancer, Combination therapy, Letrozole, Mechanism of resistance, Postmenopausal
Introduction
The proliferative, invasive, and metastatic potential of breast tumors may be largely predetermined at an early stage in the course of disease, whereas genetic alterations that accumulate during progression from in situ to metastatic disease are unpredictable and result in specific phenotypic changes and loss of sensitivity to treatments [1]; for example, although the estrogen receptor-positive (ER+) phenotype may be largely stable over time [2], hormone therapy-sensitive breast tumors may develop resistance and progress to a hormone-independent state [3]. In addition, progression to hormone independence may be associated with alterations in the expression of other regulatory genes, such as human epidermal growth factor receptor 2 (HER2) [4]. In the clinical setting, a quantitative decrease in ER expression was found in over 900 patients with primary breast cancer when HER2 was amplified [5].
Recent advances in translational research studies have highlighted the complexity of ER signaling, including differential roles for the ERα and ERβ subtypes [6], and multiple regulatory interactions between steroid hormone, growth factor, and other tyrosine kinase signaling pathways [7–10]. Greater understanding of tumor biology is beginning to help physicians to individualize treatment selection based on clinical, pathologic, molecular, and genetic profiling, and to rationally design novel combinations to improve efficacy and safety.
This article reviews novel approaches with the more potent third-generation aromatase inhibitor (AI) letrozole (Femara®; Novartis Pharmaceuticals) in combination strategies with agents targeting other growth factor pathways. The review explores the hypothesis that combining letrozole with specifically targeted therapies may delay or overcome endocrine therapy resistance in postmenopausal women with hormone receptor-positive (HR+) breast cancer.
Endocrine therapy resistance
It is well established that breast cancer cells can adapt to low concentrations of estrogen by becoming hypersensitive to estradiol [11]. Long-term exposure to tamoxifen induces hypersensitivity to estradiol [12] and this adaptive change can result in resistance to endocrine therapy [13]. It has been postulated that tamoxifen is more susceptible than AIs to this phenomenon because of its intrinsic agonist properties [12]. Moreover, it has been suggested that highly potent AIs are required to block estrogen synthesis when breast cancer tumors are hypersensitive to small amounts of estradiol [13].
Recent research has provided a compelling explanation for the development of resistance [13, 14]. Experiments using MCF-7 and other breast cancer model systems have identified alternative intracellular signaling pathways used by breast tumors to enhance and activate ER signaling, thus allowing cells to escape from the inhibitory effects of endocrine therapies [15, 16]. It has been shown that long-term estrogen deprivation upregulates ERα and growth factor signaling pathways such as mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K), and the mammalian target of rapamycin (mTOR) pathways [14–19]. Of note, Jeng et al. [15] reported that a specific inhibitor of MAPK (PD98059) could block the elevation of activated MAPK observed in MCF-7 cells exposed to long-term estrogen deprivation. Furthermore, studies in both wild-type and long-term estrogen-deprived MCF-7 cells suggested that mTOR has a key role in breast cancer cell proliferation and showed that mTOR inhibition by farnesylthiosalicylic acid (FTS) can reduce proliferation and induce apoptosis [19].
Growth factor pathways
The role of nongenomic pathways has been highlighted in resistance to antiestrogen therapy [20]. Classically, estrogens bind to nuclear ER to enhance transcription of genes important in breast cancer proliferation and survival (genomic pathway) [21]; however, estrogen may also act through ER located in or near the cell membrane [22]. Nongenomic actions include activation of various growth signaling pathways, including MAPK [15]. In addition, ER may indirectly activate epidermal growth factor receptors (EGFR) via coactivators, including src, leading to activation of EGFR [23–25]. Subsequently, dimerization of activated EGFR with other HER family receptors, particularly HER2, activates intracellular signaling pathways, which in turn may enhance nuclear ER signaling [4], thus completing a vicious cycle of events. Cross-talk between ER and HER2 pathways has been implicated in clinical resistance to tamoxifen [4, 7]. Shou et al. [4] reported that tamoxifen behaves as an agonist in MCF-7 breast cancer cells that express high levels of the coactivator AIB1 (src3) and HER2, resulting in de novo resistance. Interestingly, addition of an anti-EGFR tyrsosine kinase inhibitor eliminated cross-talk and restored tamoxifen’s antitumor activity [4].
It has been postulated that AIs may be more effective than selective estrogen-receptor modulators (SERMs) [26] because they can block genomic and nongenomic activities of ER [27]. Elucidation of ER biology and interactions with growth factor signaling pathways will help to identify potential therapeutic targets for HR+ breast cancer [4, 28].
Combination therapy
Several strategies to inhibit growth factor signaling and signal transduction in breast cancer have been tested in the preclinical setting (see Fig. 1). The humanized monoclonal antibody trastuzumab specifically targets the extracellular domain of HER2 [29, 30]. Amplification of HER2 occurs in approximately 25% of breast tumors and is associated with more aggressive disease and a poor prognosis [31]. Trastuzumab has been shown to restore sensitivity to estrogen and tamoxifen in estrogen-independent HER2-transduced MCF-7 cells [32]. Furthermore, trastuzumab blocked HER2 heterodimer formation and phosphorylation, reduced ERK1/2 activity, and strongly inhibited cell growth in MCF-7 cells overexpressing EGFR and HER2 and resistant to tamoxifen [18]. Of note, synergistic inhibition of the BT474 breast cancer cell line was observed with trastuzumab and the HER dimerization inhibitor pertuzumab [33], which targets a different domain of HER2 [34]. The combination of tamoxifen and trastuzumab in ER+, HER2+ BT-474 cells resulted in synergistic growth inhibition due to the enhancement of cell accumulation in the G0–G1 phase of the cell cycle, and a decreased number of cells in S phase [35].
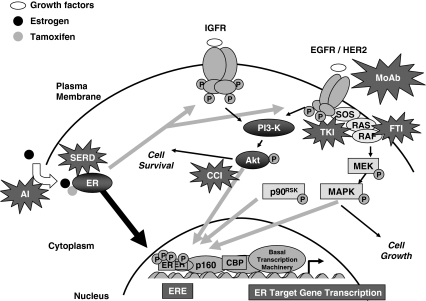
Fig. 1.
Cross-talk between signal transduction pathways and ER signaling in endocrine resistant breast cancer, with opportunities for targeted intervention. Estrogen (E2)-liganded ER activates E2-regulated genes in classical pathway (thick black arrow), but following long-term tamoxifen therapy resistance can develop with bidirectional cross-talk (gray arrows) between ER and growth factor receptors, with association of membrane bound ER with growth factor receptors, and/or IGFR or EGFR/HER2 activation of ER phosphorylation. Stars show various targeted therapies. AI aromatase inhibitor, SERD selective estrogen receptor down-regulator, MoAb monoclonal antibodies, TKI tyrosine kinase inhibitor, FTI farnesyltransferase inhibitor, CCI cell cycle inhibitor. Reprinted from [28] with permission from the American Association for Cancer Research
HER signaling pathways can also be targeted by inhibiting receptor phosphorylation by intracellular tyrosine kinases [36]. Gefitinib and erlotinib both specifically inhibit the EGFR tyrosine kinase and have demonstrated inhibitory activity in models of hormone-resistant breast cancer [37, 38]. Interestingly, pretreatment of HER2+, hormone-resistant MCF-7 cells with gefitinib eliminated the agonist effects of tamoxifen and restored its antitumor activity [4]. In ER+ breast tumors, targeted therapy with single-agent gefitinib was found to be less effective than endocrine therapy [39]; however, tamoxifen plus gefitinib had greater activity than tamoxifen alone in vivo in hormone-sensitive cells [39]. Data also support the use of EGFR tyrosine kinase inhibitors in combination with HER2 antibodies, such as trastuzumab, against breast tumors that express EGFR and high levels of HER2 [37]. Alternatively, the tyrosine kinase inhibitor lapatinib provides single-agent targeting of both EGFR and HER2 [40, 41]. Using a panel of 31 human breast tumors, Konecny et al. [41] showed that response to lapatinib was significantly correlated with HER2 expression and its ability to inhibit HER2, Raf, Akt, and ERK phosphorylation. In vivo studies showed that lapatinib had a sustained antitumor effect that was further prolonged by combination with trastuzumab [41]. Another study showed that the combination of AEE788 (an EGFR/HER2 tyrosine kinase inhibitor) with letrozole enhanced antiproliferative effects of these agents by 20–30% in MCF-7 and ZR75.1 cell lines and by 60–70% in the BT474 cell line [42]. In a model system of acquired resistance to letrozole, AEE788 partially restored sensitivity to letrozole, whereas rapamycin was not effective, suggesting that letrozole resistance and mTOR activation may not be connected in this model [43]. The authors concluded that inhibition of both HER2-mediated signaling and mTOR-dependent translation may restore responsiveness to letrozole in breast cancer refractory to this AI [43].
Strategies to inhibit downstream signal pathways have also been developed. Farnesyl transferase inhibitors block the first and most important step in the activation of Ras signaling pathways [44]. Aberrant function of the Ras signal transduction pathway is common in breast cancer as a result of upstream activation via HER2 or EGFR [45]. The farnesyl transferase inhibitor R11577 (tipifarnib) was found to have antitumor activity against MCF-7 xenografts [44]. Another interesting therapeutic target is mTOR, a central regulator of G1 cell cycle protein synthesis, that precedes commitment to normal cellular replication [46]. Treatment of MCF-7 Arom-1 cells with letrozole and the mTOR inhibitor RAD001 resulted in a further 50% reduction in proliferation compared with letrozole alone [47]. Another set of experiments, developed to test the hypothesis that Akt kinase confers resistance to endocrine therapy through suppression of ASK1/JNK pathway, showed that combining RAD001 with letrozole restored activation of the ASK/JNK pathway and increased the sensitivity of MCF-7 cells with constitutively active Akt to endocrine therapy [48]. Studies have also targeted the selective estrogen receptor down-regulator (SERD) fulvestrant, which indirectly inhibits growth factor pathways by down-regulating ER [3]. In MCF-7Ca xenografts, combined treatment with fulvestrant and letrozole prevented increases in HER2 and activation of MAPK and inhibited tumor growth [49].
These preclinical models (see Table 1) suggest that treatments to reduce growth factor signaling pathways may be useful in the treatment of human breast cancer [52]. Specifically, novel combination strategies may be developed to prevent or delay the development of endocrine therapy resistance [3, 39], to restore sensitivity to endocrine therapy [53], and to treat hormone-resistant tumors [18, 46]. Anti-vascular endothelial growth factor (VEGF) therapy with bevacizumab may be able to overcome resistance to endocrine therapy and improve efficacy in HR+ metastatic breast cancer [54], and preclinical models have shown that the estrogen-induced increase in VEGF expression may be counteracted by aromatase inhibition. Inhibition of growth factor signaling and angiogenesis pathways may be rationally combined with conventional endocrine strategies for breast cancer [4, 35, 50, 55, 56].
Table 1.
Summary of letrozole in combination with growth factor signaling inhibitors in preclinical models
| Target for growth factor inhibitor | Combination regimen | Summary of key findings | References |
|---|---|---|---|
| EGFR/HER2 | Letrozole + AEE78 | Combination enhanced antiproliferative effects in MCF-7 (ER+ HER2−), ZR75.1 (ER+ HER2+), and BT474 (ER+ HER2+) cell lines | [42] |
| Partial restoration of growth inhibitory effects of letrozole in refractory cell lines (LTLT-Ca; long-term letrozole treated) | [43] | ||
| mTOR | Letrozole + RAD001 (everolimus) | Letrozole + RAD001 significantly increased apoptosis compared with either agent alone | [50] |
| Co-treatment increased sensitivity to letrozole in resistant MCF-7 cells with constitutively active Akt | [48] | ||
| RAD001 increased antiproliferative effects of letrozole in MCF-7 Arom 1 cell line | [47] | ||
| IGFBP | Letrozole + rhIGFBP-3 | rhIGFBP-3 Enhanced letrozole activity in MCF-7-Ca cells in vitro and in vivo | [51] |
rhIGFBP recombinant human insulin-like growth factor binding protein
The activity of inhibitors of growth factor signaling depends on the presence of specific cellular aberrations, such as overexpression of HER2 [57] or mutations of EGFR [58]. Consequently, targeted therapies may have limitations as single agents because the target is active in a restricted subset of patients, and breast tumors may undergo adaptive changes to render the target redundant. Since tamoxifen exhibits agonist effects on breast cancer cells exposed to long-term estrogen deprivation [12], it may be better to combine AIs with inhibitors of growth factor signaling. Letrozole is one of the most potent AIs and is one of the most extensively studied AIs in combination with new agents (see Table 1).
Clinical trials of letrozole in combination with inhibitors of growth factor signaling pathways
Based on results from preclinical studies, several clinical trials of novel combinations are under way, with the aim of improving efficacy and safety of endocrine therapy with letrozole (see Table 2). Many of these trials are being conducted in patients with locally advanced or metastatic breast cancer who have failed prior tamoxifen or have a suboptimal response to letrozole. This represents a high-risk, difficult-to-treat population who are candidates for cytotoxic chemotherapy. Preliminary results have shown that letrozole can be safely combined with trastuzumab, lapatinib, everolimus, tipifarnib, bevacizumab, and imatinib. It is too early, however, to make definitive conclusions about efficacy and clinical benefits with these novel combinations.
Table 2.
Clinical studies of letrozole and inhibitors of growth factor signaling pathways
| Target for growth factor inhibitor | Combination regimen | Study type and patient population | Summary of key findings | References |
|---|---|---|---|---|
| HER2 | Letrozole + trastuzumab | Phase II Metastatic BC, postmenopausal, ER+ and/or PR+, HER2+ (n = 31) |
ORR 26%; median TTP 5.8 months | [59] |
| EGFR/HER2 | Letrozole + lapatinib | Phase I Advanced BC (ER+ or PR+) or other tumors (n = 36) |
Letrozole + lapatinib safely combined at recommended single agent doses | [60] |
| Phase III Advanced/metastatic BC (n = 1,200 target accrual) |
Ongoing trial; primary end point TTP | [61] | ||
| mTOR | Letrozole + RAD-001 (everolimus) | Phase Ib Advanced BC pts with suboptimal response to letrozole (n = 6) |
RAD001 pharmacokinetics not altered by letrozole | [62] |
| Phase II Presurgical therapy in patients with newly diagnosed ER+ BC (n = 255 planned) |
Ongoing trial of efficacy and biomarkers | [63] | ||
| Letrozole + CCI-779 (temsirolimus) | Phase II Advanced or metastatic BC (n = 92) |
No difference in ORR, but trend to longer PFS with letrozole + temsirolimus (30 mg) | [64] | |
| Phase III Advanced or metastatic breast cancer (n = 1,236 planned) |
Terminated | [65] | ||
| Farnesyl transferase | Letrozole + tipifarnib | Randomized phase II Advanced or metastatic BC that has progressed on tamoxifen (n = 121) |
ORR 38% for letrozole and 26% for letrozole + tipifarnib (NS) | [66] |
| Randomized, placebo-controlled phase II Advanced or metastatic BC that has progressed on antiestrogen therapy (n = 120) |
No longer recruiting | [67] | ||
| VEGF | Letrozole + bevacizumab (anti-VEGF monoclonal antibody) | Phase II Metastatic BC, postmenopausal, candidates for AI (n = 28) |
Letrozole + bevacizumab is well-tolerated | [54] |
| Endocrine therapy (tamoxifen or aromatase inhibitor) + bevacizumab | Phase III placebo-controlled First-line therapy in ER+/PR+ Metastatic BC (n = TBC) |
Planned trial | Planned CALGB trial [54] | |
| Bcr-abl | Letrozole + imatinib | Phase II Metastatic BC, postmenopausal ER+ and/or PR+ (n = 15) |
Letrozole + imatinib is feasible | [68] |
BC breast cancer, ORR overall response rate, TTP time to progression, PFS progression-free survival, AI aromatase inhibitor, TBC to be confirmed
In a clinical trial [69] designed to test whether combination therapy with letrozole and bevacizumab was possible, patients with ER+ or progesterone receptor-positive (PR+) metastatic or locally advanced breast cancer were treated with letrozole (2.5 mg daily) and bevacizumab (15 mg/kg IV every 3 weeks) [54]. The majority of patients had received prior therapy with a nonsteroidal AI. The combination of bevacizumab and letrozole was found to be well-tolerated. Common drug-related toxicities reported were hypertension, fatigue, headache, and joint pain. Median progression-free survival was reported to be 10 months, and this compares favorably with the published data on median time to progression with first-line letrozole (9.4 months) [70]. However, analysis of efficacy and biomarker data was confounded by the long duration of prestudy aromatase inhibition [54, 71]. Nevertheless, when the data were corrected for duration of previous AI therapy, the study did determine that changes in circulating endothelial cell (CEC) levels may be a biomarker of response or progression on anti-angiogenic therapy [71]. Based on these findings, a randomized, double-blind, placebo-controlled trial of bevacizumab combined with endocrine therapy in patients with ER+ or PR+ metastatic breast cancer has been initiated by the Cancer and Leukemia Group B (CALGB) (see Fig. 2) [54]. The primary end point of the trial is progression-free survival.
Fig. 2.
Planned CALGB trial of first-line endocrine therapy (tamoxifen or aromatase inhibitor) with or without bevacizumab
Letrozole plus trastuzumab produced durable responses in about one in four patients with ER/PR+, HER2+ metastatic breast cancer, but early progression occurred in one in two patients [59]. This suggests that common resistance pathways may be responsible for relapse [59]. Targeting multiple pathways may reduce the risk of resistance. The combination of letrozole and the dual EGFR/HER2 inhibitor lapatinib was found to be feasible and well-tolerated in a phase I study [60], and this regimen is currently being compared with letrozole plus placebo in a phase III study in women with ER/PR+ advanced or metastatic breast cancer [61] (see Fig. 3). Future studies will focus on finding the right combination or sequence of agents for different patients in specific treatment settings. Furthermore, studies in the neoadjuvant setting in patients with locally advanced breast cancer will allow correlative biomarker assessment, such as the proliferation marker Ki-67, to determine the efficacy of combination therapies. For example, everolimus and letrozole are being studied as preoperative therapy of primary breast cancer in postmenopausal women [63]. In this phase II trial, patients are randomized to receive letrozole in combination with everolimus or placebo, an adaptive design strategy, so that identification of biomarkers can be used to optimize patient selection for a future phase III trial of first-line combination therapy in patients with advanced breast cancer. Even if a biomarker is not identified, the trial is adequately powered to demonstrate a statistically significant difference in treatment effect of the combination in the overall population.
Fig. 3.
Letrozole and lapatinib phase III trial design. Target recruitment: 1,280 patients
Combination strategies may also change the conventional approach for treatment selection based on HR status. Interestingly, it has been suggested that treatment of HER2+, ER− metastatic breast cancer with trastuzumab may transform the tumor phenotype to become hormone responsive [72]. The hypothesis-generating study found that three of ten patients demonstrated ER overexpression at 9, 12, and 37 weeks, respectively, following the initiation of trastuzumab. Two of these patients were subsequently treated with endocrine therapy alone and one received letrozole for 3 years without evidence of progression. Further trials of letrozole used in combination with trastuzumab or sequentially in patients with HER2+, ER− tumors appear warranted.
Microarray/gene profiling studies and optimization of treatment with letrozole
The growing importance of biomarkers in oncology has been reflected in the US Food and Drug Administration (FDA), the National Cancer Institute (NCI), and the Centers for Medicare and Medicaid Services (CMS) Oncology Biomarker Qualification Initiative (OBQI), designed to improve the development of cancer therapies and the outcomes for cancer patients through biomarker development and evaluation [73]. Not only are biomarkers potentially useful as prognostic and predictive factors but they also serve as surrogate end points for long-term outcomes. For example, Dowsett et al. found that changes in Ki-67 in the neoadjuvant setting may be used to predict likely benefit (improved disease-free and overall survival) of AIs in the adjuvant setting [74], potentially expediting clinical development. Therefore, identifying biomarkers that predict endocrine resistance is essential for the optimal use of letrozole. In addition, biomarker studies that help to delineate the oncogenic pathways may be particularly useful for the development of novel combination therapies with letrozole.
Gene expression analysis of tumors represents a novel approach for biomarker development that promises to increase the understanding of breast cancer heterogeneity and facilitate the development of individualized treatment strategies. Microarray analysis, such as the Affymetrix Human Genome Gene Chips, is an exciting development in breast cancer diagnostics that allows the expression of genes in tumors to be quantified using RNA retrieved from breast cancer biopsies. Using cDNA microarray and unsupervised clustering analysis, ER+ breast cancer can be subdivided into at least two subtypes, luminal A and luminal B, with distinct gene expression patterns and clinical outcomes [75–77]. Supervised analysis, comparing gene expression patterns between tumors that relapsed and those that did not, generated a “gene signature” as an independent prognostic parameter for lymph-node negative disease [78, 79]. The National Surgical Adjuvant Breast and Bowel Project (NSABP) has developed a multigene profiling assay, using reverse transcriptase polymerase chain reaction (RT-PCR) to quantify the expression of 21 genes [80]. The 21-gene assay (Oncotype DX, Genomic Health) can predict risk of recurrence in patients with HR+ early breast cancer receiving tamoxifen [80, 81] or chemotherapy [82]. Naderi et al. [83] have also reported validation of gene expression signatures that may have predictive value in the clinic. Furthermore, newly identified interactions between ER and other signaling pathways have been studied using microarray analysis [84, 85]. For example, Bex1 and Bex2 genes have been identified as novel breast cancer-related genes and identify a subtype of ER+ tumors associated with estrogen response and nuclear factor kappa B (NF-κB) pathways [84]. Advances in gene profiling will provide further insights into tumor biology and improve prediction of likely response to specific therapies in the clinic.
Microarray analysis has shown to be valuable in predicting response to neoadjuvant endocrine therapy and was used in a study of neoadjuvant letrozole 2.5 mg/day administered to postmenopausal women with large operable or locally advanced breast cancers for 3 months [86, 87]. Changes in patterns of gene expression were assessed from tissue samples taken at diagnosis, 14 days, and 3 months (N = 58). Changes in gene expression level with treatment were identified as early as 14 days and involved classical markers of estrogen action (trefoil factors 1 and 3, LIV-1, KIAA0101) as well as tumor proliferation (cyclin D1, cyclin B2, CSK2, cell division cycle 2). The objective clinical response to neoadjuvant letrozole was 71% (N = 52 assessable). Of note, the observed changes in gene expression, when clustered, were predictive of response in all cases except one, whereas classical markers of estrogen action were not predictive [87]. In another study, postmenopausal women with primary operable breast cancer were randomized to 2 weeks of presurgical treatment with letrozole 2.5 mg/day or anastrozole 1 mg/day [88]. Microarray gene expression profiling (Breakthrough Centre cDNA chips) of biopsies taken before and during treatment identified differences in gene expression patterns between the two AIs, although the clinical significance of these preliminary findings remains to be clarified. Itoh et al. [89] also found differences between letrozole, anastrozole, and tamoxifen using microarray analysis on MCF-7 cells stably transfected with the aromatase gene (MCF-7Aro). Gene expression patterns revealed a high correlation between the AIs (letrozole and anastrozole) and a clear difference between AIs and tamoxifen [89].
Emerging data therefore suggest that estrogen-responsive genes are candidate biomarkers [90] and may be useful in the clinic as predictive factors of benefit from AI treatment [89]. The value of gene expression profiling is now being evaluated in prospective studies. MINDACT (Microarray In Node negative Disease may Avoid ChemoTherapy), an ongoing three-part randomized trial of 6,000 patients with node-negative breast cancer, is comparing the efficacy of selection of breast cancer patients for adjuvant chemotherapy based on either clinical criteria or the 70-gene microarray prognosis profile [91]. In one part of the trial, 3,500 node-negative, HR+ positive patients will be randomized to receive either 7 years of letrozole or 2 years of tamoxifen followed by 5 years of letrozole. In the US, the TAILORx Trial [92] is comparing hormone therapy with or without combination chemotherapy as adjuvant therapy for node-negative, ER+ breast cancer. The objective of this randomized phase III trial is to determine the best individual therapy using Oncotype DX gene profiling.
One of the more recent advances in transcriptional profiling addresses its potential application as an identification tool for “oncogenic pathway signatures” that could be used to guide targeted therapy. Oncogenic pathway signatures, developed in cell-line models, have been shown to predict sensitivity to therapeutic agents in vitro [93]. This offers an opportunity to identify pathway-specific drugs in endocrine therapy-resistant tumors and creates an opportunity for the rational design of combination therapy with letrozole. In addition to the RNA-based gene expression profiling approach, high throughput analysis at the DNA level, such as genome-wide microarray comparative genomic hybridization (aCGH) and DNA sequencing, has made it possible to decipher the genetic anomalies that drive a particular tumor phenotype. The use of these molecular approaches for biomarker development is still in its infancy but is now feasible with recent advances in genomic technologies.
Conclusions
Hormone-sensitive breast cancer can be regarded as a chronic disease with a persistent risk of escape from effective endocrine control. Activation of growth factor signaling pathways has been implicated in progression of HR+ breast cancer to an estrogen-independent phenotype and the development of resistance to endocrine therapy, particularly tamoxifen. It has been hypothesized that combining endocrine therapy with targeted signal transduction inhibitors may circumvent hormone-independent signaling pathways, so that patients may experience prolonged disease control.
Letrozole is one of the most potent AIs. As such, it may be more effective than tamoxifen for patients with tumor profiles associated with a high risk of developing hormone resistance (e.g., tumors with HER2 gene amplification) and represents an ideal combination partner for agents that inhibit growth signaling pathways implicated in hormone resistance. The efficacy of letrozole is currently being investigated with a variety of signal transduction inhibitors with different mechanisms of action, including monoclonal antibodies against HER family receptors, receptor tyrosine kinase inhibitors, and downstream signaling pathway inhibitors.
Gene expression profiling has been validated as a useful new tool to predict risk of relapse in patients treated with hormone therapy and chemotherapy. This approach will help physicians to identify which patient will likely benefit from specific therapies, such as letrozole. Tailoring therapy to individual patient profiles (clinical, histologic, pathologic, and genetic) will become more sophisticated in the future, helping to maximize the benefits of endocrine therapy throughout the breast cancer continuum; letrozole will undoubtedly become an integral part of the next generation of tailored combination regimens for the treatment of breast cancer.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s10549-007-9856-5
References
- 1.Lacroix M, Toillon RA, Leclercq G (2004) Stable ‘portrait’ of breast tumors during progression: data from biology, pathology and genetics. Endocr Relat Cancer 11:497–522 [DOI] [PubMed]
- 2.Robertson JF (1996) Oestrogen receptor: a stable phenotype in breast cancer. Br J Cancer 73:5–12 [DOI] [PMC free article] [PubMed]
- 3.Brodie A, Jelovac D, Sabnis G, Long B, Macedo L, Goloubeva O (2005) Model systems: mechanisms involved in the loss of sensitivity to letrozole. J Steroid Biochem Mol Biol 95:41–48 [DOI] [PubMed]
- 4.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R (2004) Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst 96:926–935 [DOI] [PubMed]
- 5.Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, Wilson C, Rong HM, Bauerfeind I, Felber M, Wang HJ, Beryt M, Seshadri R, Hepp H, Slamon DJ (2003) Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst 95:142–153 [DOI] [PubMed]
- 6.Shaw LE, Sadler AJ, Pugazhendhi D, Darbre PD (2006) Changes in oestrogen receptor-alpha and -beta during progression to acquired resistance to tamoxifen and fulvestrant (Faslodex, ICI 182,780) in MCF7 human breast cancer cells. J Steroid Biochem Mol Biol 99:19–32 [DOI] [PubMed]
- 7.Osborne CK, Bardou V, Hopp TA, Chamness G, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R (2003) Role of estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 95:353–361 [DOI] [PubMed]
- 8.Kirkegaard T, Witton CJ, McGlynn LM, Tovey SM, Dunne B, Lyon A, Bartlett JM (2005) AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J Pathol 207:139–146 [DOI] [PubMed]
- 9.Shin I, Miller T, Arteaga CL (2006) ErbB receptor signaling and therapeutic resistance to aromatase inhibitors. Clin Cancer Res 12:1008s–1012s [DOI] [PubMed]
- 10.Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, Elledge RM (2005) Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst 97:1254–1261 [DOI] [PubMed]
- 11.Masamura S, Santner SJ, Heitjan DF, Santen RJ (1995) Estrogen deprivation causes estradiol hypersensitivity in human breast cancer cells. J Clin Endocrinol Metab 80:2918–2925 [DOI] [PubMed]
- 12.Berstein LM, Wang JP, Zheng H, Yue W, Conaway M, Santen RJ (2004) Long-term exposure to tamoxifen induces hypersensitivity to estradiol. Clin Cancer Res 10:1530–1534 [DOI] [PubMed]
- 13.Santen RJ, Song RX, Zhang Z, Kumar R, Jeng MH, Masamura S, Lawrence J Jr, MacMahon LP, Yue W, Berstein L (2005) Adaptive hypersensitivity to estrogen: mechanisms and clinical relevance to aromatase inhibitor therapy in breast cancer treatment. J Steroid Biochem Mol Biol 95:155–165 [DOI] [PubMed]
- 14.Santen RJ, Song RX, Zhang Z, Kumar R, Jeng MH, Masamura A, Lawrence J Jr, Berstein L, Yue W (2005) Long-term estradiol deprivation in breast cancer cells up-regulates growth factor signaling and enhances estrogen sensitivity. Endocr Relat Cancer 12(Suppl 1):S61–S73 [DOI] [PubMed]
- 15.Jeng MH, Yue W, Eischeid A, Wang JP, Santen RJ (2000) Role of MAP kinase in the enhanced cell proliferation of long term estrogen deprived human breast cancer cells. Breast Cancer Res Treat 62:167–175 [DOI] [PubMed]
- 16.Yue W, Wang JP, Conaway M, Masamura S, Li Y, Santen RJ (2002) Activation of the MAPK pathway enhances sensitivity of MCF-7 breast cancer cells to the mitogenic effect of estradiol. Endocrinology 143:3221–3229 [DOI] [PubMed]
- 17.Martin LA, Farmer I, Johnston SR, Ali S, Marshall C, Dowsett M (2003) Enhanced estrogen receptor (ER) alpha, ERBB2, and MAPK signal transduction pathways operate during the adaptation of MCF-7 cells to long term estrogen deprivation. J Biol Chem 278:30458–30468 [DOI] [PubMed]
- 18.Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, Barrow D, Wakeling AE, Nicholson RI (2003) Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology 144:1032–1044 [DOI] [PubMed]
- 19.Yue W, Wang J, Li Y, Fan P, Santen RJ (2005) Farnesylthiosalicylic acid blocks mammalian target of rapamycin signaling in breast cancer cells. Int J Cancer 117:746–754 [DOI] [PubMed]
- 20.Song RX, McPherson RA, Adam L, Bao Y, Shupnik M, Kumar R, Santen RJ (2002) Linkage of rapid estrogen action to MAPK activation by ERalpha-Shc association and Shc pathway activation. Mol Endocrinol 16:116–127 [DOI] [PubMed]
- 21.Clark JH, Peck EJ (1976) Nuclear retention of receptor–oestrogen complex and nuclear acceptor sites. Nature 260:635–637 [DOI] [PubMed]
- 22.Li L, Haynes MP, Bender JR (2003) Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci USA 100:4807–4812 [DOI] [PMC free article] [PubMed]
- 23.Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ (2002) Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci USA 99:14783–14788 [DOI] [PMC free article] [PubMed] [Retracted]
- 24.Filardo EJ, Quinn JA, Bland KI, Frackelton AR Jr (2000) Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via transactivation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14:1649–1660 [DOI] [PubMed]
- 25.Filardo EJ, Quinn JA, Frackelton AR Jr, Bland KI (2002) Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol 16:70–84 [DOI] [PubMed]
- 26.Howell A, Dowsett M (2004) Endocrinology, hormone therapy in breast cancer: aromatase inhibitors versus antioestrogens. Breast Cancer Res 6:269–274 [DOI] [PMC free article] [PubMed]
- 27.Osborne CK, Schiff R (2005) Aromatase inhibitors: future directions. J Steroid Biochem Mol Biol 95:183–187 [DOI] [PubMed]
- 28.Johnston SR (2005) Combinations of endocrine and biological agents: present status of therapeutic and presurgical investigations. Clin Cancer Res 11:889s–899s [PubMed]
- 29.Fendly BM, Winget M, Hudziak RM, Lipari MT, Napier MA, Ullrich A (1990) Characterization of murine monoclonal antibodies reactive to either the human epidermal growth factor receptor or HER2/neu gene product. Cancer Res 50:1550–1558 [PubMed]
- 30.Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, Rowland AM, Kotts C, Carver ME, Shepard HM (1992) Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA 89:4285–4289 [DOI] [PMC free article] [PubMed]
- 31.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A et al (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244:707–712 [DOI] [PubMed]
- 32.Witters L, Engle L, Lipton A (2002) Restoration of estrogen responsiveness by blocking the HER-2/neu pathway. Oncol Rep 9:1163–1166 [PubMed]
- 33.Nahta R, Hung MC, Esteva FJ (2004) The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res 64:2343–2346 [DOI] [PubMed]
- 34.Adams CW, Allison DE, Flagella K, Presta L, Clarke J, Dybdal N, McKeever K, Sliwkowski MX (2006) Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol Immunother 55:717–727 [DOI] [PMC free article] [PubMed]
- 35.Argiris A, Wang CX, Whalen SG, DiGiovanna MP (2004) Synergistic interactions between tamoxifen and trastuzumab (Herceptin). Clin Cancer Res 10:1409–1420 [DOI] [PubMed]
- 36.King CR, Borrello I, Bellot F, Comoglio P, Schlessinger J (1988) Egf binding to its receptor triggers a rapid tyrosine phosphorylation of the erbB-2 protein in the mammary tumor cell line SK-BR-3. EMBO J 7:1647–1651 [DOI] [PMC free article] [PubMed]
- 37.Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL (2001) Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res 61:8887–8895 [PubMed]
- 38.Sabnis GJ, Jelovac D, Long B, Brodie A (2005) The role of growth factor receptor pathways in human breast cancer cells adapted to long-term estrogen deprivation. Cancer Res 65:3903–3910 [DOI] [PubMed]
- 39.Gee JM, Harper ME, Hutcheson IR, Madden TA, Barrow D, Knowlden JM, McClelland RA, Jordan N, Wakeling AE, Nicholson RI (2003) The antiepidermal growth factor receptor agent gefitinib (ZD1839/Iressa) improves antihormone response and prevents development of resistance in breast cancer in vitro. Endocrinology 144:5105–5117 [DOI] [PubMed]
- 40.Xia W, Mullin RJ, Keith BR, Liu LH, Ma H, Rusnak DW, Owens G, Alligood KJ, Spector NL (2002) Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 21:6255–6263 [DOI] [PubMed]
- 41.Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, Untch M, Rusnak DW, Spehar G, Mullin RJ, Keith BR, Gilmer TM, Berger M, Podratz KC, Slamon DJ (2006) Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res 66:1630–1639 [DOI] [PubMed]
- 42.Hauge-Evans AC, Evans DB, Dowsett M, Martin L-A (2004) Combining the RTK inhibitor AEE788 with tamoxifen or letrozole results in enhanced growth inhibition of hormone-dependent human breast cancer cells. Presented at the 27th Annual San Antonio Breast Cancer Symposium, 8–11 December 2004. Abstract 308
- 43.Sabnis GJ, Jelovac D, Long BJ, Schayowitz A, Belosay A, Brodie AMH (2006) Mammalian target of rapamycin (mTOR) as a target in human breast cancer cells that have acquired resistance to aromatase inhibitor letrozole. Poster presented at the proceedings of the 97th American Association for Cancer Research Annual Meeting, Washington, DC, 1–5 April 2006. Abstract 2316
- 44.Kelland LR, Smith V, Valenti M, Patterson L, Clarke PA, Detre S, End D, Howes AJ, Dowsett M, Workman P, Johnston SR (2001) Preclinical antitumor activity and pharmacodynamic studies with the farnesyl protein transferase inhibitor R115777 in human breast cancer. Clin Cancer Res 7:3544–3550 [PubMed]
- 45.Clark GJ, Der CJ (1995) Aberrant function of the Ras signal transduction pathway in human breast cancer. Breast Cancer Res Treat 35:133–144 [DOI] [PubMed]
- 46.Yu K, Toral-Barza L, Discafani C, Zhang WG, Skotnicki J, Frost P, Gibbons JJ (2001) mTOR, a novel target in breast cancer: the effect of CCI-779, an mTOR inhibitor, in preclinical models of breast cancer. Endocr Relat Cancer 8:249–258 [DOI] [PubMed]
- 47.Farmer I, Evans DB, Lane HA, Lykkesfeldt AE, Dowsett M, Martin L (2006) Preclinical studies of the combination of RAD001 with tamoxifen or letrozole in breast cancer. Eur J Cancer 4(2 Suppl):142. Abstract 324
- 48.Silva JM, Lin Y, Nielsen A, Friedrichs WE, Beeram M, Tekmal R et al (2006) RAD001 relieves Akt-induced suppression of the ASK/JNK pathway in resistant breast cancer cells. In: Abstracts of the 97th American Association for Cancer Research Annual Meeting, Washington, DC, 1–5 April 2006. Abstract 5068
- 49.Jelovac D, Sabnis G, Long BJ, Macedo L, Goloubeva OG, Brodie AM (2005) Activation of mitogen-activated protein kinase in xenografts and cells during prolonged treatment with aromatase inhibitor letrozole. Cancer Res 65:5380–5389 [DOI] [PubMed]
- 50.Boulay A, Rudloff J, Ye J, Zumstein-Mecker S, O’Reilly T, Evans DB, Chen S, Lane HA (2005) Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res 11:5319–5328 [DOI] [PubMed]
- 51.Alami N, Banerjee K, Page V, Brossard M, Shiry L, Brodie A, Leyland-Jones B (2005) Synergistic interaction between recombinant human insulin-like growth factor-binding protein 3 (rhIGFBP-3) and letrozole in estrogen positive breast cancer. Breast Cancer Res Treat 94(Suppl 1):S234. Abstract 5081
- 52.Nicholson RI, Hutcheson IR, Hiscox SE, Knowlden JM, Giles M, Barrow D, Gee JM (2005) Growth factor signaling and resistance to selective oestrogen receptor modulators and pure anti-oestrogens: the use of anti-growth factor therapies to treat or delay endocrine resistance in breast cancer. Endocr Relat Cancer 12(Suppl 1):S29–S36 [DOI] [PubMed]
- 53.Massarweh S, Shou J, Mohsin SK, Ge M, Wakeling AE, Osborne CK, Schiff R (2002) Inhibition of epidermal growth factor/HER2 receptor signaling using ZD1839 (“Iressa”) restores tamoxifen sensitivity and delays resistance to estrogen deprivation in HER2-overexpressing breast tumors. J Clin Oncol 21:33a. Abstract 130
- 54.Traina TA, Rugo H, Caravelli J, Yeh B, Panageas K, Bruckner J, Norton L, Park J, Hudis C, Dickler M (2006) Letrozole (L) with bevacizumab (B) is feasible in patients (pts) with hormone receptor-positive metastatic breast cancer (MBC). J Clin Oncol 24(18S):133s. Abstract 3050
- 55.Doisneau-Sixou SF, Cestac P, Faye JC, Favre G, Sutherland RL (2003) Additive effects of tamoxifen and the farnesyl transferase inhibitor FTI-277 on inhibition of MCF-7 breast cancer cell-cycle progression. Int J Cancer 106:789–798 [DOI] [PubMed]
- 56.Rudloff J, Boulay A, Zumstein-Mecker S, Evans DB, O’Reilly T, Lane HA (2004) The mTOR pathway in estrogen response: a potential for combining the rapamycin derivative RAD001 with the aromatase inhibitor letrozole (Femara®) in breast carcinomas. Proc Am Assoc Cancer Res 45:1298. Abstract 5619
- 57.Mass RD, Press MF, Anderson S, Cobleigh MA, Vogel CL, Dybdal N, Leiberman G, Slamon DJ (2005) Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer 6:240–246 [DOI] [PubMed]
- 58.Ji H, Zhao X, Yuza Y, Shimamura T, Li D, Protopopov A, Jung BL, McNamara K, Xia H, Glatt KA, Thomas RK, Sasaki H, Horner JW, Eck M, Mitchell A, Sun Y, Al-Hashem R, Bronson RT, Rabindran SK, Discafani CM, Maher E, Shapiro GI, Meyerson M, Wong KK (2006) Epidermal growth factor receptor variant III mutations in lung tumorigenesis and sensitivity to tyrosine kinase inhibitors. Proc Natl Acad Sci USA 103:7817–7822 [DOI] [PMC free article] [PubMed]
- 59.Marcom PK, Isaacs C, Harris L, Wong ZW, Kommarreddy A, Novielli N, Mann G, Tao Y, Ellis MJ (2007) The combination of letrozole and trastuzumab as first or second-line biological therapy produces durable responses in a subset of HER2 positive and ER positive advanced breast cancers. Breast Cancer Res Treat 102(1):43–49 [DOI] [PubMed]
- 60.Chu Q, Goldstein L, Murray N, Rowinsky E, Cianfrocca M, Gale M, Ho P, Paul E, Loftiss J, Pandite L (2005) A phase I, open-label study of the safety, tolerability and pharmacokinetics of lapatinib (GW572016) in combination with letrozole in cancer patients. J Clin Oncol 23(16S):192s. Abstract 3001
- 61.National Cancer Institute (2006) Study comparing GW572016 and letrozole versus letrozole in subjects with advanced or metastatic breast cancer. http://www.clinicaltrials.gov/show/NCT00073528. Cited 25 Aug 2006
- 62.Awada A, Cardoso F, Fontaine C, Dirix L, De Grève J, Sotiriou C, Steinseifer J, Wouters C, Tanaka C, Ressayre-Djaffer C, Piccart M (2004) A phase Ib study of the mTOR inhibitor RAD001 (everolimus) in combination with letrozole (Femara®), investigating safety and pharmacokinetics in patients with advanced breast cancer stable or slowly progressing on letrozole. Breast Cancer Res Treat 88(Suppl 1):S234. Abstract 6043
- 63.National Cancer Institute (2006) Everolimus and letrozole as preoperative therapy of primary breast cancer in post-menopausal women. http://www.clinicaltrials.gov/ct/show/NCT00107016. Cited 25 Aug 2006
- 64.Baselga J, Roché H, Fumoleau P, Campone M, Colomer R, Cortes-Funes H, Gil M, Chan S, Boni J, Kong S, Cincotta M, Moore L (2005) Treatment of postmenopausal women with locally advanced or metastatic breast cancer with letrozole alone or in combination with temsirolimus: a randomized, 3-arm, phase 2 study. Breast Cancer Res Treat 94(Suppl 1):S62. Abstract 1068
- 65.National Cancer Institute (2006) Study evaluating CCI-779 and letrozole in post-menopausal women with breast cancer. http://www.clinicaltrials.gov/show/NCT00083993. Cited 25 Aug 2006
- 66.Johnston SRD, Semiglazov V, Manikhas G, Spaeth D, Romieu G, Dodwell D et al; on behalf of the R115777 INT-22 Investigators (2005) A randomised, blinded, phase II study of tipifarnib (Zarnestra®) combined with letrozole in the treatment of advanced breast cancer after antiestrogen therapy. Breast Cancer Res Treat 94(Suppl 1):S237. Abstract 5087
- 67.National Cancer Institute (2006) Study of letrozole plus ZARNESTRA or placebo in the treatment of advanced breast cancer. http://www.clinicaltrials.gov/show/NCT00050141. Cited 25 Aug 2006
- 68.Aun B, Dice K, ALbarracin C, Rivera E, Walters R, Theriault R, Booser D, Bast R, Cristofanili M, Sahin A, Smith TL, Hortobagyi GN (2004) The combination of letrozole and imatinib mesylate for metastatic breast cancer. Breast Cancer Res Treat 88(Suppl 1):S235. Abstract 6046
- 69.National Cancer Institute (2006) Bevacizumab and letrozole in treating postmenopausal women with locally advanced or metastatic breast cancer that cannot be removed by surgery. http://www.clinicaltrials.gov/show/NCT00305825. Cited 25 Aug 2006
- 70.Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, Apffelstaedt J, Smith R, Sleeboom HP, Jaenicke F, Pluzanska A, Dank M, Becquart D, Bapsy PP, Salminen E, Snyder R, Chaudri-Ross H, Lang R, Wyld P, Bhatnagar A (2003) Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 21:2101–2109 [DOI] [PubMed]
- 71.Rugo HS, Dickler MN, Traina TA, Scott JH, Moore DH, Bruckner J, Hudis C, Park JW (2006) Change in circulating endothelial cells (CEC) predicts progression free survival (PFS) in patients (pts) with hormone receptor positive metastatic breast cancer (MBC) receiving letrozole (L) and bevacizumab (B). J Clin Oncol 24(18S):130s. Abstract 3039
- 72.Munzone E, Curigliano G, Rocca A, Bonizzi G, Renne G, Goldhirsch A, Nole F (2006) Reverting estrogen-receptor-negative phenotype in HER-2-overexpressing advanced breast cancer patients exposed to trastuzumab plus chemotherapy. Breast Cancer Res 8:R4 [DOI] [PMC free article] [PubMed]
- 73.FDA press release (2006) New federal health initiative to improve cancer therapy. 14 February 2006. http://www.fda.gov/bbs/topics/news/2006/NEW01316.html. Cited 22 Aug 2006
- 74.Dowsett M, A’Hern R, Smith I et al on behalf of the IMPACT Trialists (2005) Ki67 after 2 weeks’ endocrine treatment predicts relapse-free survival (RFS) in the IMPACT trial. Presented at the 28th Annual San Antonio Breast Cancer Symposium, 8–11 December 2005. Abstract 45
- 75.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406:747–752 [DOI] [PubMed]
- 76.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borrensen-Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874 [DOI] [PMC free article] [PubMed]
- 77.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borrensen-Dale AL, Botstein D (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100:8418–8423 [DOI] [PMC free article] [PubMed]
- 78.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, Jatkoe T, Berns EM, Atkins D, Foekens JA (2005) Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 365:671–679 [DOI] [PubMed]
- 79.van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhius S, Rutgers ET, Friend SH, Bernards R (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347:1999–2009 [DOI] [PubMed]
- 80.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerman DL, Bryant J, Wolmark N (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817–2826 [DOI] [PubMed]
- 81.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Watson D, Bryant J, Costantino J, Wolmark N (2005) Expression of the 21 genes in the recurrence score assay and tamoxifen clinical benefit in the NSABP study B-14 of node negative, estrogen receptor positive breast cancer. J Clin Oncol 23(16S):6s. Abstract 510
- 82.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE Jr, Wickerman DL, Wolmark N (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24:3726–3734 [DOI] [PubMed]
- 83.Naderi A, Teschendorff AE, Barbosa-Morais NL, Robertson JFR, Ellis IO, Brenton JD, Caldas C (2006) Robust identification of novel prognostic gene-signatures in breast cancer. Eur J Cancer 4(2 Suppl):135. Abstract 300
- 84.Naderi A, Teschendorff A, Beigel J, Cariati M, Ellis I, Brenton J, Caldas C (2006) Bex2 identifies a novel subtype of breast cancer associated with estrogen-response and NGF/NFKB pathway. Eur J Cancer 4(2 Suppl):125. Abstract 267
- 85.Van Laere S, Van der Auwera I, Van den Eynden G, Huygelen V, Elst H, van Dam P, Van Marck E, Vermeulen P, Dirix L (2006) Inflammatory breast cancer: at the cross-roads of NFkB and estrogen receptor signalling pathways? Eur J Cancer 4(2 Suppl):136. Abstract 305
- 86.Miller WR, Renshaw L, Larionov A, Anderson TJ, White S, Hampton G, Walker JR, Ho S, Krause A, Evans DB, Dixon MJ (2005) Prediction of hormone response in breast cancer by microarray analysis of sequential tumour biopsies from patients receiving neoadjuvant therapy with letrozole. J Clin Oncol 23(16S):198S. Abstract 3025
- 87.Miller W, Renshaw L, Larionov A, Anderson T, White S, Hampton G et al (2006) Using changes in gene expression as assessed by microarray analysis of sequential tumour biopsies to predict response to neoadjuvant therapy with letrozole. Eur J Cancer 4(2 Suppl):129. Abstract 282
- 88.Urruticoechea A, Dowsett M, Mackay A, Dexter T, Young O, Miller WR, Evans DB, Dixon GM (2005) Molecular characterisation of ER+ breast cancer before and during treatment with the aromatase inhibitors, letrozole and anastrozole. J Clin Oncol 23(16S):850S. Abstract 9554
- 89.Itoh T, Karlsberg K, Kijima I, Yuan YC, Smith D, Ye J, Chen S (2005) Letrozole-, anastrozole-, and tamoxifen-responsive genes in MCF-7aro cells: a microarray approach. Mol Cancer Res 3:203–218 [DOI] [PubMed]
- 90.Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS (2003) Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 144:4562–4574 [DOI] [PubMed]
- 91.van ’t Veer LJ (2006) Molecular strategies improve therapeutic decisions. Eur J Cancer 4(2 Suppl):2. Extended Abstract E1
- 92.National Cancer Institute (2006) Hormone therapy with or without combination chemotherapy in treating women who have undergone surgery for node-negative breast cancer (The TAILORx Trial). http://www.clinicaltrials.gov/ct/show/NCT00310180. Cited 25 Aug 2006
- 93.Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D, Joshi MB, Harpole D, Lancaster JM, Berchuck A, Olson JA Jr, Marks JR, Dressman HK, West M, Nevins JR (2006) Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 439:353–357 [DOI] [PubMed]