Abstract
Phrenic long-term facilitation (LTF) has been extensively studied in anesthetized animals under well-defined physiological conditions but the factors underlying its possible manifestation under clinically relevant conditions are not well understood. Here, we examined the stability of LTF in the face of hypercapnic or hypocapnic challenges in anesthetized, paralyzed and mechanically ventilated rats. Sixty minutes after induction of phrenic LTF by intermittent hypoxia the animal was exposed to one of four conditions for 5 min with or without positive end-expiratory pressure (PEEP, 3-4 cmH2O): hypocapnic apnea, hypocapnia (5 torr below resting level), 5% CO2 and 10% CO2. LTF at 60 min post-intermittent hypoxia was ∼39% above baseline. Following the above CO2 tests, LTF almost invariably returned to the corresponding pre-test level after recovery for 20 min. The only exception was the combination of hypocapnic apnea and PEEP, which resulted in a marked decrease in mean arterial pressure (to 38-55 mmHg) during test and a subsequent paradoxical sustained attenuation of LTF (to ∼8% above baseline) even after mean arterial pressure had fully recovered. The results suggest that LTF, once developed, is highly robust to changes in CO2 levels and is attenuated only after severe hypotension secondary to excessive hyperventilation under PEEP.
Keywords: Intermittent hypoxia, long-term facilitation, apnea, plasticity, hypocapnia, hypotension, PEEP
1. Introduction
Phrenic long-term facilitation (LTF) is a serotonin- and NMDA receptor- dependent augmentation of phrenic nerve activity following intermittent hypoxia in anesthetized animals (Bach & Mitchell, 1996; McGuire et al., 2004, 2005). Recently, the potential clinical relevance of LTF has received increasing attention. LTF can be elicited during NREM sleep in patients with airway flow limitation or obstructive sleep apnea (OSA) (Aboubakr et al., 2001; Babcock et al., 2003). It has been suggested that loss of LTF might be related to the onset of OSA (Behan et al., 2002).
OSA patients experience profound hypercapnia and hypoxia during airway obstruction, and to obviate such adverse effects these patients are often treated with all-night continuous positive airway pressure (CPAP) with consequent elevation of end-expiratory lung volume. Positive end-expiratory pressure (PEEP) is also routinely administered in mechanical ventilation, where a mild degree of hyperventilation is sometimes allowed in order to suppress spontaneous respiratory effort as an alternative to the use of sedatives and muscle relaxants. The combination of changing CO2 levels, apnea and elevated lung volume could potentially affect the manifestation of LTF in these patients.
To test this hypothesis, we investigated whether the maintenance of LTF was influenced by changes in CO2 levels, hyperventilation and PEEP in an anesthetized animal model. Our results showed that LTF, once developed, evidenced remarkable robustness except after excessive hyperventilation under PEEP.
2. Methods
Experiments were conducted on adult male Sprague-Dawley rats (Charles River, Wilmington, MA). All protocols had been approved by Massachusetts Institute of Technology Committee on Animal Care.
2.1 General procedures
The rats were anesthetized (urethane 1.5 g/kg, i.p. supplemented as needed), vagotomized, paralyzed (pancuronium bromide, 2.5 mg/kg, i.v. supplemented as needed) and mechanically ventilated (CWE Inc., Ardmore, PA, USA). The left femoral artery and vein were cannulated for blood pressure measurement and drug/fluid administration, respectively. Rectal temperature was maintained near 37 °C with a servo-controlled heated blanket. End-tidal CO2 partial pressure (PETCO2) was monitored using a CO2 analyzer (CapStar-100, CWE Inc. Ardmore, PA, USA). Inspired gases were 40% O2 (balance N2) during baseline and other non-hypoxia periods. At the end of experiments, rats were euthanized via urethane overdose.
The left phrenic nerve was isolated via a ventral approach and placed on a bipolar platinum wire electrode. The phrenic nerve activity was filtered, amplified and integrated with a Paynter filter (time constant 15 msec). The integrated phrenic signals were digitized at 10 kHz and acquired with computer software (LabView 7.0, National Instruments Corporation, USA) and analyzed.
2.2 Experimental protocols
Induction of LTF
Following 1-hour stabilization the CO2-recruitment threshold (Boden et al., 1998) was determined and baseline PETCO2 was set to be 3 torr above this recruitment threshold. The latter is defined as the PETCO2 at which respiratory rhythmic activity resumes from previous, hypocapnic silence in phrenic nerve recording when PETCO2 fell below the apneic threshold (which was typically lower than the recruitment threshold in our vagotomized animals). Following baseline phrenic nerve activity measurement, one of 3 protocols was applied: 1) intermittent hypoxia (IH): three 5-min episodes of 12% O2 (isocapnic) separated by 5-min intervals of 40% O2; 2) sustained hypoxia (SH): one 25-min episode of 12% O2; and 3) time control (TC): no hypoxia given.
CO2 tests
At 60 min post-hypoxia (P60) (or equivalent in time controls) the phrenic nerve responses to one of the following tests were measured: 5 min of hypercapnia (by adding 5% or 10% CO2 to inhalate), hypocapnia (PETCO2 at ∼5 torr below baseline) or hypocapnic apnea (no phrenic activity) attained by increasing the ventilator frequency until PETCO2 fell below the apneic threshold, to ∼7 torr below baseline. Recovery of phrenic activity was monitored at 20 min after termination of the tests. Each rat was given either the hypercapnic (5%/10% CO2) or the hypocapnic (low PETCO2 /apnea) tests.
Positive end-expiratory pressure
The above CO2 tests were carried out first without PEEP, then with PEEP introduced by submerging the expiratory tube of the ventilator circuitry into water (3-4 cmH2O).
2.3 Data Analysis
Amplitude of integrated phrenic activity was normalized relative to baseline. In the IH group, hypoxic response data were recorded during the last 2 min of hypoxia when phrenic amplitude reached a plateau, and were averaged over 3 hypoxia episodes. In the SH group, data were recorded during the last 2 min of the 25-min episode and averaged. The TC data were measured during the period equivalent to hypoxia exposures in the IH and SH groups. Data were analyzed in rats whose mean arterial pressure was kept >80 mmHg in the post-hypoxia hyperoxia period (up to P60).
Differences in phrenic activity and blood pressure values between varying experimental conditions were analyzed using one-way ANOVA at 5% significance level. All values are expressed as means ± SE.
3. Results
Baseline recruitment threshold values were similar in TC (n=7), SH (n=8) and IH (n=12) groups (PETCO2: 30.7 ± 0.9, 32.0 ± 0.8 and 31.9 ± 0.6 torr, respectively; P=0.452). Baseline mean arterial pressures were also similar (118.8 ± 3.7, 116.7 ± 6.8 and 111.5 ± 2.6 torr; P= 0.460) in TC (n=7), SH (n=8) and IH (n=12) groups. The phrenic responses to 12% O2 were not significantly different (P=0.552) between SH (123.3 ± 7.3 % above baseline) and IH (134.3 ± 13.9) groups.
As expected, phrenic activity at P60 was significantly increased (average 38.8 ± 4.6% above baseline, see Figs. 1 and 2 LTF columns) in the IH group but not in the TC and SH groups. In 3 separate rats not subjected to other test protocols, LTF persisted up to 90 min post-IH with similar magnitude compared to P60 (data not shown).
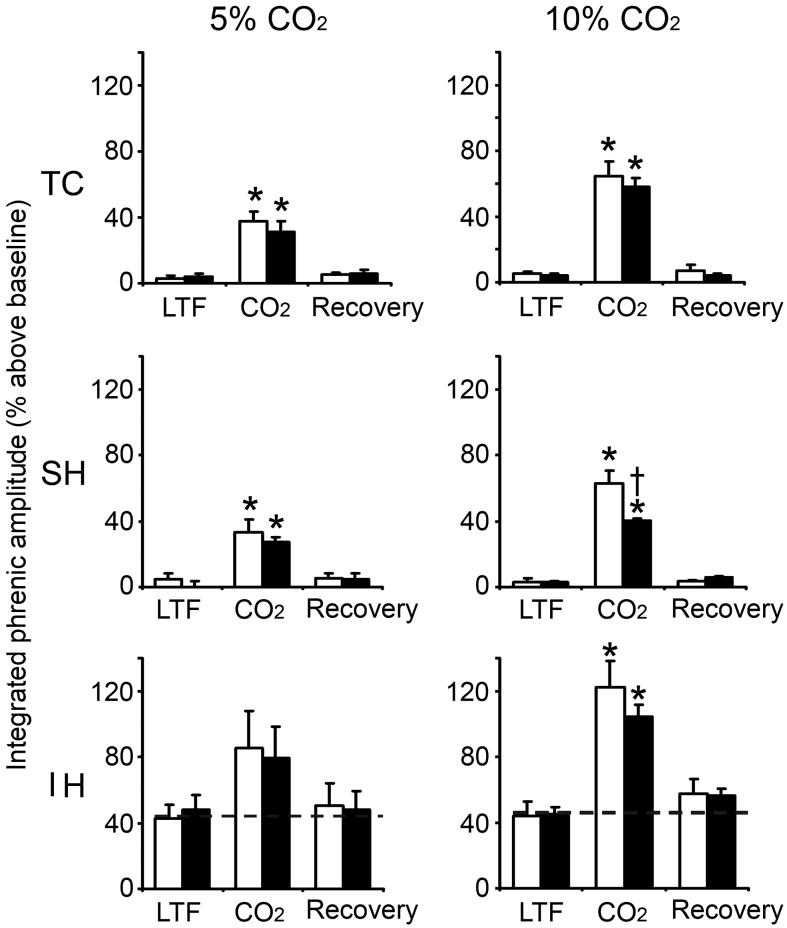
Fig. 1.
Phrenic LTF at 60 min post-hypoxia in TC, SH and IH groups before, during and after hypercapnic test without PEEP (white column) or with PEEP (black column). Left panel: Effect of 5% CO2 on LTF, n=3 for TC, n=4 for SH and IH control and n=5 for IH PEEP. Right panel: Effect of 10% CO2 on LTF, n=3 for TC, n=4 for SH and n-5 for IH. Data (means ± SE) expressed as percentage above baseline. n=4 in all groups except IH with PEEP (n=5). * indicates significant difference from baseline; † indicates significant difference from non-PEEP.
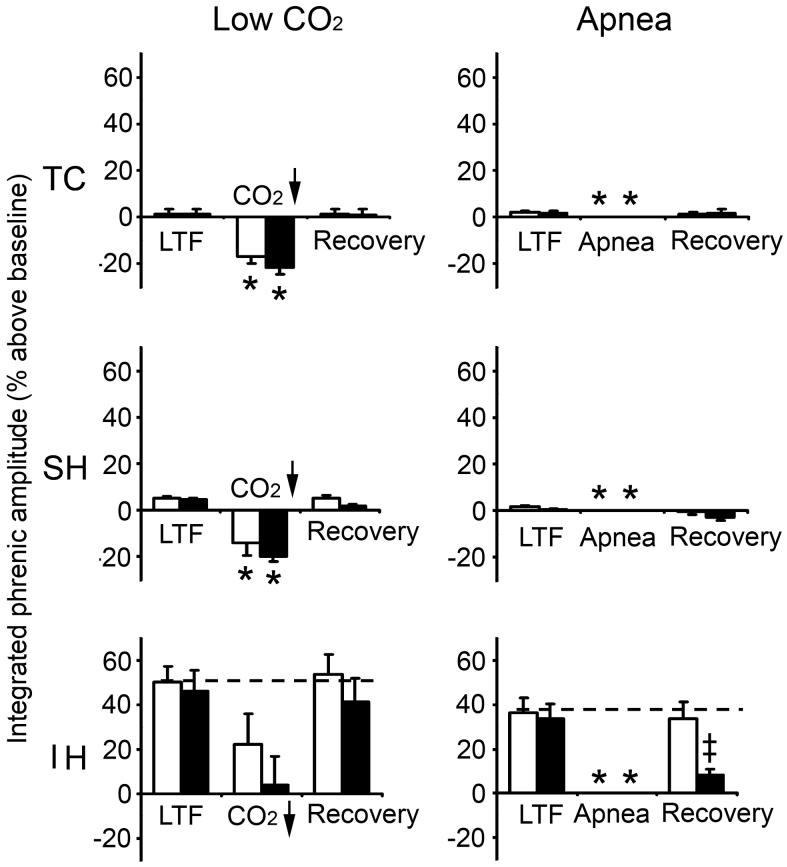
Fig. 2.
Phrenic LTF at 60 min post-hypoxia in TC, SH and IH groups before, during and after hypocapnic test without PEEP (white column) or with PEEP (black column). Left panel: Effect of low CO2 (5 torr below control) on LTF, n=4 for all groups except IH with PEEP (n=5). Right panel: Effect of apnea on LTF. n=4 for all groups except IH (n=7). Data (means ± SE) expressed as percentage above baseline. The apnea data (-100% below baseline) are not plotted. * indicates significant difference from baseline; ‡ indicates significant difference from non-PEEP and LTF.
In all (IH, SH, TC) groups, phrenic activity significantly increased or decreased in a dose-dependent manner during hypercapnic (5% or 10% CO2) or hypocapnic challenge (5 torr below baseline PETCO2) under PEEP or non-PEEP conditions. In all cases phrenic activity returned to the corresponding pre-test levels after 20-min recovery (Figs. 1, 2).
By contrast, during hypocapnic apneic challenge following IH, SH or TC, phrenic activity vanished under both PEEP and non-PEEP conditions. In almost all cases, however, phrenic activity also returned to the corresponding pre-test levels after 20-min recovery (Figs. 2). The only exception was the IH group subjected to hypocapnic apnea under PEEP, where phrenic activity failed to return to pre-apneic LTF level after 20-min recovery (Fig. 2, Panel B, IH graph).
In all groups, mean arterial blood pressure showed little or no changes during hypercapnic or hypocapnic challenges under PEEP or non-PEEP conditions compared to corresponding LTF and recovery periods (Table 1). However, in all groups mean arterial blood pressure was slightly decreased (to 92-98 mmHg) during hypocapnic apnea under non-PEEP condition and was markedly decreased (to 38-55 mmHg) during hypocapnic apnea under PEEP. In all cases mean arterial pressure was not significantly different between the LTF and recovery (pre- and post-test) periods.
Table 1.
Mean arterial pressures before (LTF), during and after (recovery) CO2 tests with and without PEEP.
| Time Control | Sustained Hypoxia | Intermittent Hypoxia | ||||
|---|---|---|---|---|---|---|
| CO2 Test | non-PEEP | PEEP | non-PEEP | PEEP | non-PEEP | PEEP |
| Hypercapnia | ||||||
| LTF | 104.3 ± 3.8 (3) | 96.0 ± 1.5 (3) | 101.3 ± 7.3 (4) | 92.0 ± 5.9 (4) | 115.3 ± 2.3 (4) | 104.2 ± 5.8 (5) |
| 5% CO2 | 110.7 ± 4.7 (3) | 97.7 ± 4.3 (3) | 105.0 ± 8.7 (4) | 93.0 ± 4.8 (4) | 119.5 ± 4.8 (4) | 109.0 ± 4.6 (5) |
| Recovery | 105.0 ± 4.6 (3) | 96.7 ± 1.7 (3) | 102.8 ± 8.0 (4) | 92.3 ± 6.6 (4) | 116.5 ± 3.9 (4) | 106.4 ± 8.4 (5) |
| LTF | 105.3 ± 5.4 (3) | 95.7 ± 3.8 (3) | 105.5 ± 8.4 (4) | 97.5 ± 11.9 (4) | 111.0 ± 6.2 (5) | 101.0 ± 4.4 (5) |
| 10% CO2 | 117.3 ± 6.7 (3) | 103.7 ± 5.2 (3) | 123.0 ± 11.5 (4) | 106.3 ±11.9 (4) | 130.2 ± 8.9 (5) | 116.6 ± 3.0 (5) |
| Recovery | 108.0 ± 6.1 (3) | 94.3 ± 2.9 (3) | 109.5 ± 8.3 (4) | 97.8 ± 9.9 (4) | 113.8 ± 6.1 (5) | 101.8 ± 4.5 (5) |
| Hypocapnia | ||||||
| LTF | 109.8 ± 4.9 (4) | 102.8 ± 11.3 (4) | 103.5 ± 4.7 (4) | 95.8 ± 2.2 (4) | 105.5 ± 6.4 (4) | 94.8 ± 1.4 (5) |
| Low CO2 | 102.8 ± 5.0 (4) | 91.0 ± 10.9 (4) | 99.0 ± 4.2 (4) | 87.5 ± 1.3 (4) | 102.8 ± 5.4 (4) | 88.2 ± 1.5†‡ (5) |
| Recovery | 108.0 ± 5.1 (4) | 104.8 ± 12.2 (4) | 103.3 ± 4.5 (4) | 95.3 ± 1.6 (4) | 105.5 ± 5.9 (4) | 94.6 ± 1.5 (5) |
| LTF | 108.0 ± 5.1 (4) | 103.3 ± 12.7 (4) | 98.0 ± 4.5 (4) | 90.3 ± 4.1 (4) | 103.3 ± 3.8 (7) | 92.7 ± 2.0† (7) |
| apnea | 98.3 ± 9.8 (4) | 55.0 ± 14.6†‡ (4) | 92.0 ± 3.4 (4) | 38.3 ± 6.9†‡ (4) | 93.3 ± 5.3 (7) | 45.6 ± 1.7†‡ (7) |
| Recovery | 107.0 ± 4.6 (4) | 100.8 ± 12.5 (4) | 100.3 ± 4.2 (4) | 89.3 ± 3.8 (4) | 101.5 ± 3.7 (7) | 91.9 ± 2.9 (7) |
Data are means ± SE (number of rats), in mmHg.
indicates significant difference from non-PEEP
indicates significant difference from LTF and recovery.
4. Discussion
The present results confirmed that phrenic LTF is induced by intermittent hypoxia but not sustained hypoxia, as reported previously (Dwinell et al., 1997; Baker & Mitchell, 2000). Remarkably, our results showed that LTF, once established, remained largely stable and was robust to pronounced hypercapnic or hypocapnic challenges with or without PEEP. The only exception was hypocapnic apneic challenge with PEEP where recovery of LTF was greatly attenuated.
The sustained maintenance of LTF and its striking robustness to changes in CO2 level and PEEP is in contrast to the reported variability of LTF induction (Jansen & Fregosi, 2000; Baker & Mitchell, 2001). This is in agreement with the notion that different factors underlie the induction and maintenance of LTF (Fuller et al., 2001).
The mechanism underlying the attenuation of LTF (from ∼39% to ∼8% above baseline) after hypocapnic apnea with PEEP is unclear. Since the animals were vagotomized, effects of changes in lung-volume feedback secondary to the increased mechanical ventilation and PEEP may be ruled out. It is also unlikely that the hypocapnic condition per se was the culprit, since neither moderate hypocapnia (5 torr below baseline) nor hypocapnic apnea without PEEP attenuated the LTF. Although apnea may disrupt LTF-related changes in connectivity of brainstem respiratory neurons (Morris et al., 2000), it was found to have little or no effect on LTF maintenance except when paired with PEEP.
Everything considered, a likely factor contributing to the observed attenuation of LTF appears to be the marked decrease in mean arterial pressure secondary to hypocapnic apnea with PEEP. The sustained attenuation of LTF even after mean arterial pressure had fully recovered (Table 1) is surprising because a decrease in arterial baroreceptor activation alone, if anything, should disinhibit respiration hence augmenting phrenic activity (Grunstein et al., 1975). Apparently, the mechanism underlying the maintenance of LTF is susceptible to severe hypotension secondary to hyperventilation under PEEP. Increased mechanical ventilation and PEEP are known to have adverse hemodynamic effects (Lin, 1981) whereas hypocapnia may elicit vasodilation (in most vascular beds except the heart and brain) via reversal of the sympathetic chemoreflex (Guyenet and Koshiya, 1995), further aggravating the hypotension. The present results suggest that these cardiopulmonary risk factors when acting in combination may also attenuate LTF.
Acknowledgments
We thank Dr. L. Ling for helpful discussions. This work was supported by National Institutes of Health grants HL067966 and HL072849.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboubakr SE, Taylor A, Ford R, Siddiqi S, Badr MS. Long-term facilitation in obstructive sleep apnea patients during NREM sleep. J Appl Physiol. 2001;91(6):2751–7. doi: 10.1152/jappl.2001.91.6.2751. [DOI] [PubMed] [Google Scholar]
- Babcock M, Shkoukani M, Aboubakr SE, Badr MS. Determinants of long-term facilitation in humans during NREM sleep. J. Appl. Physiol. 2003;94(1):53–59. doi: 10.1152/japplphysiol.00476.2002. [DOI] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir. Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baker TL, Fuller DD, Zabka AG, Mitchell GS. Respiratory plasticity: differential actions of continuous and episodic hypoxia and hypercapnia. Respir. Physiol. 2001;129(12):25–35. doi: 10.1016/s0034-5687(01)00280-8. [DOI] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Mitchell GS. Age and gender effects on serotonin-dependent plasticity in respiratory motor control. Respir. Physiol. Neurobiol. 2002;131:65–77. doi: 10.1016/s1569-9048(02)00038-1. [DOI] [PubMed] [Google Scholar]
- Boden AG, Harris MC, Parkes MJ. Apneic threshold for CO2 in the anesthetized rat: fundamental properties under steady-state conditions. J. Appl. Physiol. 1998;85(3):898–907. doi: 10.1152/jappl.1998.85.3.898. [DOI] [PubMed] [Google Scholar]
- Dwinell MR, Janssen PL, Bisgard GE. Lack of long-term facilitation of ventilation after exposure to hypoxia in goats. Respir. Physiol. 1997;108(1):1–9. doi: 10.1016/s0034-5687(96)02522-4. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Grunstein MM, Derenne JP, Milic-Emili J. Control of depth and frequency of breathing during baroreceptor stimulation in cats. J. Appl. Physiol. 1975;39:295–404. doi: 10.1152/jappl.1975.39.3.395. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Koshiya N. Working model of the sympathetic chemoreflex in rats. clin. Exp. Hypertens. 1995;17(12):167–79. doi: 10.3109/10641969509087063. [DOI] [PubMed] [Google Scholar]
- Jansenn PL, Fregosi RF. No evidence for long-term facilitation after episodic hypoxia in spontaneously breathing, anesthetized rats. J. Appl. Physiol. 2000;89:1345–1351. doi: 10.1152/jappl.2000.89.4.1345. [DOI] [PubMed] [Google Scholar]
- Lin C-Y. Cardiovascular and pulmonary effects of mechanical ventilation. In: Rattenborg CC, Via-Reque E, editors. Clinical Use of Mechanical Ventilation. Year Book Medical Publishers; Chicago, IIllinois: 1981. pp. 243–249. [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Serotonin receptor subtypes required for ventilatory long-term facilitation and its enhancement after chronic intermittent hypoxia in awake rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;286(2):R334–41. doi: 10.1152/ajpregu.00463.2003. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Phrenic long-term facilitation requires NMDA receptors in the phrenic motoneucleus in rats. J. Physiol. 2005;567(Pt 2):599–611. doi: 10.1113/jphysiol.2005.087650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KF, Baekey DM, Shannon R, Lindsey BG. Respiratory neural activity during long-term facilitation. Respir. Physiol. 2000;121:119–133. doi: 10.1016/s0034-5687(00)00123-7. [DOI] [PubMed] [Google Scholar]