Abstract
Statement of problem
It remains unclear which shade guide system is most representative of the shades found in the human dentition.
Purpose
The purpose of this study was to determine and to compare the coverage errors (CEs) of 3 different shades in a selected population.
Material and methods
The coverage errors of the following shade guide systems were evaluated to determine which shade guide system is most effective in producing the best visual shade match: (1) Vita Lumin, (2) Chromascop, (3) Vitapan 3D Master, and (4) a combination of the 3 shade guide systems. The spectral reflectance values of the central one ninth (1-mm diameter) of each shade tab (without a backing) were measured with a spectroradiometer and an external light source at wavelengths from 380 nm to 780 nm at 2-nm intervals. All spectral reflectance measurements were made using 0-degree observer and 45-degree illumination and then converted to CIE values. The color values of 359 anterior teeth were measured with the same protocol. The CEs for each of the 359 anterior teeth for each shade guide system, and with all 3 shade guide systems, were determined and averaged. Repeated measure ANOVA was used to evaluate the mean minimum CEs within-subject (shade guide system) and between-subject (age) difference as well as the interaction between these variables (α=.05). Then, a post hoc multiple comparison was performed using the Tukey-Kramer test.
Results
A significant difference (P<.001) was found among the mean minimum CEs of the 3 shade guide systems and their combination, but not between age groups (P=.384). An interaction was found between shade guide systems and age (P<.001). The Tukey-Kramer test revealed that the mean minimum CEs for Vita Lumin (5.39 ΔE) and Chromoscop (5.28 ΔE) shade guide systems were not significantly different from each other. However, the combination of all 3 shade guide systems (3.69 ΔE) and Vitapan 3D Master (3.93 ΔE) were significantly different from the Vita Lumin and Chromoscop shade guide system. The rankings of the shade guide systems within each age group were similar between the age groups.
Conclusions
The Vitapan 3D Master shade guide system resulted in the lowest coverage errors compared to the Vita Lumin or Chromascop shade guide systems. Coverage errors for the Vitapan 3D Master shade guide system did not differ significantly from the coverage errors when all 3 shade guide systems were combined.
Clinical Implications.
The Vitapan 3D Master shade guide system provides a high potential for a good visual shade match compared to the Vita Lumin or Chromascop shade guide systems. The use of the Vita 3D Master shade guide system alone is as effective as using a combination of Vitapan 3D Master, Vita Lumin, and Chromascop shade guide systems.
In restorative dentistry, the clinician commonly encounters the challenge of replicating the color of natural teeth. The goal of an esthetic restoration is to achieve morphological, optical, and biological beauty which results in social acceptance.1,2 Clinically, the color replication process for dental porcelain consists of shade selection followed by the shade duplication phase. Shade selection can be made using either visual assessment or instrumental color analysis.3 Although the most popular and traditional method of shade selection in dentistry is through the use of visual selection with a prefabricated shade guide, color duplication with this process is plagued by unreliable and inconsistent results.4-6 Color matching with shade guides is considered subjective and difficult at the chair side because of variable viewer interpretation and environmental influences such as fatigue of the human eye, aging, emotion, lighting conditions, level of experience, and physiological variables such as color blindness.4,7,8
Culpepper6 reported inconsistencies among individual dentists in matching natural tooth shades and the inability of some dentists to duplicate their own shade selections reliably from one occasion to another. Errors in color matching mentioned by Culpepper6 are attributed to human variables, but other errors are due to the inadequacy of available guides. In addition, the dissimilarities between the center and sides of a tooth in terms of color, shape, structure, and gloss may be interpreted differently by each observer. There are several other reported disadvantages of shade guides, such as inadequate range of available shades and their nonuniformity.4 The common shade guides currently used clinically include the Vita Lumin shade guide (Vita Zahnfabrik, Bad Sackingen, Germany), Chromoscop shade guide (Ivoclar Vivadent, Amherst, NY) and Vitapan 3D Master shade guide (Vita Zahnfabrik). The Vita Lumin classic shade guide is divided into 4 groups, with primary group division based on hue. Group A is reddish brown, group B is reddish yellow, group C is gray, and group D is reddish gray. Within the groups, tab arrangement is based on increasing chroma; the more chromatic tabs are designated with higher numbers.9 The Chromoscop shade guide is primarily divided into groups, also, according to the hue criterion. There are 5 groups: group 100 is white, group 200 is yellow, group 300 is light brown, group 400 is gray, and group 500 is dark brown. Within the groups, tabs are arranged according to increasing chroma (the more chromatic tabs have higher numbers).9 The Vitapan 3D Master shade guide consists of 26 tabs divided into 5 groups according to lightness. Within the groups, tabs are arranged according to the chroma (vertically) and hue (horizontally). Tabs are marked in the following manner: the numbers (1, 2, 3, 4, and 5) in front of the letters indicate group number and lightness level; a lower number corresponds to greater lightness. The number below the group number designates chroma level (1, 1.5, 2, 2.5, and 3) and the more chromatic tabs have higher numbers. The letter M designates the middle hue in each group, whereas the letters L and R designate greener (left) and redder (right) tabs, respectively, as compared with the M tab.9
The successful achievement of a clinically acceptable color match between a tooth and a restoration is closely related to spectral coverage of a shade guide, clinician experience, and the lighting conditions in which the shade match occurs.10 The range of available shades in shade guides is inadequate, illogically distributed, inconsistent with natural tooth color, and does not cover all the possible values found for tooth chromaticities.6,11
Reports have indicated that commercially available shade guides do not provide sufficient spectral coverage of colors present in teeth.12-14 Moreover, shade tab colors may not be distributed uniformly throughout the color space of natural teeth, resulting in close matches for some shades and gross mismatches for others.15 Preston and Miller16,17 stated many of the errors associated with the use of commercial shade guides and indicated a lack of red shades based on spectrophotometric measurements of extracted teeth reported by Sproull.18 When the natural tooth color was evaluated with shade guides, the most frequently chosen shades were of reddish brown hues A3 and A2.19 The frequent selection of the reddish brown hues is in broad agreement with spectrophotometric work investigating the color of human teeth.20 Shades in the D range were rarely selected.
Comparison of different shade guides and the color range of natural teeth may also be performed using coverage error (CE).21 CE is the index that shows the mean value of the minimal color differences among the specimens of one set (in this case, shade guides) to each specimen of another set (in this case, teeth). The average of these color differences is defined as the CE. O’Brien21 evaluated 2 shade guides and reported that the CEs for Bioform (2.99) and Vita Lumin (3.02) shade guides were not significantly different from each other, but their combination showed a CE value of 2.54, which was significantly lower. Limitations of this study include the use of a color measuring instrumentation that will result in edge loss22,23 when measuring translucent shade tabs. In addition, the color data used in their study was from a previously published study18 which used a different color measurement set-up that also results in edge loss for translucent materials.
Instrumental color analysis offers objective and quantified data. Tooth and shade guide color21 can be measured with an instrument possessing a small window for illumination and measurement, but these translucent specimens are subject to edge loss of the light, resulting in systematic errors in color coordinates.24 Edge loss is the phenomenon that occurs when scattered light transmitted through the translucent material, which originally would be seen by the eye, is simply not measured by the instrument due to the configuration of the illuminant, sensor, and aperture. This occurs during conventional reflectance measurements of translucent materials when both the illuminant and observation light path travels through an aperture. Edge loss does not depend upon the measuring methods, but upon the measurement configuration. Therefore, these losses are not considered in the comparison between different measuring methods. Edge loss can be avoided by ensuring that there is no aperture between the external light source, spectroradiometer, and the object.24
In a more recent study, Analoui et al25 studied extracted teeth, which have different spectral characteristics than nonextracted teeth, and demonstrated that the Vitapan 3D Master guide has the lowest average color difference (ΔE) among the 3 commercially available shade guides for extracted teeth. Although the color measurement optical configuration was ideal in terms of measurement of translucent specimens without edge loss, extracted teeth, as previously mentioned, have different spectral characteristics compared to vital natural dentition.
In 1931, the International Commission on Illumination, or Commission Internationale de l’Eclairage (CIE), an organization devoted to standardization in areas such as color and appearance, defined a standard light source, developed a standard observer, and enabled the calculation of tristimulus values, which represent how the human visual system responds to a given color.26 The CIE Lab color space represents a uniform color space, with equal distances corresponding to equally perceived color differences. In this 3-dimensional color space, the 3 axes are L*, a*, and b*. The L* value is a measure of the lightness of an object and is quantified on a scale such that a perfect black has an L* value of zero and a perfect reflecting diffuser an L* value of 100. The a* value is a measure of redness (+a*) or greenness (-a*). The b* value is a measure of yellowness (+b*) or blueness (-b*). The a* and b* coordinates approach zero for achromatic colors (white, grays) and increase in magnitude for more saturated or intense colors. The advantage of the CIE Lab system is that color differences can be expressed in units that can be related to visual perception and clinical significance.27 Correlates of chroma are defined by converting the rectangular a* and b* axes into polar coordinates. The following formula is used for determination of chroma.9
To date, no published study has presented appropriate color measuring instrumentation to measure translucent shade guides and teeth to obtain CEs. Therefore, the purposes of this clinical study were to determine and compare the CEs of 3 different shade guide systems and the combination of the 3 shade guide systems when selecting shades for anterior vital teeth. The null hypothesis was that the CE for the 3 shade guide systems for the vital teeth would be similar.
MATERIAL AND METHODS
Human subject approval was obtained from The Ohio State University Institutional Review Board. A total of 120 human subjects over the age of 18 years were recruited. Potential subjects responded to the advertisements posted near The Ohio State University Medical Center by calling the laboratory and were screened using a telephone screening form. This screening process ensured that potential subjects satisfied the inclusion and exclusion criteria for this study. Included in the study were generally healthy subjects between the ages of 18 and 85 years of age. Other inclusion criteria were: the presence of at least 1 maxillary central incisor, 1 lateral incisor, and 1 canine, willingness to brush teeth for 3 minutes prior to color measurement and to spend approximately an hour to participate in the study, and signed informed consent and HIPAA forms. Excluded from the study were subjects with any direct or complete coverage restorations on both similar types of maxillary anterior teeth, external surface staining on both similar types of maxillary anterior teeth, with any intrinsic staining on both similar types of maxillary anterior teeth (for example, tetracycline stains, or fluorosis), severe attrition resulting in incisal enamel wear, spontaneous bleeding from the gingiva due to periodontal disease, pregnant subjects (eliminating the possibility of any misunderstanding that the color measurement instrument may cause harm to the unborn child), subjects with psychiatric, cognitive, or social conditions (for example, alcoholism or drug abuse) that would interfere with giving consent and cooperation, and prisoners.
The maxillary central and lateral incisors and canines of the subjects were measured. The color of 5 sets of shade guides of 3 conceptually different shade guide systems was also measured. The mean CEs of each of the 3 shade guide systems and all shade guide systems combined were then calculated and statistically compared.
The color measurement apparatus consisted of a spectroradiometer (PR 705; Photo Research Inc, Chatsworth, Calif) and fiber optic light cable (model 70050; Newport Stratford Inc, Stratford, Conn) fixed on an optical table (Mecom Inc, Rising Sun, Ohio). The fiber optic light cable was connected to a xenon arc lamp (300W; Newport Stratford Inc). The spectroradiometer and the optic light cable, positioned at a 45-degree angle inferior to the horizontal plane, provided an optical configuration of 0-degree observation and 45-degree illumination to the object. For all color measurements in this study, spectral reflectance was obtained from 380 nm to 780 nm, with a 2-nm interval (Spectrawin 2.0; Photo Research Inc), and subsequently converted to CIELAB values (D65 illumination and 2-degree observer). The spectroradiometer was standardized to 8 cm from the measured object with a measurement aperture size of 1 mm. Edge loss is avoided in this color measurement experimental design, as there is no aperture between the external light source, spectroradiometer, and the object, thus no shadows are cast on the object. Color measurement protocol, validity, and reliability are published in previous studies.28,29
One hundred twenty subjects were recruited at the University’s Health Science Center to serve as a convenience sample. Six subjects with an equal gender balance (3 men and 3 women) from 4 racial/ethnic groups (white, black, Mongol/Pacific Islander, and others, which included Middle Eastern/Indian and American Indian/Alaskan Native) were recruited into each of the following 5 age groups: 18-29 years, 30-39 years, 40-49 years, 50-59 years, and 60-85 years. Three teeth of each subject were measured on the central one ninth of the tooth. The vital teeth were without any restorations or internal or external stains. The data from 1 of the 360 teeth could not be used (central incisor for A50-1M, a 50-year-old Asian man); thus, only 359 teeth were used. The CIELAB values for the anterior teeth measurements were published in a previous study that used the same color measurement protocol, instrumentation, and optical configuration as this study.28
Five sets of the 3 shade guide systems: Vita Lumin (Vita Zahnfabrik), Chromascop (Ivoclar Vivadent), and Vitapan 3D Master (Vita Zahnfabrik) were collected from the Ohio State Dental School. The shade tabs were first cleaned with a disinfecting towel (DisCide Ultra; Palmero Health Care, Stratford, Conn) and placed in a specimen holder fabricated specifically to hold the shade tabs for color measurements. A specimen holder ensured that the surface being measured (central one ninth) of the shade tab was perpendicular to the color measurement axis of the spectroradiometer. Four shade tabs were selected to assess reliability of the experiment. Each shade tab was measured 10 times after removing the tab from the specimen holder and then replacing it to the holder. The spectral reflectance data were then converted to CIELAB values and the pool standard deviations were calculated.
The mean CIELAB values for the 5 sets of shade guides for a particular shade guide system were calculated. The CE21 was calculated for each of the 3 shade guide systems and the combination of all 3 shade guide systems. For a particular shade guide system, a CIELAB color difference was calculated for each anterior tooth measured (359 teeth) and all mean CIELAB values (5 sets of shade guides) for all the shade tabs in that shade guide system. For each of the 359 teeth, the shade tab with the smallest ΔE was determined for the shade guide system. The average minimum ΔE for that shade guide system was then computed. The CE for a shade guide system was therefore the average ΔE between each of the 359 teeth and the corresponding shade tab with the minimum ΔE to that tooth (Equation 1). The following formula was used for calculation of average ΔE and is an index of the CE of the shade guide system21:
where ΔL*, Δa*, and Δb* are the differences in color parameters between individual teeth and closest shade tab colors selected by a computer algorithm. The total sample size, n, was 359 for the pooled data. The average error, therefore, is the average difference between the tooth colors and the nearest shade tab match.
Parametric statistical analyses were carried out at a 95% confidence interval (CI) using statistical software (SAS, 10th edition; SAS Institute Inc, Cary, NC). The mean minimum CEs were statistically analyzed using a repeated measures analysis of variance (ANOVA) to evaluate within-subject (3 shade guides system) and between-subject (age) differences, as well as the interaction between these variables (shade guides system and age). Thereafter, post hoc multiple comparisons of data were analyzed with the Tukey-Kramer test (α=.05).
RESULTS
The pooled standard deviation for the 10 repeated comparisons involving the 4 selected shade tabs showed good experimental design repeatability: L*=0.85, a*=0.03, and b*=0.52. Average CIELAB values for the 3 shade guide systems are listed in Tables I, II, and III. The mean CEs for the 3 shade guide systems tested, with standard deviations, are given in Table IV. The mean CEs and standard deviations of color differences of shade guide systems in different age groups are given in Table V. As shown in Table V, the smallest CE was determined in the age group of 40 for Vitapan 3D Master (3.4 ΔE), and the combination of all 3 shade guide systems (3.2 ΔE).
Table I.
Means (standard deviations) of CIE L*, a*, and b* values for Vita Lumin shade guide
| Shade | L* | a* | b* |
|---|---|---|---|
| A1 | 82.4 (1.9) | −1.4 (0.4) | 14.3 (0.7) |
| A2 | 79.1 (1.1) | 0.6 (0.3) | 19.2 (0.5) |
| A3 | 77.6 (0.9) | 1.0 (0.3) | 21.0 (0.9) |
| A3.5 | 73.4 (1.2) | 2.3 (0.1) | 24.5 (0.6) |
| A4 | 69.0 (0.9) | 2.4 (0.6) | 25.4 (0.8) |
| B1 | 80.1 (2.3) | −1.9 (0.5) | 12.6 (0.9) |
| B2 | 80.1 (2.2) | −1.0 (0.5) | 18.2 (1.0) |
| B3 | 74.8 (1.4) | 0.9 (0.5) | 25.0 (0.9) |
| B4 | 75.5 (2.7) | 1.0 (0.2) | 26.1 (1.8) |
| C1 | 76.6 (0.9) | −0.7 (0.2) | 14.2 (0.8) |
| C2 | 72.7 (0.4) | 0.2 (0.3) | 20.0 (0.4) |
| C3 | 70.5 (0.9) | 0.8 (0.1) | 19.1 (0.5) |
| C4 | 64.2 (1.2) | 2.6 (0.2) | 22.1 (0.5) |
| D2 | 74.9 (1.5) | −0.4 (0.4) | 13.2 (0.8) |
| D3 | 74.7 (2.6) | 1.1 (0.4) | 18.3 (0.9) |
| D4 | 73.5 (0.7) | −0.6 (0.2) | 21.1 (0.5) |
Table II.
Means (standard deviations) of CIE L*, a*, and b* values for Chromoscop shade guide
| Shade | L* | a* | b* |
|---|---|---|---|
| 110 | 82.5 (1.0) | 0.1 (0.1) | 18.3 (0.3) |
| 120 | 80.2 (1.8) | 0.7 (0.1) | 19.7 (0.6) |
| 130 | 78.2 (0.8) | 0.1 (0.1) | 20.2 (0.5) |
| 140 | 78.9 (1.1) | 1.6 (0.2) | 23.7 (0.5) |
| 210 | 77.4 (1.5) | 1.8 (0.1) | 25.6 (0.8) |
| 220 | 76.4 (2.5) | 3.4 (0.0) | 23.4 (0.7) |
| 230 | 74.7 (1.8) | 3.7 (0.2) | 25.6 (0.9) |
| 240 | 73.8 (0.6) | 5.6 (0.1) | 28.2 (0.5) |
| 310 | 73.6 (1.0) | 1.2 (0.1) | 28.1 (0.7) |
| 320 | 71.4 (1.6) | 2.7 (0.1) | 28.2 (0.8) |
| 330 | 71.5 (1.4) | 3.4 (0.1) | 31.1 (0.5) |
| 340 | 68.3 (2.0) | 4.9 (0.2) | 28.9 (0.8) |
| 410 | 73.5 (1.2) | 2.2 (0.2) | 20.2 (0.7) |
| 420 | 72.1 (0.9) | 1.7 (0.1) | 20.5 (0.3) |
| 430 | 72.2 (0.9) | 0.6 (0.1) | 20.8 (0.7) |
| 440 | 69.1 (1.0) | 0.9 (0.1) | 21.1 (0.3) |
| 510 | 69.9 (1.5) | 1.9 (0.1) | 22.5 (0.6) |
| 520 | 67.6 (1.0) | 2.7 (0.2) | 24.7 (0.9) |
| 530 | 67.2 (0.2) | 3.4 (0.1) | 26.8 (1.0) |
| 540 | 64.0 (1.2) | 7.6 (0.1) | 26.2 (0.6) |
Table III.
Means (standard deviations) of CIE L*, a*, and b* values for Vitapan 3D Master shade guide
| Shade | L* | a* | b* |
|---|---|---|---|
| 1M1 | 83.1 (0.9) | −0.1 (0.3) | 12.5 (0.4) |
| 1M2 | 84.0 (0.8) | − 0.2 (0.5) | 18.8 (0.9) |
| 2L1.5 | 79.0 (1.0) | 0.0 (0.2) | 18.5 (0.2) |
| 2L2.5 | 79.5 (0.8) | 0.2 (0.2) | 24.5 (0.7) |
| 2M1 | 78.0 (0.6) | 0.8 (0.3) | 14.0 (0.6) |
| 2M2 | 78.7 (0.6) | 0.9 (0.4) | 19.9 (0.5) |
| 2M3 | 79.2 (0.8) | 0.7 (0.2) | 25.3 (0.4) |
| 2R1.5 | 77.8 (1.0) | 1.5 (0.2) | 16.3 (0.7) |
| 2R2.5 | 79.5 (1.1) | 1.7 (0.3) | 23.3 (0.6) |
| 3L1.5 | 73.1 (0.9) | 1.5 (0.2) | 20.3 (0.4) |
| 3L2.5 | 73.9 (1.1) | 1.9 (0.2) | 26.2 (0.8) |
| 3M1 | 73.4 (0.6) | 1.8 (0.3) | 15.4 (0.5) |
| 3M2 | 74.6 (1.0) | 2.0 (0.4) | 21.5 (0.8) |
| 3M3 | 75.0 (1.4) | 2.6 (0.2) | 27.9 (0.8) |
| 3R1.5 | 73.4 (1.1) | 2.7 (0.3) | 17.9 (0.6) |
| 3R2.5 | 73.6 (1.0) | 3.5 (0.3) | 25.9 (0.7) |
| 4L1.5 | 69.2 (0.8) | 2.8 (0.3) | 21.7 (0.3) |
| 4L2.5 | 69.1 (0.8) | 3.7 (0.4) | 28.5 (0.7) |
| 4M1 | 68.3 (0.9) | 2.9 (0.2) | 17.0 (0.5) |
| 4M2 | 70.1 (1.4) | 3.7 (0.4) | 23.7 (0.6) |
| 4M3 | 69.5 (0.7) | 4.8 (0.3) | 30.7 (0.4) |
| 4R1.5 | 69.6 (0.6) | 4.3 (0.2) | 20.8 (0.3) |
| 4R2.5 | 69.2 (1.1) | 5.1 (0.2) | 26.3 (0.4) |
| 5M1 | 64.4 (0.6) | 4.2 (0.2) | 19.4 (0.5) |
| 5M2 | 65.1 (1.0) | 5.7 (0.2) | 26.3 (0.8) |
| 5M3 | 65.9 (0.5) | 7.0 (0.4) | 33.4 (1.3) |
Table IV.
Mean, standard deviation, minimum, and maximum coverage errors for 3 shade guide systems tested (n=359)
| Variable | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| Vita Lumin | 5.39 | 3.14 | 1.01 | 27.67 |
| Chromoscop | 5.28 | 3.54 | 0.85 | 26.75 |
| Vitapan 3D Master | 3.93 | 2.91 | 0.60 | 27.45 |
| Combination of 3 shade guides | 3.69 | 2.88 | 0.60 | 26.75 |
Table V.
Mean coverage error and standard deviations (SD) of color difference of shade guide systems in different age groups (n=72)
| Age Groups | Guide | Coverage Error | SD |
|---|---|---|---|
| 18-29 | 3 guides combined | 4.2 | 3.3 |
| Chromoscop | 5.8 | 3.9 | |
| Vita 3D Master | 4.3 | 3.3 | |
| Vita Lumin | 5.4 | 3.4 | |
|
| |||
| 30-39 | 3 guides combined | 3.7 | 3.3 |
| Chromoscop | 5.0 | 3.6 | |
| Vita 3D Master | 3.9 | 3.3 | |
| Vita Lumin | 5.1 | 3.4 | |
|
| |||
| 40-49 | 3 guides combined | 3.2 | 1.3 |
| Chromoscop | 5.0 | 2.6 | |
| Vita 3D Master | 3.4 | 1.3 | |
| Vita Lumin | 4.8 | 1.7 | |
|
| |||
| 50-59 | 3 guides combined | 3.6 | 2.2 |
| Chromoscop | 5.5 | 3.3 | |
| Vita 3D Master | 3.8 | 2.2 | |
| Vita Lumin | 5.3 | 2.5 | |
|
| |||
| 60-85 | 3 guides combined | 3.9 | 3.6 |
| Chromoscop | 5.2 | 4.2 | |
| Vita 3D Master | 4.3 | 3.7 | |
| Vita Lumin | 6.3 | 4.1 | |
Results of the repeated measures ANOVA indicated that a significant difference (P<.001) was found with the shade guide systems used but not between age groups. An interaction was found between the shade guide system used and age (P<.001) (Table VI). Figure 1 represents the (a*, b*) coordinates of 359 anterior teeth of human subjects and average color of the 3 shade guide systems, and Figure 2 shows the coordinates (L, Chroma) of 359 human anterior teeth and the average color of the 3 shade guide systems.
Table VI.
Results of ANOVA for different age groups
| Source | Degrees of
Freedom |
Mean
Square |
F | P | |
|---|---|---|---|---|---|
| Tests of hypothesis for
between-subjects effect |
Age | 4 | 37.22 | 1.04 | .384 |
| Error | 354 | 35.64 | |||
|
| |||||
| Univariate tests of hypotheses
or within-subject effects |
Guide | 3 | 284.11 | 249.57 | <.001 |
| Guide × age | 12 | 5.79 | 5.09 | <.001 | |
| Error (guide) | 1062 | 1.14 | |||
1.
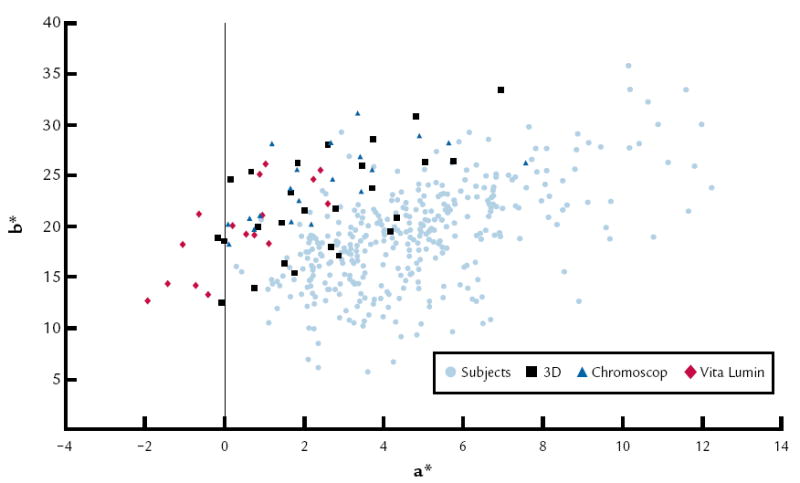
a* vs. b* for 359 anterior human teeth and average color of 3 shade guide systems (62 tabs).
2.
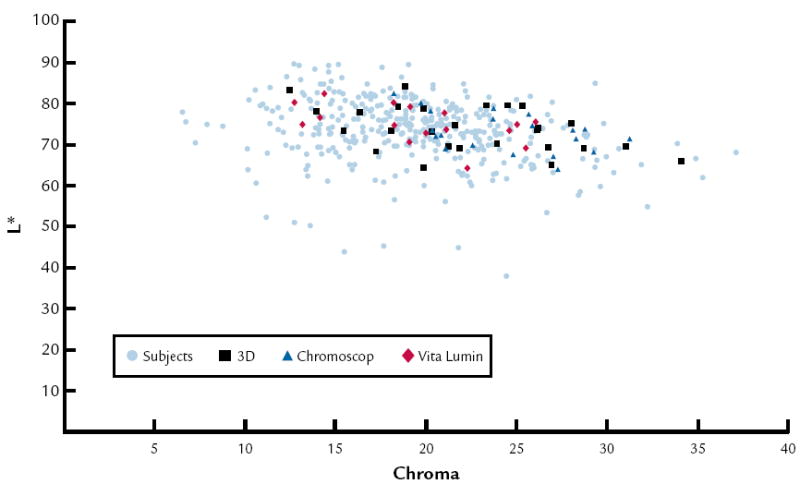
L* vs. chroma for 359 anterior teeth of human subjects and average color of 3 shade guide systems (62 tabs).
Although there is an interaction between the shade guide system and age, the Tukey-Kramer test reveals similar differences between guides within the group range (Table VI). The CEs of the Vitapan 3D Master shade guide system did not differ significantly from the CEs when all guides were combined. The CE of the combined (all 3) shade guide systems, 3.69 ΔE, was significantly lower than the Vita Lumin and Chromoscop shade guides, as a result of having a greater number and wider distribution of shades. CEs for the Vita Lumin (5.39 ΔE) and Chromoscop (5.28 ΔE) shade guide systems were not significantly different from each other. Conversely, all 3 shade guide systems (3.69 ΔE) and the Vitapan 3D Master (3.93 ΔE) were statistically different from these 2 shade guide systems. All 3 shade guide systems and the Vitapan 3D Master resulted in the lowest CEs compared to the 2 other shade guides. The rankings of the shade guide systems within each age group were similar between the age groups.
The frequencies of selection in the shade guide systems are presented in Figure 3 to 5. In this study, it was determined that the most common colors are D3, 410, and 3R1, 5 in Vita Lumin, Chromoscop, and Vitapan 3D-Master shade guide systems, respectively. In addition, for all shade guide systems combined, the most common color was determined to be 4R1, 5. The frequency of selection for the combination of all shade guide systems is presented in Figure 6.
3.
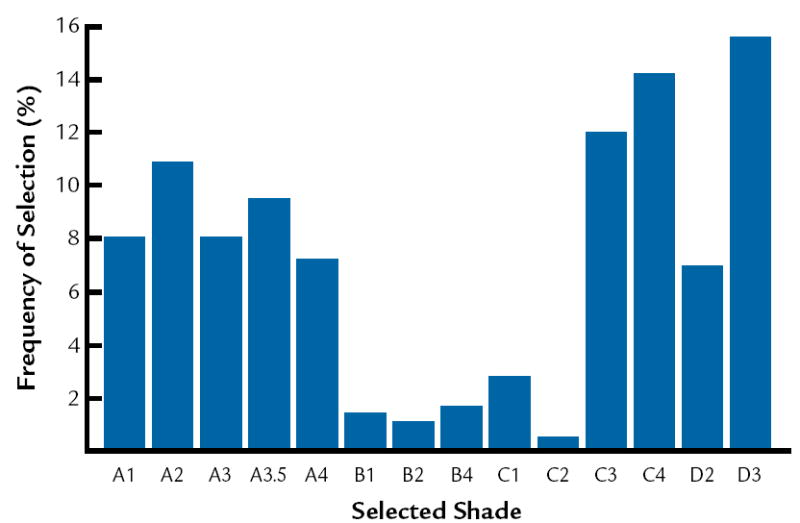
Frequency of selection for Vita Lumin shade guide.
5.
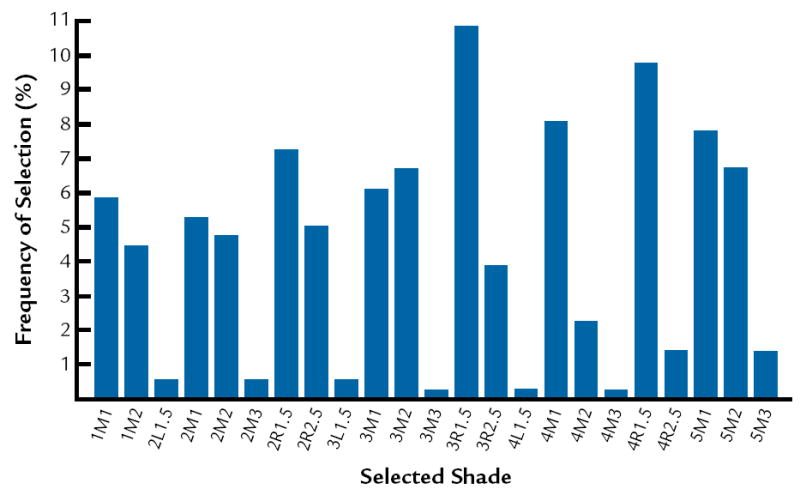
Frequency of selection for Vitapan 3D Master shade guide.
6.
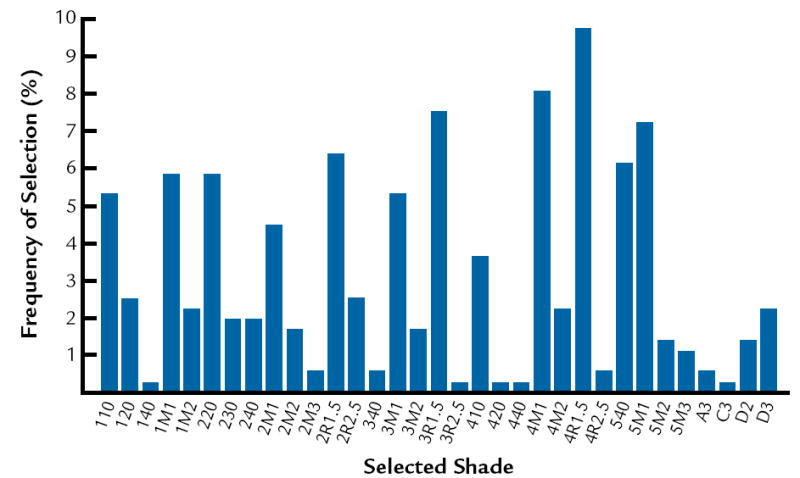
Frequency of selection for combination of all 3 guide systems: Chromoscop, Vita Lumin, and Vitapan 3D Master shade guides.
DISCUSSION
The data and analysis of this study support rejecting the null hypothesis. In this study, it was determined that subject effects were statistically significant (P<.001), and the potential best matches were obtained with the Vitapan 3D Master shade guide system (ΔE 3.93) compared to the other 2 shade guide systems tested. The Vitapan 3D system uses 36 shade tabs compared to the Vita Lumin 16 shade tabs, which may account for the difference. According to the manufacturer of the Vitapan 3D guide, the guide was designed to include a uniform coverage of shade tabs in virtually all existing natural tooth shades. It is purported to be systematically arranged in a 3-dimensional color space that makes shade selection simpler and more accurate. In addition, several important characteristics have been improved with the Vitapan 3D shade guide: the lightness range is broader, more chromatic tabs are included, the shade tabs are more uniformly spaced, the hue range is extended in the direction of the reddish spectra, group division is better, and, although certain disharmony still exists, the overall tab arrangement is much better compared with the Vitapan classic shade guide.9 In view of these results, it appears that the use of the Vitapan 3D Master shade guide alone is just as effective as using all 3 shade guides combined, which is less clinically practical. Nevertheless, it is important to note that none of the shade guides, including the 3D Master, appear to effectively cover the red range of the tooth spectrum, as indicated in Figure 1.
O’Brien et al21 reported the CE of the Vita Lumin shade guide to be ΔE=3.02, which is different from that reported in the present study (ΔE=5.39). This difference may be related to various factors. The O’Brien et al21 study used a color measurement instrument that was subject to edge loss, and thus, the color measurements were lower. In addition, the lingual portion of their shade tabs was coated with barium sulfate, which may not be similar to the color clinically. The shade tabs in the present study were not coated.
The results agree with Analoui et al24 with respect to the ability of the Vitapan 3D Master to provide better coverage compared to the Vita Lumin. The results do not agree with Smith and Wilson,28 who demonstrated that the 5 shades most frequently chosen were A3, closely followed by shades A2, C2, B2, and B3. The results from the present study indicate that the most common shades are D3, 410, and 3R1, 5 in Vita Lumin, Chromoscop, and Vitapan 3D Master shade guides, respectively. This may relate to basic differences in the hue of teeth between different population groups.
Some CEs will always exist, because dental color standards are schematic representations of tooth color space, and the number of the shade tabs is limited.3 Currently used shade guides have obvious inadequacies. Therefore, straightforward and specific written instructions, shade diagrams, casts, and clinical photographs should be used to communicate and produce consistently acceptable esthetic results.9
Limitations of this study include the secondary validity of the results of the study, since the sample of anterior teeth used in this study is only from a stratified convenience sample in 1 state in the United States. Sampling a population that is representative of the general population in the United States would be ideal, but difficult to perform. Interestingly, there was an interaction between age and the shade guide systems, which shows that each age group might not be weighted the same. The results did not indicate that the shade guide systems provided different CEs for different age groups. Another limitation of this study was that there was no attempt to ensure that the color of the Vita Lumin shade guide system used in this study was similar to new Vita Lumin guides. There is a possibility that the older Vita Lumin guides may have been discolored due to years of disinfection, compared to the newer Chromascop and Vitapan 3D Master shade guide systems.
Further studies might include determining which is the best method to reduce the CEs of currently used shade guide systems or developing a guide with reduced CEs. More research is necessary on the clinical use of visual shade selection so as to improve the color replication progress.
CONCLUSIONS
Within the limitations of this study, age group was found to have a significant interaction with shade guide systems, but the ranking of the shade guide systems was similar between the age groups. The Vitapan 3D Master shade guide system (ΔE 3.93) and the group consisting of all 3 shade guides (ΔE 3.69) were not statistically significantly different from each other. The CEs of the Vitapan 3D Master and the combined shade guide systems were significantly lower than 2 other shade guide systems (Vita Lumin, ΔE=5.39; Chromoscop, ΔE=5.28), which were not significantly different from each other.
4.
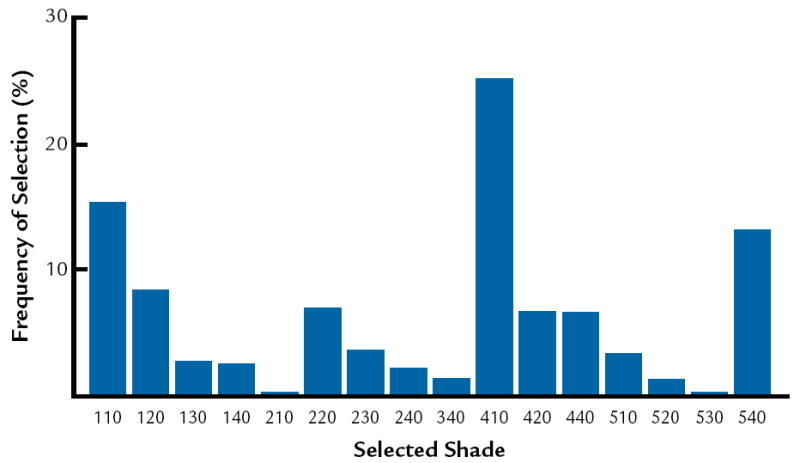
Frequency of selection for Chromoscop shade guide.
Acknowledgments
This research was supported in part by the National Eye Institute, a National Institute of Health Grant (R15 EY013527), as well as the Awards and Grants Program of the Editorial Council of The Journal of Prosthetic Dentistry, 2003.
Footnotes
This study was presented in part at the 80th General Session of the International Association for Dental Research, San Diego, Calif, March 2002.
References
- 1.Goodkind RJ, Loupe MJ. Teaching of color in predoctoral and postdoctoral dental education in 1988. J Prosthet Dent. 1992;67:713–7. doi: 10.1016/0022-3913(92)90177-c. [DOI] [PubMed] [Google Scholar]
- 2.Paravina RD, Powers JM, Fay RM. Dental color standards: shade tab arrangement. J Esthet Restor Dent. 2001;13:254–63. doi: 10.1111/j.1708-8240.2001.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 3.Wee AG. Description of color, color replication process and esthetics. In: Rosenstiel SF, Land MF, Fujimoto J, editors. Contemporary fixed prosthodontics. 4. St. Louis: Mosby; 2006. p. 712. [Google Scholar]
- 4.Okubo SR, Kanawati A, Richards MW, Childress S. Evaluation of visual and instrument shade matching. J Prosthet Dent. 1998;80:642–8. doi: 10.1016/s0022-3913(98)70049-6. [DOI] [PubMed] [Google Scholar]
- 5.Cal E, Sonugelen M, Guneri P, Kesercioglu A, Kose T. Application of a digital technique in evaluating the reliability of shade guides. J Oral Rehabil. 2004;31:483–91. doi: 10.1111/j.1365-2842.2004.01197.x. [DOI] [PubMed] [Google Scholar]
- 6.Culpepper WD. A comparative study of shade-matching procedures. J Prosthet Dent. 1970;24:166–73. doi: 10.1016/0022-3913(70)90140-x. [DOI] [PubMed] [Google Scholar]
- 7.Berns RS, Billmeyer FW, Saltzman M. Billmeyer and Saltzman’s principles of color technology. 3. New York: John Wiley & Sons; 2000. pp. 13–20. [Google Scholar]
- 8.Rosenstiel SF, Land MF, Fujimoto J. Contemporary fixed prosthodontics. 4. St Louis: Mosby; 2006. pp. 712–25. [Google Scholar]
- 9.Paravina RD, Powers JM. Esthetic color training in dentistry. St Louis: Elsevier Health Sciences; 2004. pp. 39–44. [Google Scholar]
- 10.Bentley C, Leonard RH, Nelson CF, Bentley SA. Quantitation of vital bleaching by computer analysis of photographic images. J Am Dent Assoc. 1999;130:809–16. doi: 10.14219/jada.archive.1999.0304. [DOI] [PubMed] [Google Scholar]
- 11.Rubino M, Garcia JA, Jimenez del Barco L, Romero J. Colour measurement of human teeth and evaluation of a colour guide. Color Res Appl. 1994;19:19–22. [Google Scholar]
- 12.Milleding P, Haag P, Neroth B, Renz I. Two years of clinical experience with Procera titanium crowns. Int J Prosthodont. 1998;11:224–32. [PubMed] [Google Scholar]
- 13.Ferreira D, Monard LA. Measurement of spectral reflectance and colorimetric properties of Vita shade guides. J Dent Assoc S Afr. 1991;46:63–5. [PubMed] [Google Scholar]
- 14.Horn DJ, Bulan-Brady J, Hicks ML. Sphere spectrophotometer versus human evaluation of tooth shade. J Endod. 1998;24:786–90. doi: 10.1016/S0099-2399(98)80002-2. [DOI] [PubMed] [Google Scholar]
- 15.Bergen SF, McCasland J. Dental operatory lighting and tooth color discrimination. J Am Dent Assoc. 1977;94:130–4. doi: 10.14219/jada.archive.1977.0264. [DOI] [PubMed] [Google Scholar]
- 16.Preston JD. Current status of shade selection and color matching. Quintessence Int. 1985;16:47–58. [PubMed] [Google Scholar]
- 17.Miller L. Organizing color in dentistry. J Am Dent Assoc. 1987:26E–40E. doi: 10.14219/jada.archive.1987.0315. [DOI] [PubMed] [Google Scholar]
- 18.Sproull RC. Color matching in dentistry. Part II. Practical applications of the organization of color.1973. J Prosthet Dent. 2001;86:458–64. doi: 10.1067/mpr.2001.119828. [DOI] [PubMed] [Google Scholar]
- 19.Smith PW, Wilson NH. Shade selection for single-unit anterior metal ceramic crowns: a 5-year retrospective study of 2,500 cases. Int J Prosthodont. 1998;11:302–6. [PubMed] [Google Scholar]
- 20.Lund TW, Schwabacher WB, Goodkind RJ. Spectrophotometric study of the relationship between body porcelain color and applied metallic oxide pigments. J Prosthet Dent. 1985;53:790–6. doi: 10.1016/0022-3913(85)90158-1. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien WJ, Boenke KM, Groh CL. Coverage errors of two shade guides. Int J Prosthodont. 1991;4:45–50. [PubMed] [Google Scholar]
- 22.Hasegawa A, Ikeda I, Kawaguchi S. Color and translucency of in vivo natural central incisors. J Prosthet Dent. 2000;83:418–23. doi: 10.1016/s0022-3913(00)70036-9. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa A, Motonomi A, Ikeda I, Kawaguchi S. Color of natural tooth crown in Japanese people. Color Res Appl. 2000;25:43–8. [Google Scholar]
- 24.Bolt RA, Bosch JJ, Coops JC. Influence of window size in small-window colour measurement, particularly of teeth. Phys Med Biol. 1994;39:1133–42. doi: 10.1088/0031-9155/39/7/006. [DOI] [PubMed] [Google Scholar]
- 25.Analoui M, Papkosta E, Cochran M, Matis B. Designing visually optimal shade guides. J Prosthet Dent. 2004;92:371–6. doi: 10.1016/j.prosdent.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 26.McLaren K. Colour space, colour scales and colour difference. In: McDonald R, editor. Colour physics for industry. Huddersfield: H Charlesworth & Co Ltd; 1987. pp. 97–115. [Google Scholar]
- 27.O’Brien WJ, Hemmendinger H, Boenke KM, Linger JB, Groh CL. Color distribution of three regions of extracted human teeth. Dent Mater. 1997;13:179–85. doi: 10.1016/S0109-5641(97)80121-2. [DOI] [PubMed] [Google Scholar]
- 28.Gozalo-Diaz DJ, Lindsey DT, Johnston WM, Wee AG. Measurement of color for craniofacial structures using a 45/0-degree optical configuration. J Prosthet Dent. 2007;97:45–53. doi: 10.1016/j.prosdent.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wee AG, Lindsey DT, Kuo S, Johnston WM. Color accuracy of calibrated digital cameras in dentistry. Dent Mater. 2006;22:553–9. doi: 10.1016/j.dental.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


