Abstract
Third-generation nonsteroidal aromatase inhibitors (AIs), letrozole and anastrozole, are superior to tamoxifen as initial therapy for early breast cancer but have not been directly compared in a head-to-head adjuvant trial. Cumulative evidence suggests that AIs are not equivalent in terms of potency of estrogen suppression and that there may be differences in clinical efficacy. Thus, with no data from head-to-head comparisons of the AIs as adjuvant therapy yet available, the question of whether there are efficacy differences between the AIs remains. To help answer this question, the Femara versus Anastrozole Clinical Evaluation (FACE) is a phase IIIb open-label, randomized, multicenter trial designed to test whether letrozole or anastrozole has superior efficacy as adjuvant treatment of postmenopausal women with hormone receptor (HR)- and lymph node-positive breast cancer. Eligible patients (target accrual, N = 4,000) are randomized to receive either letrozole 2.5 mg or anastrozole 1 mg daily for up to 5 years. The primary objective is to compare disease-free survival at 5 years. Secondary end points include safety, overall survival, time to distant metastases, and time to contralateral breast cancer. The FACE trial will determine whether or not letrozole offers a greater clinical benefit to postmenopausal women with HR+ early breast cancer at increased risk of early recurrence compared with anastrozole.
Keywords: Anastrozole, Aromatase inhibitors, Breast cancer, Femara, Letrozole, Tamoxifen
Introduction
The aromatase inhibitors (AIs) have proven to be a powerful drug class for use in hormone-sensitive breast cancer and have shown superiority over the selective estrogen-receptor modulator (SERM) tamoxifen in preclinical models of hormone-dependent breast cancer [1] and in randomized controlled trials in patients with advanced breast cancer [2] and early breast cancer [3, 4]. Initial adjuvant therapy with either letrozole (Femara®) or anastrozole (Arimidex®) was shown to be significantly more effective than tamoxifen in both the Breast International Group (BIG) 1-98 and Anastrozole and Tamoxifen Alone or in Combination (ATAC) randomized controlled trials in postmenopausal women with localized breast cancer [3, 4].
In the BIG 1-98 primary core analysis, patients with hormone receptor-positive (HR+) tumors randomized to receive letrozole initially were compared with those assigned to receive tamoxifen initially (N = 8,010). After a median follow-up of 25.8 months, 351 events had occurred in the letrozole group (n = 4,003) and 428 events in the tamoxifen group (n = 4,007), with 5–year disease-free survival (DFS) estimates of 84.0% and 81.4%, respectively. Letrozole significantly reduced the risk of breast cancer recurrence (hazard ratio = 0.81; 95% confidence interval [CI] 0.70, 0.93; P = 0.003), especially the risk of distant recurrence (hazard ratio = 0.73; 95% CI 0.60, 0.88; P = 0.001) [3]. An analysis limited to patients randomized to either letrozole-only or tamoxifen-only arms (N = 4,922) was recently published and allows for more direct comparisons with results from other trials of continuous therapy with a single endocrine agent [5]. Results from the letrozole-only or tamoxifen-only arms were consistent with those published for the primary core analysis and showed that letrozole significantly reduced the risk of DFS events (hazard ratio = 0.82; 95% CI 0.71, 0.95; P = 0.007) [5, 6] and the risk of distant metastases. After a median follow-up of 51 months, 352 DFS events (14.3%) were observed in the letrozole-only group (n = 2,463), compared with 418 DFS events (16.9%) in the tamoxifen-only group (n = 2,459) [5, 6].
The ATAC trial, which compared anastrozole with tamoxifen for 5 years, did not have receptor positivity as a study requirement. After a median follow-up of 68 months (n = 6,186), anastrozole significantly prolonged DFS (575 events with anastrozole vs. 651 events with tamoxifen; hazard ratio = 0.87, 95% CI 0.78, 0.97; P = 0.01) and time-to-recurrence (402 vs. 498 events; hazard ratio = 0.79, 95% CI 0.70, 0.90; P = 0.0005), and significantly reduced the risk of developing distant metastases (324 vs. 375 events; hazard ratio = 0.86, CI 0.74, 0.99; P = 0.04) and contralateral breast cancers (35 vs. 59 events; 42% reduction, 95% CI 12, 62; P = 0.01) in the intent-to-treat (ITT) population [4]. However, neither the time to distant recurrence nor distant DFS (DDFS) were significantly improved with anastrozole in the HR+ population [7].
While both letrozole and anastrozole have been evaluated extensively in early breast cancer, no head-to-head trial of these two AIs has been conducted in this setting. This report will focus on the design of the Femara versus Anastrozole Clinical Evaluation (FACE) trial, and describe how it will prospectively address potential efficacy and safety differences between the two AIs.
Rationale for head-to-head trial
An American Society of Clinical Oncology technology assessment concluded that AIs should be included in the adjuvant treatment of postmenopausal women with HR+ breast cancer [8] but did not recommend one AI over another. The National Comprehensive Cancer Network has also recommended initial adjuvant therapy with an AI (specifically letrozole or anastrozole) as an alternative to tamoxifen [9]. Some evidence suggests that AIs may not be equivalent, even though they belong to the same pharmacologic class of agents; differences have been reported in terms of potency, suppression of aromatization, antitumor effects, and pharmacogenomics. However, whether or not one AI is superior in treating early breast cancer is not known.
Relative potency
Bhatnagar and colleagues compared the aromatase-inhibiting potency of letrozole and anastrozole in a variety of aromatase-containing cellular endocrine and tumor models [10, 11]. While letrozole and anastrozole were approximately equipotent in a cell-free aromatase system (human placental microsomes), letrozole was found to be more potent than anastrozole in inhibiting intracellular aromatase in intact rodent cells (50% inhibitory concentration [IC50] 20 vs. 600 nM, respectively), normal human adipose fibroblasts (0.8 vs. 14 nM), and human cancer cell lines (MCF-7Ca 0.07 vs. 0.82 nM and JEG-3 0.07 vs. 0.99 nM). Miller and colleagues reported that letrozole and anastrozole were more potent than aminoglutethimide in vitro against tumor samples obtained from postmenopausal women with breast cancer, with letrozole demonstrating the lowest IC50 (2 nM, 8 nM, and 20 μM, respectively) [12]. Letrozole was compared with anastrozole in vivo in athymic mice inoculated with MCF7 cells [13]. Tumor volumes increased to 145.9% in controls and decreased to 22.4% with letrozole 10 μg, and to 95.6% or 78.2% with anastrozole 10 or 60 μg, respectively. These results are consistent with a higher in vitro potency of letrozole in cell-based assays [13].
Suppression of aromatization
The effects of letrozole and anastrozole on suppression of total-body aromatization and plasma estrogen concentrations have been compared in patients with metastatic breast cancer [14, 15]. Levels of aromatase were detectable in 11 of 12 patients during treatment with anastrozole (mean percentage inhibition in the whole group, 97.3%) but in none of the 12 patients during treatment with letrozole (> 99.1% suppression in all patients; Wilcoxon, P = 0.0022, comparing the two drug regimens). Suppression of estrone and estrone sulfate was found to be significantly greater during treatment with letrozole compared with anastrozole (P = 0.019 and P = 0.0037, respectively). Another study conducted in 54 postmenopausal women with invasive breast cancer showed that more complete inhibition of aromatase was achieved by 2.5 mg of letrozole than 1 mg of anastrozole, resulting in significantly greater suppression of estradiol (P < 0.0001) [15]. Thus, letrozole reduces estradiol levels to a greater degree than anastrozole, but it is not known whether this difference is clinically relevant.
Breast cancer proliferation
Biological changes in breast tumors occurring within 14 days of starting treatment may predict the efficacy of different endocrine agents in the adjuvant setting and could prove to be useful surrogate markers to compare drug efficacy [16]. A study of neoadjuvant endocrine therapy compared the effects of letrozole and anastrozole on the expression of HRs and markers of tumor proliferation in postmenopausal women with estrogen receptor (ER)-positive breast cancer [16]. Neoadjuvant letrozole and anastrozole decreased overall ER expression (Allred score) after 14 days, but more cases showed a reduction in progesterone receptor (PgR) expression following letrozole treatment (75/106) than with anastrozole treatment (65/102). Furthermore, only letrozole significantly reduced proliferation at lower Allred ER expression levels (scores 2–5). This is a potentially important finding, because it has been suggested that a greater suppression in proliferation may lead to improved DFS [17].
Clinical activity
Letrozole and anastrozole have not been directly compared in the adjuvant setting, but data from a randomized, head-to-head trial in patients with advanced breast cancer are available [18]. Postmenopausal women with advanced breast cancer (N = 713) that had progressed either during antiestrogen therapy or within 12 months of completing that therapy were randomized to receive letrozole (2.5 mg per day) or anastrozole (1 mg per day). Letrozole was significantly superior to anastrozole in terms of overall response rate (19.1% vs. 12.3%, P = 0.013), but there were no significant differences in median time to progression, the primary end point of the trial. Both agents were well-tolerated, and there were no significant differences in safety.
Anastrozole and letrozole in the adjuvant setting have demonstrated superiority to tamoxifen in significantly reducing the risk of recurrence. In the ATAC trial, at 68 months’ median follow-up, anastrozole significantly reduced the risk of distant metastases in the ITT population by 14% (P = 0.04) but not significantly in the HR+ patient subgroup (hazard ratio = 0.84; P = 0.06) [4]. A recent study of the recurrence rates after 2.5 and 5 years from the ATAC study showed that there were fewer recurrence events with anastrozole at these time points due to reductions in contralateral, primary, loco-regional, and distant recurrences [19]. In addition, at 25.8 months’ median follow-up in the BIG 1-98 trial, letrozole significantly reduced the risk of distant metastases by 27% (P = 0.001) in the HR+ population, and another analysis of the early risk of relapse in 5,980 patients, with a median follow-up of 25 months, showed that letrozole reduced distant recurrences early on [3, 20]. The recently reported analysis of letrozole-only and tamoxifen-only arms in the BIG 1-98 trial showed that the time to distant metastases advantage for letrozole was consistent with these findings from the primary core analysis [5]. These data are potentially important, because the development of distant metastases directly translates into decreased survival. The ATAC trial showed a 3% relative improvement in overall survival (OS) (P = 0.7) with anastrozole at 68 months of follow-up, while a 9% relative improvement in OS (P = 0.35) was seen with letrozole at 51 months of follow-up [3, 5].
Subset analyses of randomized trials comparing letrozole or anastrozole with either tamoxifen or placebo demonstrated differences between these AIs and suggested that specific patient populations may derive differing degrees of benefit from a particular AI. In the trial, retrospective subgroup analyses with a median follow-up of 33 months revealed no significant benefit of anastrozole over tamoxifen in patients with node-positive tumors and with prior chemotherapy [21], and these findings were confirmed in the 4-year update of the ATAC trial. Thus, the hazard ratio for risk of recurrence in patients with four or more positive nodes was 0.96 (95% CI 0.72, 1.25), and in patients with prior chemotherapy, it was 0.98 (95% CI 0.76, 1.28), indicating no differences between treatments [22, 23]. No analyses in similar subgroups were presented in the 68-month update [4].
Prospectively planned subgroup analysis revealed a benefit of letrozole over tamoxifen in patients who had received chemotherapy and in those with node-positive tumors [3, 5]. In the former subset, letrozole reduced the risk of recurrence after 5 years (hazard ratio 0.70; 95% CI 0.54, 0.92; P = 0.01). In the node-positive subset, letrozole reduced the risk of an event ending a period of DFS by 29% (hazard ratio 0.71, 95% CI 0.59, 0.85; P < 0.001). The advantage for letrozole in these patient subsets was confirmed in the recent analysis of the letrozole-only and tamoxifen-only arms of BIG 1-98 [5]. Interestingly, with longer follow-up of 51 months in this monotherapy analysis, there was an emerging benefit in the node-negative group, as letrozole reduced the relative risk of recurrence by 12% in this patient population. The MA.17 trial, evaluating the efficacy of extended adjuvant letrozole therapy, although not positive for OS in the overall population, demonstrated that OS was statistically significantly improved with letrozole among lymph node-positive breast cancer patients compared with placebo (hazard ratio 0.61; 95% CI 0.38, 0.98; P = 0.04) [24].
The question of whether one third-generation AI is superior for the adjuvant treatment of postmenopausal women with HR+ breast cancer remains, as does the question of whether there are any specific patient populations who derive particular benefit from a specific AI. Patients with early breast cancer can be assigned to risk groups on the basis of clinical and pathological characteristics. In the St. Gallen Guidelines [25], node-positive patients are considered to be in the intermediate- or high-risk group depending on the number of positive nodes and human epidermal growth factor receptor-2 (HER2) expression (see Table 1). The guidelines state that endocrine therapy with an AI is a recommended option for patients with node-positive tumors who are in the intermediate- or high-risk groups. As high-risk patients are at greater risk of relapse, a drug specifically effective in this patient population would provide the oncology community with valuable information that may alter the outcomes of these patients.
Table 1.
Risk categories for early breast cancer according to the St. Gallen Guidelines. Reprinted from [25], with permission from the European Society for Medical Oncology
| Low risk | Steroid hormone receptors expression, node-negative, and all of the following features: |
| pT ≤ 2 cm | |
| Grade 1 | |
| No peritumoral vascular invasion | |
| HER2/neu gene neither overexpressed nor amplified | |
| Age ≥35 years | |
| Intermediate risk | Node-negative and at least one of the following features: |
| pT > 2 cm | |
| Grade 2–3 | |
| Peritumoral vascular invasion | |
| HER2/neu gene either overexpressed or amplified | |
| Age < 35 years | |
| Node positive (1–3 involved nodes) and HER2/neu gene neither overexpressed nor amplified | |
| High risk | Node positive (1–3 involved nodes) and HER2/neu gene either overexpressed or amplified |
| Node-positive (4 or more involved nodes) |
HER2 human epithelial growth factor receptor 2
pT pathological tumor size (i.e. size of the invasive component)
Is one AI superior in early breast cancer?
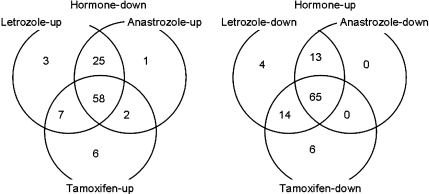
Microarray analysis was used to study the effects of various hormone therapies on ER+ MCF-7 cells, stably transfected with the aromatase gene (MCF-7aro cells) [26]. The study found that hormonal stimulation of gene expression can be counteracted by treatment with AIs (letrozole and anastrozole) and an anti-estrogen (tamoxifen), but that each agent had its own unique effects on gene expression (see Fig. 1), suggesting possible differences between letrozole and anastrozole [26]. Although differences between letrozole and anastrozole have been demonstrated in preclinical models, it is widely recognized that preclinical findings do not always translate into clinical results, and that comparisons in one treatment setting or subpopulation cannot be extrapolated to another. A prospective trial is therefore needed to address the question of whether one AI is superior to another.
Fig. 1.
Changes in inhibitor-responsive genes after treatment with letrozole, anastrozole, or tamoxifen. The Venn diagrams show the numbers of genes responsive to individual inhibitors in hormone-regulated genes. Reprinted from [26], with permission from the American Association for Cancer Research
FACE was designed to test whether there is a preferable AI for the adjuvant treatment of postmenopausal women with HR+ and lymph node-positive cancer [27]. Node-positive patients were selected, because this population has a higher risk of relapse, and recurrence events occur earlier than in node-negative patients [20, 28, 29]. Thus, conducting the FACE trial in patients with lymph node-positive early breast cancer will provide an answer more quickly than conducting a trial in a broader population that includes patients with node-negative tumors.
FACE trial design
FACE is a phase IIIb open-label, randomized, multicenter trial [30]. The primary objective of the trial is to compare DFS at 5 years for letrozole and anastrozole. Secondary objectives are to assess safety, OS, time to distant metastases, and time to contralateral breast cancer [27].
Patients
The trial is recruiting 4,000 patients from up to 250 international sites. Eligible patients are postmenopausal women with HR+ and lymph node-positive tumors who have recently undergone surgery for primary breast cancer (pathologic or clinical stage IIA, IIB, or IIIA). All patients must provide written informed consent.
HR+ tumors are defined as tumors with any detectable ER or PgR expression by institutional standards. Patients who are PgR+ and ER− are eligible for the trial. Pathologic assessment of axillary lymph nodes is determined by sentinel node biopsy and/or axillary lymph node dissection. Patients are stratified according to the number of involved lymph nodes and HER2 tumor status. Adjuvant trastuzumab is permitted in patients with HER2+ tumors. Other inclusion criteria include World Health Organization performance status of 0 or 1, lipid panel (fasting total cholesterol and triglycerides) ≤ grade 1 (National Cancer Institute Common Terminology Criteria for Adverse Events v3.0), and adequate hematologic, hepatic, and renal function.
Patients with T4 tumors, metastatic disease, contralateral breast cancer including ductal carcinoma in situ, or evidence of disease progression are excluded. Other exclusion criteria include prior neoadjuvant endocrine therapy; hormone replacement therapy (except intravaginal estradiol preparations) not stopped at least 4 weeks before randomization; adjuvant anti-estrogen therapy for > 1 month immediately following surgery, radiotherapy, and/or chemotherapy; breast cancer chemoprevention with anti-estrogens if < 18 months between stopping and diagnosis of breast cancer; and therapy with any hormonal agent, such as raloxifene, for management of osteoporosis.
Randomized trial design and treatments
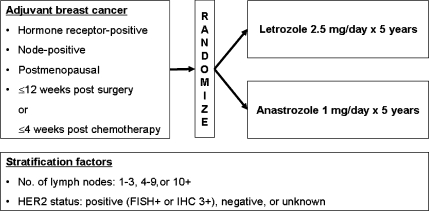
Eligible patients are randomized to receive either letrozole 2.5 mg or anastrozole 1 mg daily for up to 5 years (see Fig. 2). The date of randomization must be no more than 12 weeks from completion of surgery or 4 weeks after completion of adjuvant chemotherapy. Treatment assignments are balanced based on the number of lymph nodes (1–3, 4–9, 10+) and HER2 status (positive, negative, or unknown). Treatment will commence within 30 days of randomization and following the completion of standard chemotherapy (if given) and concurrently with radiotherapy (if given). Patients receive treatment with the allocated AI for up to 5 years or until disease recurrence/relapse. Recurrence and survival will be assessed every 12 months.
Fig. 2.
FACE randomized trial design
Efficacy end points
The primary end point is DFS, defined as the time from the date of randomization to the date of the first documentation of re-occurrence of invasive breast cancer in local, regional, or distant sites; new invasive breast cancer in the contralateral breast; or death from any cause.
Secondary efficacy end points include OS, defined as the time from the date of randomization to date of death from any cause; breast cancer-free survival, defined as the time from date of randomization to the date of death due to breast cancer; time to development of distant metastases, defined as the time from date of randomization to the date of the first development of any recurrent or metastatic disease in sites other than the local mastectomy scar, the ipsilateral breast in case of breast conservation, or the contralateral breast; and time to development of contralateral breast cancer, defined as the time from date of randomization to the date of the first development of any disease in the contralateral breast.
Although the FACE trial, co-chaired by Drs. Ian Smith and Joyce O’Shaughnessy, is an open-label trial, analysis of the data in a blinded fashion will make the data from this trial comparable with that obtained in a single-blinded trial. Both patients and their physicians will know which drug is being taken, but the analysis of the data will be conducted blinded to study treatment. The sponsor of the trial will not have access to the database, and all efficacy analyses will be conducted by an independent academic organization (the Instituto Nazionale Tumori, Milan, Italy), which will receive the data in a blinded manner. The data will be reviewed by an Independent Data Monitoring Committee, chaired by Professor Martine Piccart. The Independent Data Monitoring Committee will then make recommendations to a Trial Steering Committee chaired by Dr. Kathy Pritchard. The Independent Data Monitoring Committee will decide when the data will be released. The final analysis will be performed after the expected total number of DFS events have occurred. This is anticipated to be 7 years after the start of the study, following an accrual period of about 2 years and a minimum of 5 years of further follow-up. There are two planned interim analyses, scheduled to occur after one third and subsequently after two thirds of the maximum number of events have been observed. The interim analyses will be conducted after 320 and 639 events, respectively, have been recorded. In addition, analyses of secondary end points will be conducted at the interim time points.
FACE is powered to detect a 3.5% absolute difference between the two treatment arms in DFS at 5 years. The 3.5% difference corresponds to a hazard ratio of 0.83 in favor of letrozole, corresponding to 5-year DFS values of 80.0% and 76.5% for letrozole and anastrozole, respectively.
Safety end points
General patient safety and drug tolerability will be evaluated. Adverse events are recorded at every visit and graded for severity using the National Cancer Institute Common Terminology Criteria for Adverse Events v3.0. A checklist of adverse events is used to solicit adverse event information from patients.
Safety analyses specifically include cardiovascular events and bone fracture events. All patients are evaluated clinically for osteoporosis and fracture risks. Bone mineral density testing is recommended at least every 2 years for all patients during study therapy by dual X-ray absorptiometry, peripheral dual X-ray absorptiometry, or ultrasound densitometry. Osteoporosis may be managed as clinically indicated using calcium supplements, vitamin D, or bisphosphonates. Measurements of fasting serum lipids are obtained at 6 and 12 months and then annually thereafter for the duration of the study treatment. Other laboratory assessments include hematology and blood chemistry.
Other head-to-head studies
Other trials that are directly comparing AIs are also under way. A randomized phase III trial [31] is comparing neoadjuvant therapy with exemestane, letrozole, or anastrozole in postmenopausal women undergoing surgery for stage II or stage III breast cancer. Another ongoing randomized trial, MA.27 [32], has been designed to compare the event-free survival of postmenopausal women with HR+ primary breast cancer when treated with exemestane or anastrozole. Results from these trials and ongoing pharmacogenomic studies [26] will also help individualize AI therapy for early breast cancer.
The study of inherited genetic polymorphisms that affect drug response and toxicity promises to help physicians individualize hormone treatment. For example, “slow metabolizers” of tamoxifen may have a worse outcome in the adjuvant setting with tamoxifen treatment than “fast metabolizers,” suggesting that these patients might be better treated with an AI [33–35]. Polymorphisms in tamoxifen metabolizing cytochrome P (CYP) 2D6 gene affect the plasma concentration of tamoxifen active metabolites: women with the CYP2D6 *4/*4 or wt/*4 genotype could have lower benefit of tamoxifen treatment and tend to have a higher risk of disease relapse [35, 36]. Genetic polymorphisms in the aromatase gene, CYP19, have recently been characterized [37]. Eighty-eight polymorphisms were identified, resulting in 44 haplotypes. Functional genomic studies revealed that polymorphisms may lead to changes in aromatase activity and altered affinity for AIs. These findings indicate that genetic variation in CYP19 might contribute to variation in the pathophysiology of estrogen-dependent disease. Clinical trials have been initiated to study the impact of genetic differences on response to AI therapy and may eventually lead to patient-specific selection of therapy based on optimizing efficacy and toxicity.
Conclusions
Letrozole and anastrozole have both demonstrated superior efficacy compared with tamoxifen as initial therapy for early breast cancer [3, 4]. Preclinical and clinical evidence suggests that AIs do not have identical pharmacodynamic profiles, but it is not known whether one agent may be more effective as adjuvant therapy for early breast cancer. Differences in potency in preclinical studies, and the reduction in distant metastases in the BIG 1-98 study, suggest the potential for clinical efficacy differences between AIs. Based on the results of these trials, international guidelines now recommend adjuvant hormone therapy with an AI [9, 25] in patients with an increased risk of early recurrence. The FACE trial is addressing an important medical question in the oncology community: whether or not letrozole offers greater clinical benefit to postmenopausal women with HR+ early breast cancer at increased risk of early recurrence compared with anastrozole. Results from the FACE trial may refine the treatment strategies for treating breast cancer in postmenopausal women.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s10549-007-9851-x
References
- 1.Brodie A, Jelovac D, Long BJ (2003) Predictions from a preclinical model: studies of aromatase inhibitors and antiestrogens. Clin Cancer Res 9:455S–459S [PubMed]
- 2.Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, Apffelstaedt J, Smith R, Sleeboom HP, Janicke F, Pluzanska A, Dank M, Becquart D, Bapsy PP, Salminen E, Snyder R, Lassus M, Verbeek JA, Staffler B, Chaudri-Ross HA, Dugan M (2001) Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol 19:2596–2606 [DOI] [PubMed]
- 3.Thurlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M, Smith I, Wardley A, Price KN, Goldhirsch A; Breast International Group (BIG) 1-98 Collaborative Group (2005) A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353:2747–2757 [DOI] [PubMed]
- 4.Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS; ATAC Trialists’ Group (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365:60–62 [DOI] [PubMed]
- 5.Coates AS, Keshaviah A, Thürlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Colleoni M, Lang I, Del Mastro L, Smith I, Chirgwin J, Nogaret JM, Pienkowski T, Wardley A, Jakobsen EH, Price KN, Goldhirsch A (2007) Five years of letrozole compared with tamoxifen as adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol 25:486–492 [DOI] [PubMed]
- 6.International Breast Cancer Study Group. http://www.ibcsg.org
- 7.Anastrozole package insert. Wilmington, DE; AstraZeneca Pharmaceuticals, 2002
- 8.Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, Chlebowski RT, Gelber R, Edge SB, Gralow J, Cobleigh MA, Mamounas EP, Goldstein LJ, Whelan TJ, Powles TJ, Bryant J, Perkins C, Perotti J, Braun S, Langer AS, Browman GP, Somerfield MR (2005) American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol 23:619–629 [DOI] [PubMed]
- 9.National Comprehensive Cancer Network Practice Guidelines in Oncology. Breast Cancer Version 2.2006. Available at: http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf
- 10.Bhatnagar AS, Batzl C, Hausler A, Schieweck K, Lang M, Trunet PF (1996) Pharmacology of non-steroidal aromatase inhibitors. In: Pasqualini JR, Katzenellenbogen BS (eds) Hormone-dependent cancer. Marcel Dekker, New York, pp 155–168
- 11.Bhatnager AS, Brodie AMH, Long BJ, Evans DB, Miller WR (2001) Intracellular aromatase and its relevance to the pharmacological efficacy of aromatase inhibitors. J Steroid Biochem Mol Biol 76:199–202 [DOI] [PubMed]
- 12.Miller WR (1999) Biology of aromatase inhibitors: pharmacology/endocrinology within the breast. Endocr Relat Cancer 6:187–195 [DOI] [PubMed]
- 13.Lu Q, Liu Y, Long BJ, Grigoryev D, Gimbel M, Brodie A (1999) The effect of combining aromatase inhibitors with antiestrogens on tumor growth in a nude mouse model for breast cancer. Breast Cancer Res Treat 57:183–192 [DOI] [PubMed]
- 14.Geisler J, Haynes B, Anker G, Dowsett M, Lonning PE (2002) Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer women evaluated in a randomized, cross-over study. J Clin Oncol 20:751–757 [DOI] [PubMed]
- 15.Dixon JM, Renshaw L, Young O, Murray J, Macaskill EJ, McHugh M, Folkerd E, Cameron D, Dowsett M (2006) Letrozole suppresses plasma oestradiol (E2) levels more completely than anastrozole in postmenopausal women with breast cancer. J Clin Oncol 24(18S):15s. Abstract 552 [DOI] [PubMed]
- 16.Murray J, Young O, Renshaw L White S, Prescot RJ, Krause A, Evans DB, Salem R, Cameron D, Dowsett M, Miller WR, Dixon JM (2004) Letrozole and anastrozole: a pre-operative study of their effects on ER positive breast cancers in postmenopausal women. Presented at the 27th annual San Antonio breast cancer symposium, San Antonio, 8–11 December 2004. Abstract 406
- 17.Dowsett M, A’Hern R, Smith I; on behalf of the IMPACT Trialists (2005) Ki67 after 2 weeks endocrine treatment predicts relapse-free survival (RFS) in the IMPACT trial. Presented at the 28th Annual San Antonio Breast Cancer Symposium, 8–11 December 2005. Abstract 45
- 18.Rose C, Vtoraya O, Pluzanska A, Davidson N, Gershanovich M, Thomas R, Johnson S, Caicedo JJ, Gervasio H, Manikhas G, Ben Ayed F, Burdette-Radoux S, Chaudri-Ross HA, Lang R (2003) An open randomised trial of second-line endocrine therapy in advanced breast cancer. comparison of the aromatase inhibitors letrozole and anastrozole. Eur J Cancer 39:2318–2327 [DOI] [PubMed]
- 19.Houghton J, on behalf of the ATAC (‘Arimidex’, Tamoxifen, Alone or in Combination) Trialists’ Group (2006) Initial adjuvant therapy with anastrozole (a) Reduces rates of early breast cancer recurrence and adverse events compared with tamoxifen (t). Ann Oncol 17(Suppl 9):ix94. Abstract 243PD
- 20.Mauriac L, Keshaviah A, Debled M, Mouridsen H, Forbes J, Thuerlimann B, Paridaens R, Monnier A, Lang I, WardleyA, Nogaret JM, Gelber R, Castiglione-Gertsch M, Price K, Coates A, Smith I, Viale G, Rabaglio M, Zabaznyi N, Goldhirsch A (2007) Predictors of early relapse in postmenopausal women with hormone receptor positive breast cancer in the BIG 1-98 trial. Ann Oncol 18:859–867 [DOI] [PubMed]
- 21.Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, Sahmoud T; ATAC Trialists’ Group (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359:2131–2139. Erratum in: Lancet 2002;360:1520 [DOI] [PubMed]
- 22.Buzdar AU, Guastalla JP, Nabholtz JM, Cuzick J (2006) Impact of chemotherapy regimens prior to endocrine therapy: results from the ATAC (Anastrozole and Tamoxifen, Alone or in Combination) trial. Cancer 107:472–480 [DOI] [PubMed]
- 23.Baum M, Buzdar A, Cuzick J, Forbes J, Houghton J, Howell A, Sahmoud T; The ATAC (Arimidex, Tamoxifen Alone or in Combination) Trialists’ Group (2003) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer 98:1802–1810 [DOI] [PubMed]
- 24.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Cameron DA, Palmer MJ, Pater JL (2005) Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 97:1262–1271 [DOI] [PubMed]
- 25.Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thurlimann B, Senn HJ, Panel members (2005) Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol 16:1569–1583 [DOI] [PubMed]
- 26.Itoh T, Karlsberg K, Kijima I, Yuan YC, Smith D, Ye J, Chen S (2005) Letrozole-, anastrozole-, and tamoxifen-responsive genes in MCF-7aro cells: a microarray approach. Mol Cancer Res 3:203–218 [DOI] [PubMed]
- 27.DeBoer R, Burris H, Monnier A, Mouridsen H, O’Shaughnessy J, McIntyre K, Pritchard K, Smith I, Yardley D, on behalf of the H2H trial steering committee (2006) The Head to Head trial: letrozole vs anastrozole as adjuvant treatment of postmenopausal patients with node positive breast cancer. J Clin Oncol 24(18S):582s. Abstract 10672
- 28.McArthur HL, Olivotto I, Gelmon KA, Speers CH, Chia S, Ellard S, Kennecke HF (2005) Risk of early relapse in post-menopausal women with early stage, estrogen receptor positive (ER+) breast cancer on tamoxifen. Breast Cancer Res Treat 94(Suppl 1):S124. Abstract 3001
- 29.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717 [DOI] [PubMed]
- 30.National Cancer Institute. Comparison trial of letrozole to anastrozole in the adjuvant treatment of postmenopausal women with hormone receptor and node positive breast cancer. http://www.clinicaltrials.gov/ct/show/NCT00248170
- 31.National Cancer Institute. Exemestane, letrozole, or anastrozole in treating postmenopausal women who are undergoing surgery for stage II or stage III breast cancer. http://www.clinicaltrials.gov/ct/show/NCT00265759
- 32.National Cancer Institute. Exemestane or anastrozole in treating postmenopausal women who have undergone surgery for primary breast cancer. http://www.clinicaltrials.gov/ct/show/NCT00066573
- 33.Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, Hayes DF, Desta Z, Flockhart DA (2003) Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 95:1758–1764 [DOI] [PubMed]
- 34.Jin Y, Desta Z, Stearns V, Ward B, Ho H, Lee KH, Skaar T, Storniolo AM, Li L, Araba A, Blanchard R, Nguyen A, Ullmer L, Hayden J, Lemler S, Weinshilboum RM, Rae JM, Hayes DF, Flockhart DA (2005) CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97:30–39 [DOI] [PubMed]
- 35.Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL, Flockhart DA, Desta Z, Perez EA, Ingle JN (2005) Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 23:9312–9318 [DOI] [PubMed]
- 36.Gonzalez-Santiago S, Zarate R, de la Haba-Rodriguez J, Gomez A, Bandres E, Borrega P, Garcia-Foncillas J, Aranda E (2006) Genetic polymorphism CYP2D6(*4) interaction in clinical outcomes of tamoxifen-treated breast cancer patients. Ann Oncol 17(Suppl 9):ix56–ix68. Abstract 114P
- 37.Ma CX, Adjei AA, Salavaggione OE, Coronel J, Pelleymounter L, Wang L, Eckloff BW, Schaid D, Wieben ED, Adjei AA, Weinshilboum RM (2005) Human aromatase: gene resequencing and functional genomics. Cancer Res 65:11071–11082 [DOI] [PubMed]