Abstract
The rapid processing of emotional information adaptively regulates the allocation of attention, but may also divert resources away from attention performance, particularly for those showing elevated anxiety. The temporal organization of rapid emotional processing and its implications for attention performance, however, remain unclear. Participants were 18 healthy adults (12 females) who reported on trait anxiety. Tasks-irrelevant fearful, sad, and neutral faces were presented for 50 ms prior to each trial of a cued attention task measuring alerting, orienting, and executive attention. Electroencephalographic recordings were made from 64 scalp electrodes to generate event-related potentials (ERPs) to faces. Emotional face type and trait anxiety modulated ERP responses at three early stages around 200 ms, 250 ms, and 320 ms. Although behavioral findings showed enhanced orienting and executive attention following presentation of fearful and sad faces, the degree to which these faces modulated ERP responses, particularly around 250 ms, interfered with orienting and executive attention in the high trait anxiety group, and enhanced alerting in the low trait anxiety group. Results are discussed in terms of mechanisms in the emotional capture of attention and implications for understanding attentional processes in anxiety.
Keywords: Emotional face processing, ERPs, Emotion-attention interactions, Trait anxiety
Rapid and accurate detection of negative emotional information is highly adaptive because it provides critical information about potential danger in the environment. In this way, emotional processes regulate the allocation of attention by highlighting relevant information and inhibiting irrelevant information. On the other hand, preferential attention towards negative information is implicated in the etiology and course of anxiety and mood disorders (Beck and Clark, 1997; Compton, 2003; Derryberry and Reed, 2002). Studies using scalp-recorded event-related potentials (ERPs) are able to explore the time course of the emotional capture of attention at a very high temporal resolution. Links between early stages of emotional processing and attention performance, however, are poorly understood.
The emotional face processing literature provides important information about rapid and automatic stages of emotional processing (Eimer and Holmes, 2002; Pizzagalli et al., 1999; Righart and de Gelder, 2006; Sato et al., 2001). Negative emotional faces are preferentially processed: As early as 80-100 ms, negative emotional faces compared to neutral faces elicit enhanced ERP activity (Eger et al., 2003; Smith et al., 2003) and modulate later stages of ERP responses between 200 and 300 ms (Campanella et al., 2002; Smith et al., 2003; Taylor et al., 2004). For example, posterior P200 (Carretié et al., 2001; Correll et al., 2006; Eimer et al., 2003) and P300 amplitudes, thought to reflect emotional salience processing, are greater for negative emotional faces and pictures (Cuthbert et al., 2000; Dien et al., 2004). Negative-going waveforms are also greater within 300 ms following presentation of negative versus positive faces and liked versus disliked faces (Pizzagalli et al., 1999; Pollak and Tolley-Schell, 2003; Schupp et al., 2003). In particular, a posterior face-specific component, N170, is modulated by emotion (Batty and Taylor, 2003; Eger et al., 2003; Pizzagalli et al., 1999), and is greater in the right hemisphere (Bentin et al., 1996). Other studies, however, have failed to find enhanced N170 amplitudes for negative emotional faces (Eimer and Holmes, 2002), and suggest that later occurring components are sensitive both to faces and emotional valence (Sato et al., 2001; Taylor et al., 2004). For example, in a passive viewing task, posterior N270 amplitudes were greater for emotional versus neutral faces (Sato et al., 2001).
If, instead, faces are irrelevant to performing a target task, ERP responses related to the control and inhibition of attention may emerge (Hanoch and Vitouch, 2004; Kliegel et al., 2003; Rokke et al., 2002). The N200 response, for example refers to activity over frontal midline regions 200 to 350 ms post stimulus onset, and is generated by frontal structures related to cognitive control, such as the anterior cingulate cortex. (Nieuwenhuis et al., 2003). The N200 is thought to reflect conflict monitoring and the gating of incoming information to the prefrontal cortex, thus signaling the extent to which attentional control is required. Emotional information and states are thought to bias the competition for cognitive control and processing resources measured by N200. For example, N200 amplitudes are greater following fearful, sad, and angry compared to neutral facial expressions (Campanella et al., 2002; Schutter et al., 2004) and other frontally-generated components, such as those related to error monitoring, are enhanced among individuals showing high negative affectivity (Luu et al., 2000a,b). Most ERP studies of emotional faces, however, employ passive viewing and other tasks which are unlikely to recruit cognitive control.
Studies of emotional face processing rarely relate ERPs directly to attention performance. This is surprising given that the excellent temporal resolution of ERPs provides a tool for disentangling links between attentional processing operations and attention performance (Smith et al., 2003). Behavioral studies suggest that viewing negative emotional faces can both interfere with (Eastwood et al., 2003) and facilitate (Ladouceur et al., 2006) attention performance. In a study of fearful face processing among autistic children, activity related to visual processing (N300) but not the face-specific N170, was linked to enhanced attention performance in social and joint attention tasks (Dawson et al., 2004). In other ERP research, affective processing (e.g., P200) was associated with the speed of responding in a stereotype assessment paradigm (Correll et al., 2006). Few studies, however, clarify mechanisms in the emotional capture of attention that impact multiple domains of attention performance.
Assessing the emotional capture of attention in relation to attention performance is especially relevant to understanding the interplay between anxiety and attention. Anxiety is associated with enhanced attention to threat-related stimuli such as fearful faces; but this has been found to have a negative impact on attention performance reflecting spatial orienting and top-down executive control of attention (Eastwood et al., 2003; Fenske and Eastwood, 2003; Fox et al., 2001; Mogg et al., 1992; Schupp et al., 2003). Like negative emotional information, anxious states are thought to rapidly and automatically bias the allocation of processing resources so that when there is competition for attention, emotional processing is prioritized over attention performance (Easterbrook, 1959; Hanoch and Vitouch, 2004; Leith and Baumeister, 1996; Meinhardt and Pekrun, 2003). Anxiety within an optimal range would show fewer biasing effects and might actually enhance attention performance (Gray, 2004).
Taken together, these findings highlight a critical goal in the study of emotional face processing, anxiety, and attention: determining the impact of very early stages of emotional face processing on attention in multiple domains of attention (Fan et al., 2002; Posner and Petersen, 1990). Chronometric analyses of separable attention capacities, alerting, orienting, and executive attention, have been combined into a single assessment with fully randomized conditions within blocks called the Attention Network Test (Fan et al., 2003, 2002). In this study, we modified this task in a novel way: each trial was preceded by task-irrelevant fearful, sad, and neutral faces. This design allowed us to assess the impact of emotional face processing on alerting, orienting, and executive attention when faces also recruit attentional control and inhibition because they are distracters.
In the present study, we examined whether emotional factors would bolster relatively early and automatic stages of emotional face processing (0-400 ms), and whether these rapid ERP responses would be related to subsequent attention performance. We hypothesized that ERP responses would be enhanced following negative versus neutral faces and in those showing relatively high versus low trait anxiety. We also hypothesized that links between these ERPs and attention performance would be moderated by trait anxiety. For those showing relatively high trait anxiety, enhanced emotional processing would interfere with attention, particularly orienting and executive attention, whereas among those showing relatively low trait anxiety, enhanced emotional processing would bolster attention, particularly alerting.
1. Methods
1.1. Participants
Participants were 28 adults between the ages of 19 and 34 (25 females) recruited through the psychology participant research pool at an urban college in the Northeast, and screened for identified psychological or neurological impairments. The data from 10 subjects were excluded due to experimental problems: specifically, excessive EEG artifacts (5) and performance errors on greater than 25% of trials, indicating poor engagement with the task (5). This left a total sample of 18 (13 females). This high rate of exclusion was necessary in order to retain only those participants who complied with task directions. Self-reported race/ethnicity was as follows: 6 Caucasian, 1 African American, 4 Hispanic, 5 Asian, 2 “Other.”
1.2. Procedures and measures
Participants completed the State Trait Anxiety Inventory (STAI; Spielberger, 1983) immediately after consent procedures in order to assess trait anxiety1. Participants werecategorized into low and high groups based on sample norms (50th percentile=40, low N=9, high N=9). Average trait anxiety scores were M=47.56, SD=7.42, range 41-60 in the high anxiety group and M=33.11, SD=5.40, range 22-39 in the low anxiety group. All scores were consistent with those reported for a normative sample of adults and college students (Spielberger, 1983). Low and high trait anxiety groups did not differ on any demographic variables and did not differ in the likelihood of being excluded from analyses due to computer failure, excessive EEG artifacts, or performance errors.
1.3. Attention task
The ANT, illustrated in Fig. 1, combines a cued reaction time and flanker task (Eriksen and Eriksen, 1974). It quantifies the efficiency of three attention systems by measuring how response times to the target flanker task are influenced by alerting and spatial cues and flankers. After the emotional face, a cue is presented followed by the target arrow, which randomly appears above or below the fixation cross and is surrounded on the left and right by four “flanker” stimuli. Participants indicate with one of two alternative button presses whether the central target arrow points left or right.
Fig. 1.
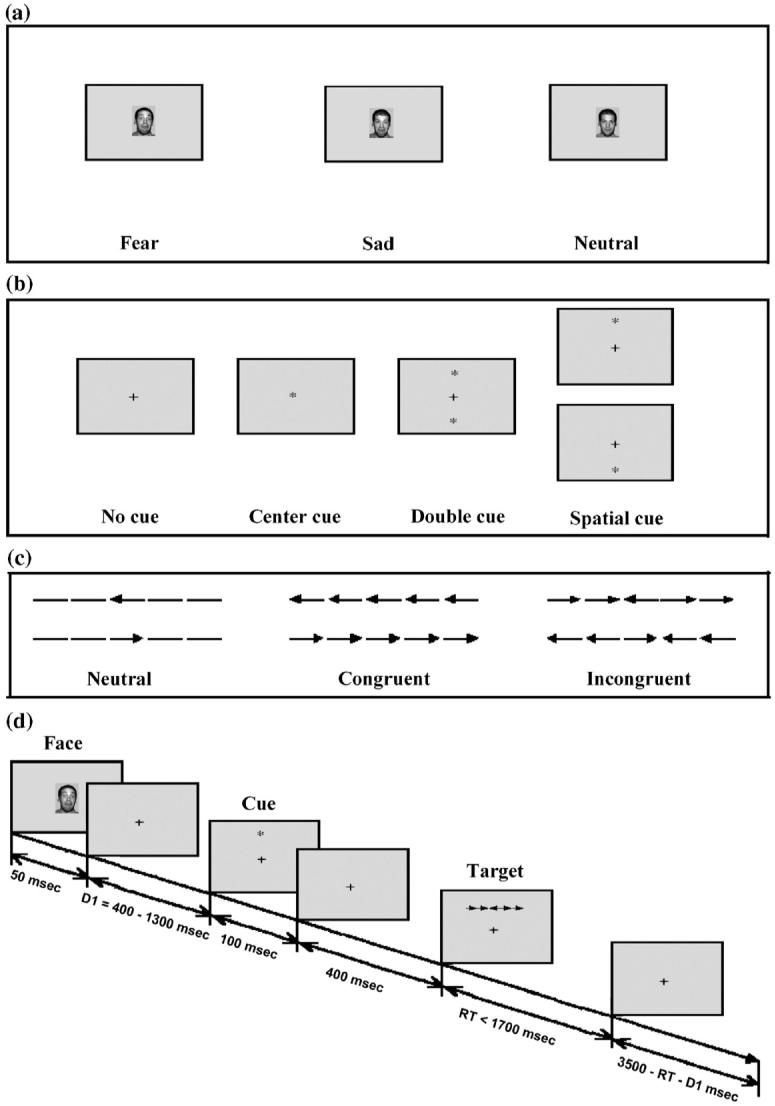
Diagram of experimental design (based on Fan et al., 2002).
Fig. 1a shows the inter-trial faces and Fig. 1b shows the cue conditions. Cues modulate whether subjects are alerted to the impending stimulus, and whether subjects are oriented ahead of time to the location of the target. Cues are no cues, double cues (asterisk appears above and below the fixation), center cues (asterisk appears superimposed over the fixation), and spatial cues (asterisk appears above or below the fixation to indicate the location of the subsequent target). Fig. 1c shows the flanker stimuli: congruent flankers point in the same direction as the central target arrow, incongruent flankers point in the opposite direction, and neutral flankers have no directional information. Consistent with the classic flanker task, congruent and neutral flankers yield faster reaction times than incongruent flankers due to reduced conflict interference (flanker incongruence;Eriksen and Eriksen, 1974; Fan et al., 2002).
The experiment consisted of a 24-trial full-feedback practice block (reaction time, whether answer was correct, and cumulative success rate) followed by two blocks of 384 feedback-free trials each, with a brief break in each block after half the trials. Each block included exactly two types of stimuli: One block contained a random mix of fear or neutral faces and the other block contained a random mix of sad or neutral faces. Faces were randomly presented without replacement within each block so that each stimulus type was presented for 50% of the trials within a block. Block order was counterbalanced across subjects. Also, throughout the experiment, the inter-trial faces were completely unrelated and uninformative for performance of the primary ANT task. Thus, this measure provides a simple but effective way to examine effects of task-irrelevant emotional stimuli on attention.
As depicted in Fig. 1d, each trial consisted of six events: (1) inter-trial stimulus (fearful, neutral, or sad face; 50 ms); (2) first fixation period (variable 400-1300 ms); (3) cue (no cue, center cue, double cue, spatial cue; 100 ms); (4) second fixation period (400 ms); (5) simultaneously presented target and flanker stimuli (terminated at response up to 1700 ms); and (6) post-target fixation period (variable, based on the first fixation minus the reaction time for that trial). Each trial lasts for 4050 ms. Subjects rested briefly after each 192-trial block.
Efficiency of the three attentional networks, alerting, orienting, and executive attention, is determined by measuring how response times to the flanker displays are influenced by alerting cues, spatial cues, and flanker type (see Fan et al., 2002 for additional details). Efficiency of alerting is calculated as RT following no cue - RT double cue. The double cue was used because it diffuses attention between the two potential target locations while alerting the participant to the arrival of the target. Higher scores indicate greater alerting efficiency due to presence of cues. The efficiency of orienting is calculated as RT following center cue - RT spatial cue. Higher scores indicate greater orienting efficiency due to presence of spatially predictive information of one cue, while controlling for alerting effects in the other. The efficiency of executive attention is calculated in terms of conflict interference: RT to incongruent flankers - RT to congruent flankers. Higher scores indicate greater conflict interference or less efficient executive attention.
1.4. Emotional faces
Emotion stimuli were fearful, sad, and neutral faces taken from a battery developed by the Research Network on Early Experience and Brain Development (Tottenham et al., 2002, April). This battery of 646 facial expression stimuli was posed by actors of varying ethnicities. Selected faces were matched for gender and ethnicity. Stimuli were presented centrally over the fixation cross between trials of the attention task. The order of the pictures was block randomized by emotion for each subject. The faces used in this study were selected based on normative ratings of the faces for fearful and neutral facial expressions, and representation of gender and ethnicity. Because arousal properties of emotional stimuli have been related to physiological and attentional changes (Lang, 1995; Lang et al., 1998a), we examined whether fearful, sad, and neutral faces were similar in terms of perceived arousal properties. Following the attention task, participants rated each face using the Self-Assessment Mannequin technique (SAM;Lang et al., 1998b). Faces were rated on a 0-5 scale, with 5 indicating highly arousing. Faces did not statistically differ in level of arousal (Mean ratings = 2.37 for fearful, 2.53 for sad, and 2.58 for neutral faces).
1.5. Psychophysiological recording and data analysis
Electroencephalogram (EEG) activity was recorded from 64 AgCl electrodes embedded in an elasticized cap and referenced to the left mastoid. The electro-oculogram (EOG) was recorded from electrodes placed above and below the left eye and from electrodes lateral to each eye. Electrode AFz served as the ground electrode. Recordings were re-referenced off-line to an average reference. Scalp impedance for each electrode was balanced and was kept below 5 kΩ. EEG and the vertical and horizontal electro-oculogram (EOG) were amplified and bandpass filtered from .1 to 70 Hz. Data were collected continuously with a sampling rate of 1000 Hz. Continuous EEG data were low-pass filtered at 30 Hz using filtering before stimulus synchronized epochs were extracted from 200 ms before until 800 ms after the stimulus onset. The pre-stimulus period of 200 ms was subtracted for baseline correction. The raw EEG epochs were passed though both computerized artifact detection batch and visual inspection. Artifacts were corrected using BESA 5.1. After correction, those trials with EEG or EOG activity remaining above ±100 μV were excluded from further analysis. A range from 60%-99% of trials was retained across all participants.
1.6. Data reduction and ERP component evaluation
To guide selection of windows for measuring ERPs, we submitted the grand-averaged waveforms (averaged across all conditions) from 0-400 ms to a Principal Components Analysis (PCA) in the brain electrical source analysis 5.1 Source Analysis module (BESA) (Berg and Scherg, 1994). The PCA yielded three discrete time windows, which accounted for 98.5% of the total variance with maximal peaks at 200 ms, 250 ms, and 320 ms. ERPs were calculated as the peak amplitudes during time windows 20 ms before and after the peak of each component: P200 (180-220), N250 (230-270), N200 (300-340) and P300 (300-340). The P200, N250, P300 (posterior electrodes), and N200 (anterior electrodes) signals were obtained from midline electrodes FCz, Cz, and Pz, and POz and from electrodes corresponding to these four positions in the right or left hemispheres to test for laterality effects, FC3, FC4, C3, C4, P7, P8, PO3, and PO4.
To aid interpretation of the N200/P300 time window, we next computed dipole source models of the observed scalp voltage distributions. If the positive and negative voltage regions at this time point (320 ms) are a polarity reversal of one primary component, we should detect neural sources in either posterior or anterior regions; if the components are distinct, we should detect neural sources in both anterior and posterior regions. The data from all 64 electrodes were 1-30 Hz bandpass filtered. Modeling was performed on data re-referenced to the average reference across a 10-ms window around the component peaks, using a three-shell spherical head model. Energy was included as a criterion to be minimized in fitting (i.e., along with residual variance). The reported regional source solutions were stable across different starting positions.
A 3-dipole solution emerged that was sufficient to achieve a satisfactory fit across the entire sampling period of 400 ms; residual variance (RV) was 7.42%. A RV of less than 10% indicates that the solution accounted for more than 90% of the variance in the signal. The solution for the N200/P300 time window consisted of two symmetrically-constrained sources in the medial posterior cortex and a third anterior source in the area of the left medial frontal cortex (see Fig. 2). The anterior source accounted for most of the variance (RV=22.98), and the three sources together accounted for all but 8.26% of the variance in this one time window. Results support at least partial independence of anterior and negative activity.
Fig. 2.
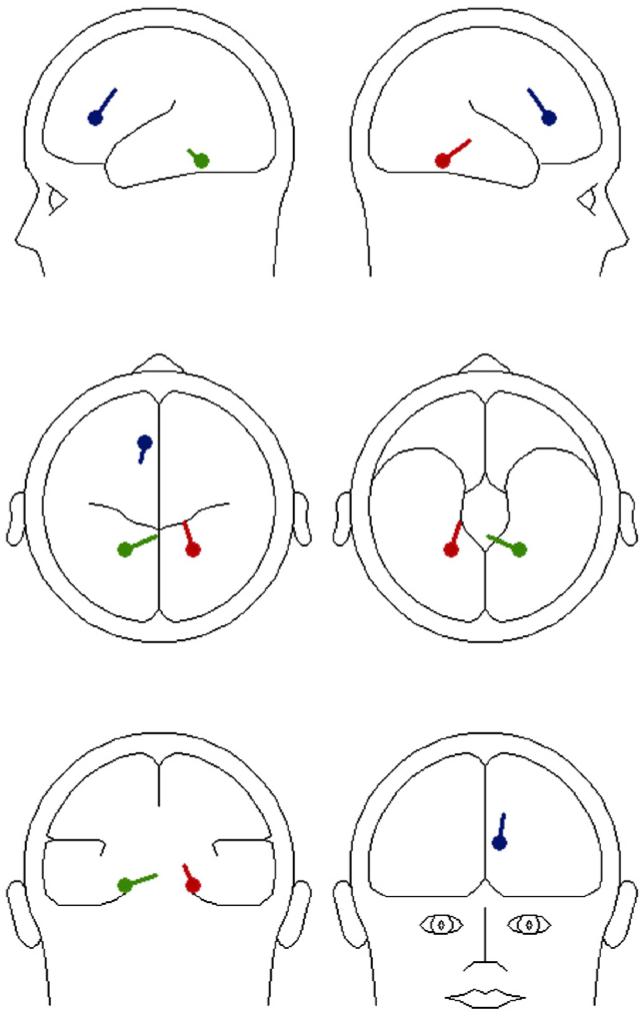
Dipole model of N200/P300 time window.
2. Results
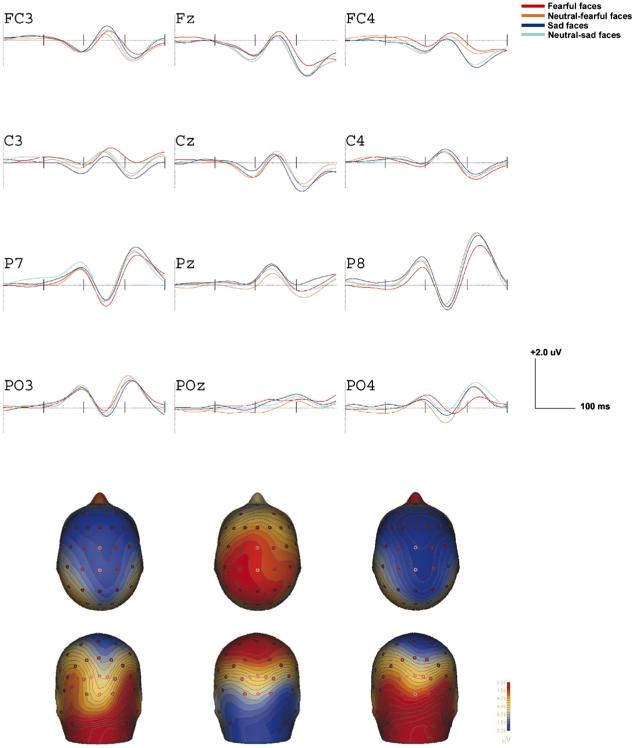
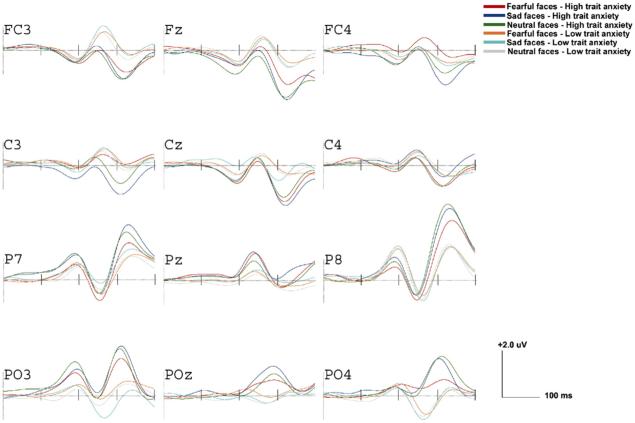
To examine whether ERPs during the first 400 ms after presentation of faces were sensitive to emotional face type and trait anxiety, we conducted four 2 × 4 × 2 × 4 MANOVAs. Trait Anxiety (low or high based on a median split) was the between-subjects variable, and Emotion (fear, neutral-f, sad, neutral-s), Laterality (right hemisphere, left hemisphere), and Electrode Placement (fronto-central, central, parietal, parietal-occipital) were the within-subjects variables. The dependent variables were peak negative amplitudes at electrodes FC3, FC4, C3, C4, P7, P8, PO3, and PO4 during each time window. Analyses were run separately for each waveform, P200, N250, N200, and P300. We report those effects in which the assumption of Sphericity was met (tested by Mauchly’s Test of Sphericity) and thus do not differ from Greenhouse-Geisser corrected effects. Significant effects were followed with LSD (least significant difference) tests or with multiple, paired t tests. Because gender differences on study variables were non-significant, gender was not included in analyses reported below. Waveforms for the entire sample are presented in Fig. 3 and topographical voltage maps in the bottom panel of Fig. 3 at 200 ms (left), 250 ms (middle), and 320 ms (right). Fig. 4 shows waveforms for high and low trait anxiety groups.
Fig. 3.
Grand-averaged ERPs at left, midline, and right electrodes. The bottom panel shows the scalp topography for each component (P200, N250, and N200/P3 from left to right). Contour lines are spaced every 0.50 μV.
Fig. 4.
Grand-averaged ERPs at left, midline, and right electrodes by trait anxiety group and emotional face type (fearful, sad, and neutral-fear/neutral-sad combined).
2.1. Effects of electrode position
As seen in Fig. 3, P200 amplitudes were maximal at posterior electrodes P7/8 (M=2.68) compared to electrodes FC3/4, C3/4, and P03/4 (Electrode F(3,14)=8.39, p<.01, η2=.64; M=0.27, 0.62, and 2.09; t-tests all p’s<.05). N250 amplitudes were also maximal in posterior electrodes P7/8 (M=-3.68) compared to electrodes FC3/4, C3/4, and PO3/4 (Electrode F(3,14)=10.02, p< <.001, η2=.68; M=-1.33, -1.53, and -1.29; all t-tests p’s<.01). There was greater anterior negativity around 320 ms at electrodes FC3/4 (-2.64) compared to P7/8 and PO3/4 (M=-0.71, and -0.10, respectively; Electrode F(3,14)= 7.01, p<.01, η2=.60; t-test all p’s<.01). Effect of Electrode on P300 amplitudes was not significant.
2.2. Effects of trait anxiety and emotion
P200 and N200 amplitudes were enhanced in the high versus low trait anxiety group (see Fig. 4). P200 amplitudes were comparable in both anxiety groups in right posterior regions, but the Electrode × Hemisphere × Trait Anxiety interaction, F(3,14)=5.99, p<.01, η2=.66, showed that in left posterior regions (P03), P200 amplitudes were greater in the high versus low trait anxiety group, M=3.52 versus 1.35, t(17)=2.96, p<.05. Also, at anterior electrodes FC3/4 N200 was enhanced in those showing high versus low trait anxiety (Electrode × Trait Anxiety F(3,14)=2.98, p<.05, η2=.16; M=-2.46 versus -0.97, t(17)=2.39, p<.05). There were no significant differences among emotional face types.
In contrast, P300 amplitudes were sensitive to emotion: the Emotion × Electrode × Hemisphere interaction, Electrode F(3,14)=2.81, p<.05, η2=.15, showed that P300 amplitudes were enhanced at right posterior sites (P8) following sad versus fearful faces, t(17)=2.01, p=.05, and compared to more posterior regions (PO4), t(17)=2.72, p<.05. Analyses with neutral faces did not reach significance. As seen in Fig. 4, this effect was enhanced in the high versus low trait anxiety group, but the overall analysis did not reach significance, p=.10.
2.3. Attention performance
Before examining links between ERPs and attention performance, we examined whether emotion face type and trait anxiety were related to attention performance in three domains, alerting, orienting, and executive attention using a 4 (Emotion) × 2 (Trait Anxiety group: low or high) ANOVA for each attention system. There was an interaction between Trait Anxiety and Emotion that just missed significance for orienting, F(3,14)=3.52, p<.06, showing that within the high trait anxiety group, orienting was more efficient following fearful versus neutral-f faces (M=52.18, SD=32.30 versus M=20.44, SD=20.98; t(8)=2.18, p=.06), but that those showing high versus low trait anxiety oriented more efficiently following sad faces, (M=55.46, SD=39.85 versus M=14.94, SD=29.58; t(17)=2.03, p=.05). For the entire sample, F(3,14)=3.59, p<.05, executive attention was enhanced following fearful versus neutral-f faces (M=107.13, SD=47.49 versus M=131.25, SD=71.17; t(17)=-2.28, p<.05). Thus, without taking ERP responses into account, orienting and executive attention were enhanced following fearful and sad faces. Alerting was not modulated by emotional factors.
2.4. Correlations between ERPs and attention performance
The goal of the next set of analyses was to assess whether the degree to which participants processed emotional faces as measured by ERPs was associated with attention performance. We examined correlations between attention scores and ERPs following fearful and sad faces at maximal left (FC3 or P7) and right (FC4 and P8) electrode sites. Correlations were conducted for the entire sample but none reached significance. Correlations were then conducted for the low and high trait anxiety groups separately to examine whether links between ERPs and attention varied by trait anxiety group. Recall that for positive amplitudes (P200, P300) correlations should be interpreted as usual, but that for negative amplitudes, the interpretation of correlations should be inverted.
In the low trait anxiety group increases in N250 following fearful faces were linked to greater alerting (r=-.85, p<.001), and, although marginally significant, increases in N200 following fearful faces were linked to greater orienting (r=-.64, p<.07). In contrast to the low trait anxiety group and analyses of attention performance alone, for the high trait anxiety group, increased processing of fearful and sad faces was associated with reduced attention performance. N250 following fearful and sad faces was linked to greater conflict interference and thus reduced executive attention (r=-.68, p<.05 and r=-.85, p<.001). In addition, although marginally significant, P200 following fearful faces was linked to reduced orienting (r=-.60, p<.07) and P300 following sad faces was linked to greater conflict interference (r=.64, p<.07). All correlations were right lateralized.
3. Discussion
The present study showed that early-developing ERP responses to emotional faces were enhanced for negative versus neutral faces and for those showing high versus low trait anxiety. Although behavioral results showed improved attention performance following presentation of negative emotional faces, even among those showing relatively high trait anxiety, ERP findings showed that the degree to which faces recruited attentional resources among the high trait anxiety group was linked to decrements in orienting and executive attention. Taken together, this suggests that the regulation of attention in multiple domains is influenced by rapid and automatic aspects of emotional face processing and that these links are sensitive to individual differences in anxiety. Results further suggest that ERPs provide a powerful tool for capturing aspects of emotion-attention interactions that behavioral studies alone may miss.
Modulation of ERP responses showed emotional specificity. P300 amplitudes were enhanced following sad faces, whereas P200 and N200 were enhanced for the high trait anxiety group. Therefore, affective discrimination around 300 ms was similar for all, but high trait anxiety specifically enhanced initial detection (P200) and top-down control of emotional stimuli (N200). This suggests that even trait anxiety in a normative range bolsters monitoring of emotional information and biases competition for processing resources to favor motivationally-significant stimuli (Compton, 2003). Indeed, because many of these effects were right lateralized, the “emotional surveillance system” reflecting fear and threat processing is implicated (Bear, 1983; Nitschke et al., 2000). Overall, this suggests that in normative and perhaps clinical levels of anxiety, emotional capture of attention is extremely rapid.
In contrast to previous studies, however, the N250 was not enhanced in emotional versus neutral faces (Sato et al., 2001). If the N250 is analogous to the face N170, as suggested by other studies and by its topographical distribution (Taylor et al., 2004; Sato et al., 2001), it may be that affective characteristics of the face are less significant than configural characteristics in eliciting this ERP response (Eimer and Holmes, 2002). Another possibility is that the very brief duration of the faces in the present study (50 ms) decreased the emotional modulation of ERP responses overall because the affective properties of the faces may have been less salient or less detectable.
What implication did these stages of emotional face processing have for attention performance? In contrast to previous research on negative emotions and attention performance (Eastwood et al., 2003), behavioral findings showed enhanced orienting and executive attention following negative emotional faces, particularly among those reporting relatively high trait anxiety. However, a very different picture emerged when ERP measures of emotional face processing and trait anxiety were taken into account. N250 responses were consistently linked to attention performance. As N250 amplitudes increased, orienting and conflict resolution became less efficient in the high trait anxiety group, but alerting became more efficient in the low trait anxiety group. Other correlations were marginally significant but suggest a similar pattern.
Due to the small sample size, results must be interpreted with caution and require replication. However, it appeared that processing negative emotional faces may “jump-start” alerting among the less anxious, but disrupt orienting and executive attention among the more anxious (Derryberry and Rothbart, 1997; Gray and McNaughton, 2000). The specificity of these effects implies that anxious arousal in a normative range may primarily influence orienting and executive attention; in clinical groups, anxious arousal might have a more generalized and intensified negative impact (Compton, 2003). These patterns could also be interpreted in terms of competition and resource allocation models (Meinhardt and Pekrun, 2003) in which the balance between affective state and emotional processing biases attention. If participants already show relatively high anxiety, further prioritizing emotional processing could over-tax the resources available for shifting and inhibiting attention, resulting in reduced performance (Compton, 2003; Hanoch and Vitouch, 2004). Low reactive individuals should have more available resources, and thus enhanced emotional processing might instead motivate and bolster general alertness. To better disentangle these effects, future research should assess emotional arousal in multiple ways, including mood inductions, examining anxious state in comparison with trait (Rutherford et al., 2004) and testing the impact of negative versus positive emotions on multiple attention systems (Ashby et al., 1999; Fredrickson and Branigan, 2005).
The present study introduced a novel methodology in that an emotional factor was integrated into a well-validated attention task yielding three distinct attention measures. This provides an excellent opportunity to simultaneously examine the impact of emotional processing on attention performance in multiple domains. Several characteristics of this experimental task should be taken into account when interpreting results. Because faces were presented very briefly and were essentially mild emotional distracters that needed to be ignored during the task, findings support the contention that the emotional capture of attention is extremely rapid and recruits both automatic and relatively voluntary attentional processes (Lewis, 2000; Scherer, 2000). Second, faces were randomly alternating in terms of emotional valence, making it unclear how results might have differed if participants took part in extended emotional processing or in the context of a sustained, block design. Future research should directly compare block versus event-based designs, and examine a more extended time course of emotional processing in order to delineate effects of automatic and effortful emotion processing, as well as earlier and later processing biases on distinct attention systems (Compton, 2003). These factors might explain why we did not detect a P1 orienting response or a posterior N170 (Righart and de Gelder, 2006; Batty and Taylor, 2003): due to the brief duration and distracting nature of emotional faces, correctly directed or selective spatial attention may not have been consistently elicited (Mangun, 1995).
In summary, all participants showed increased processing of negative emotional faces, and individuals evidencing high trait anxiety further showed enhanced processing and monitoring of emotional faces through posterior and anterior attention mechanisms, which resulted in compromised attention. The opposite occurred for the low trait anxiety group, suggesting that when anxious mood is reduced, emotional processing can facilitate attention performance. Results provide insights into the time course of the rapid emotional capture of attention related to anxiety and into ERP markers for the emotional modulation of attention.
Acknowledgements
We thank James Gordon and Bruce D. McCandliss for their roles in the development of the study, Gerard Bruder for his feedback on earlier versions of this manuscript, and Steve Alvarez, Melville Malone, and Nicole Amador for their assistance throughout the project. This research was supported by NIH Grants 5S06GM060654-04 and K01 MH075764-02, awarded to the first author.
Footnotes
State anxiety was also assessed at baseline and four additional times following each block of the attention task; baseline and subsequent assessments did not significantly differ suggesting that the task did not induce or modulate anxious mood. There was a high correlation between state and trait anxiety (r=.72, p<.001). Because we were interested in trait tendencies to experience anxious arousal, however, we did not co-vary out state anxiety because this might have inaccurately reduced effects.
References
- Ashby FG, Isen AM, Turken U. A neuropsychological theory of positive affect and its influence on cognition. Psychological Review. 1999;106:529–550. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- Batty M, Taylor MJ. Early processing of the six basic facial emotional expressions. Brain Research. Cognitive Brain Research. 2003;17:613–620. doi: 10.1016/s0926-6410(03)00174-5. [DOI] [PubMed] [Google Scholar]
- Bear DM. Hemispheric specialization and the neurology of emotion. Archives of Neurology. 1983;40:195–202. doi: 10.1001/archneur.1983.04050040025003. [DOI] [PubMed] [Google Scholar]
- Beck AT, Clark DA. An information processing model of anxiety: automatic and strategic processes. Behaviour Research and Therapy. 1997;35:49–58. doi: 10.1016/s0005-7967(96)00069-1. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg P, Scherg M. A multiple source approach to the correction of eye artifacts. Electroencephalography and Clinical Neurophysiology. 1994;90:229–241. doi: 10.1016/0013-4694(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Campanella S, Gaspard C, Debatisse D, Bruyer R, Crommelinck M, Guerit JM. Discrimination of emotional facial expressions in a visual oddball task: an ERP study. Biological Psychology. 2002;59:171–186. doi: 10.1016/s0301-0511(02)00005-4. [DOI] [PubMed] [Google Scholar]
- Carretié L, Mercado F, Tapia M, Hinojosa JA. Emotion, attention, and the ‘negativity bias’, studied through event-related potentials. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2001;41:75–85. doi: 10.1016/s0167-8760(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Compton RJ. The interface between emotion and attention: a review of evidence from psychology and neuroscience. Behavioral and Cognitive Neuroscience Reviews. 2003;2:115–129. doi: 10.1177/1534582303255278. [DOI] [PubMed] [Google Scholar]
- Correll J, Urland GR, Ito TA. Event-related potentials and the decision to shoot: the role of threat perception and cognitive control. Journal of Experimental Social Psychology. 2006;42:120–128. [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, Carver L, Panagiotides H, McPartland J. Young children with autism show atypical brain responses to fearful versus neutral facial expressions of emotion. Developmental Science. 2004;7:340–359. doi: 10.1111/j.1467-7687.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Reactive and effortful processes in the organization of temperament. Development and Psychopathology. 1997;9:633–652. doi: 10.1017/s0954579497001375. [DOI] [PubMed] [Google Scholar]
- Dien J, Spencer KM, Donchin E. Parsing the late positive complex: mental chronometry and the ERP components that inhabit the neighborhood of the P300. Psychophysiology. 2004;41:665–678. doi: 10.1111/j.1469-8986.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- Easterbrook JA. The effect of emotion on cue utilization and the organization of behavior. Psychological Review. 1959;66:183–201. doi: 10.1037/h0047707. [DOI] [PubMed] [Google Scholar]
- Eastwood JD, Smilek D, Merikle PM. Negative facial expression captures attention and disrupts performance. Perception & Psychophysics. 2003;65:352–358. doi: 10.3758/bf03194566. [DOI] [PubMed] [Google Scholar]
- Eger E, Jedynak A, Iwaki T, Skrandies W. Rapid extraction of emotional expression: evidence from evoked potential fields during brief presentation of face stimuli. Neuropsychologia. 2003;41:808–817. doi: 10.1016/s0028-3932(02)00287-7. [DOI] [PubMed] [Google Scholar]
- Eimer M, Holmes A. An ERP study on the time course of emotional face processing. NeuroReport. 2002;13:427–431. doi: 10.1097/00001756-200203250-00013. [DOI] [PubMed] [Google Scholar]
- Eimer M, Holmes A, McGlone FP. The role of spatial attention in the processing of facial expression: an ERP study of rapid brain responses to six basic emotions. Cognitive, Affective and Behavioral Neuroscience. 2003;3:97–110. doi: 10.3758/cabn.3.2.97. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. NeuroImage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Fenske MJ, Eastwood JD. Modulation of focused attention by faces expressing emotion: evidence from flanker tasks. Emotion. 2003;3:327–343. doi: 10.1037/1528-3542.3.4.327. [DOI] [PubMed] [Google Scholar]
- Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? Journal of Experimental Psychology. General. 2001;130:681–700. [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Branigan C. Positive emotions broaden the scope of attention and thought-action repertoires. Cognition and Emotion. 2005;19:313–332. doi: 10.1080/02699930441000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR. Integration of emotion and cognitive control. Current Directions in Psychological Science. 2004;13:46–48. [Google Scholar]
- Gray JA, McNaughton N. The Neuropsychology of Anxiety. 2nd ed. Oxford; New York, NY: 2000. [Google Scholar]
- Hanoch Y, Vitouch O. When less is more: information, emotional arousal and the ecological reframing of the Yerkes-Dodson law. Theory & Psychology. 2004;14:427–452. [Google Scholar]
- Kliegel M, Horn AB, Zimmer H. Emotional after-effects on the P3 component of the event-related brain potential. International Journal of Psychology. 2003;38:129–137. [Google Scholar]
- Ladouceur CD, Dahl RE, Williamson DE, Birmaher B, Axelson DA, Ryan ND, et al. Processing emotional facial expressions influences performance on a Go/NoGo task in pediatric anxiety and depression. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47:1107–1115. doi: 10.1111/j.1469-7610.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: studies of motivation and attention. American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion and attention: stop, look, and listen. Cahiers de Psychologie Cognitive/Current Psychology of Cognition. 1998a;17:997–1020. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN.International Affective Picture System (IAPS): Instruction Manual and Affective Ratings (Tech. Rep.No. A-4) 1998bUniversity of Florida, The Center for Research in Psychophysiology; Gainsville, FL [Google Scholar]
- Leith KP, Baumeister RF. Why do bad moods increase self-defeating behavior? Emotion, risk tasking, and self-regulation. Journal of Personality and Social Psychology. 1996;71:1250–1267. doi: 10.1037//0022-3514.71.6.1250. [DOI] [PubMed] [Google Scholar]
- Lewis MD, editor. Emotional Self-organization at Three Time Scales. Cambridge University Press; New York, NY: 2000. [Google Scholar]
- Luu P, Collins P, Tucker DM. Mood, personality, and self-monitoring: negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. Journal of Experimental Psychology. General. 2000a;129:43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- Luu P, Flaisch T, Tucker DM. Medial frontal cortex in action monitoring. Journal of Neuroscience. 2000b;20:464–469. doi: 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangun GR. Neural mechanisms of visual selective attention. Psychophysiology. 1995;32:4–18. doi: 10.1111/j.1469-8986.1995.tb03400.x. [DOI] [PubMed] [Google Scholar]
- Meinhardt J, Pekrun R. Attentional resource allocation to emotional events: an ERP study. Cognition and Emotion. 2003;17:477–500. doi: 10.1080/02699930244000039. [DOI] [PubMed] [Google Scholar]
- Mogg K, Mathews A, Eysenck M. Attentional bias to threat in clinical anxiety states. Cognition and Emotion. 1992;6:149–159. [Google Scholar]
- Nieuwenhuis S, Yeung N, Van Den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cognitive, Affective and Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Miller GA. Anxiety, stress, and cortical brain function. In: Borod JC, editor. The Neuropsychology of Emotion. Oxford University Press; Oxford, England: 2000. pp. 298–319. [Google Scholar]
- Pizzagalli D, Regard M, Lehmann D. Rapid emotional face processing in the human right and left brain hemispheres: An ERP study. Neuroreport: For Rapid Communication of Neuroscience Research. 1999;10:2691–2698. doi: 10.1097/00001756-199909090-00001. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Tolley-Schell SA. Selective attention to facial emotion in physically abused children. Journal of Abnormal Psychology. 2003;112:323–338. doi: 10.1037/0021-843x.112.3.323. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Righart R, de Gelder B. Context influences early perceptual analysis of faces—an electrophysiological study. Cerebral Cortex. 2006;16:1249–1257. doi: 10.1093/cercor/bhj066. [DOI] [PubMed] [Google Scholar]
- Rokke PD, Arnell KM, Koch MD, Andrews JT. Dual-task attention deficits in dysphoric mood. Journal of Abnormal Psychology. 2002;111:370–379. [PubMed] [Google Scholar]
- Rutherford EM, MacLeod C, Campbell LW. Negative selectivity effects and emotional selectivity effects in anxiety: differential attentional correlates of state and trait variables. Cognition and Emotion. 2004;18:711–720. [Google Scholar]
- Sato W, Kochiyama T, Yoshikawa S, Matsumura M. Emotional expression boosts early visual processing of the face: ERP recording and its decomposition by independent component analysis. NeuroReport. 2001;12:709–714. doi: 10.1097/00001756-200103260-00019. [DOI] [PubMed] [Google Scholar]
- Scherer KR. Emotions as episodes of subsystems synchronization driven by nonlinear appraisal processes. In: D Lewis, M., I Granic., editors. Emotion, Development, and Self-organization: Dynamic Systems Approaches to Emotional Development. Cambridge University Press; New York, NY: 2000. pp. 70–99. [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. Attention and emotion: an ERP analysis of facilitated emotional stimulus processing. Neuroreport: For Rapid Communication of Neuroscience Research. 2003;14:1107–1110. doi: 10.1097/00001756-200306110-00002. [DOI] [PubMed] [Google Scholar]
- Schutter DJLG, de Haan EHF, van Honk J. Functionally dissociated aspects in anterior and posterior electrocortical processing of facial threat. International Journal of Psychophysiology. 2004;53:29–36. doi: 10.1016/j.ijpsycho.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Smith NK, Cacioppo JT, Larsen JT, Chartrand TL. May I have your attention, please: electrocortical responses to positive and negative stimuli. Neuropsychologia. 2003;41:171–183. doi: 10.1016/s0028-3932(02)00147-1. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory Manual. Mind Garden Inc; Redwood City, CA: 1983. [Google Scholar]
- Taylor MJ, Batty M, Itier RJ. The faces of development: a review of early face processing over childhood. Journal of Cognitive Neuroscience. 2004;16:1426–1442. doi: 10.1162/0898929042304732. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Borscheid A, Ellertsen K, Marcus DJ, Nelson CA. Categorization of facial expressions in children and adults: establishing a larger stimulous set; Paper Presented at the Meeting of Cognitive Neuroscience Society; San Francisco. April.2002. [Google Scholar]