Abstract
We investigated whether puberty influences the morphology of the medial nucleus of the amygdala (MeA) by comparing Siberian hamsters (Phodopus sungorus) that had been raised from birth in either long day (LD; 16:8 h light:dark) or short day (SD; 8:16) photoperiods. Hamsters were sacrificed at 42 – 49 days of age, at which point all LD hamsters were reproductively mature, as evidenced by adult-like testes weights (mean: 657 mg). In contrast, the testes weights of the SD hamsters were low (mean: 31 mg), indicating that the SD photoperiod had delayed puberty. The regional volume and mean soma size of the four MeA subnuclei was estimated bilaterally by stereological procedures. In the posterior dorsal and ventral MeA subnuclei, regional volume was 22–25% larger, and mean soma size 18% larger, in LD males than SD males. Unbiased cell counts in the posterior dorsal MeA showed that LD and SD hamsters have equivalent neuron numbers. In the anterior MeA subnuclei, regional volumes and soma sizes from LD and SD hamsters were equivalent. Additionally, the regional volume of the posteroventral subnucleus was larger in the right hemisphere than the left, but this laterality did not respond to photoperiod manipulation. These results suggest that the extant neurons within the posterior MeA, a steroid-sensitive nucleus implicated in socio-sexual behavior, grow in response to the elevated levels of circulating androgen accompanying puberty, and that photoperiodic regulation of puberty affects morphological maturation of this nucleus.
Keywords: Puberty, regional volume, morphological plasticity, androgen, trophic factor
Introduction
Puberty represents the final stage of sexual maturation. After puberty, the neuroendocrine and gonadal mechanisms that enable the production and/or release of gametes and the secretion of adult-like levels of sex steroids, have fully matured, making the animal capable of sexual reproduction. However, in addition to gonadal maturation, behavioral development must also occur, such that conspecifics become capable of recognizing, and responding appropriately to, fertile conspecifics (Sisk and Foster, 2004). The Siberian hamster, Phodopus sungorus, restricts its breeding to the spring and summer months. Hoffman showed that rearing P. sungorus from birth in short days (SD) representative of winter delays the onset of gonadal maturation by roughly two months (Hoffman, 1978), which in the wild would block reproductive capacity during the winter when conditions are unfavorable for reproduction. Because puberty is delayed in SD, one can evaluate the effect of puberty independent of age by comparing age-matched hamsters raised from birth in SD versus long days (LD). By using this approach, our laboratory showed that P. sungorus raised from birth in SD had a reproductively immature spinal nucleus of the bulbocavernosus (a sexually dimorphic spinal cord system controlling penile reflexes during copulation) relative to age-matched hamsters raised in LD (Hegstrom and Breedlove, 1998). This experiment showed that the gonadal and physiognomic maturation observed by Hoffman in LD-reared hamsters is matched by a maturation of a spinal center required for male sexual reproduction.
The medial nucleus of the amygdala (MeA) has been implicated in the appetitive aspects of reproductive behavior in rats and Syrian hamsters (Veening, et al., 2005; Wood and Coolen, 1997). The MeA receives input from the accessory olfactory bulbs (Scalia and Winans, 1975) and projects to the medial preoptic area, mediobasal hypothalamus, and midbrain (Canteras, et al., 1995). Because of these connections, the MeA is positioned to monitor the external world for socio-sexual cues as well as to regulate the endocrine and motivational aspects of socio-sexual behavior. In rats and hamsters, the posterior MeA intensely expresses androgen receptors as well as both α and β estrogen receptors, particularly within the posterodorsal subnucleus (MeApd). In contrast, the expression of gonadal steroid receptors in the anterior MeA is relatively sparse (Simerly, et al., 1990; Wood and Newman, 1995). Consistent with the expression of androgen and estrogen receptors there, the adult MeApd displays structural plasticity in response to changing levels of circulating androgens. In rats and Siberian hamsters, castration reduces the regional volume of the MeApd, while testosterone replacement maintains MeApd volume at pre-castrate levels. These manipulations likewise modify the average soma size of MeApd neurons (Cooke, et al., 2002a; Cooke, et al., 1999). In contrast, the anterior subnuclei of the MeA do not display detectable effects of gonadal steroid manipulations in adult rats or hamsters (Cooke, et al., 2003; Cooke et al., 2002a). These observations suggest that the pubertal rise in circulating androgen may influence MeApd morphology. To evaluate this hypothesis, we analyzed the morphology of the MeA in 42 – 49 day old male Siberian hamsters raised from birth in either SD or LD. These animals were also used in a study of the spinal nucleus of the bulbocavernosus (Hegstrom and Breedlove, 1998).
Materials and Methods
Animals were obtained from the breeding colony of Siberian hamsters maintained in the Psychology Department of the University of California, Berkeley, since 1985. From this colony, 12 male and 12 female hamsters were conceived and raised under long day (LD) photoperiods (16:8 h light/dark cycle; lights on at 2 a.m.) until adulthood (at least 3 months of age). These animals were then paired with a nonsibling member of the opposite sex. Half of the pairs were moved immediately into short day (SD) photoperiods (8:16 h light/dark cycle; lights on at 10 a.m.), while the rest were maintained under LD conditions. All the animals were housed at 22ºC with continuous access to food and water and were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Procedures were approved by the University of California, Berkeley, Institutional Animal Care and Use Committee.
Male offspring from these pairs were maintained in the photoperiod of their birth in single-sex groups, 2–3 per cage. At 42 – 49 days after birth, eight male offspring from SD and ten from LD were weighed, administered a lethal intraperitoneal injection of 39 mg pentobarbital and intracardially perfused with phosphate buffered saline (PBS, pH 7.2) followed by 10% phosphate buffered formalin for 20 minutes. Brains were removed, weighed, scored on the right cerebral hemisphere, coded, and stored in 10% phosphate buffered formalin for at least 30 days. Brains were submerged in 20% sucrose / PBS overnight, then frozen sectioned coronally through the MeA at 60 and 30 μm. Every section was mounted on gelatin-coated slides, dried, stained with cresyl violet, dehydrated in ethanol, cleared in xylene, and coverslipped with Permount.
As in previous studies of P. sungorus (Cooke et al., 2002a; Cooke, et al., 2002b), the analysis of regional volume of MeA subnuclei was aided with the use of a rat atlas (Paxinos and Watson, 1998). Nissl-stained 60 μm-thick sections containing the MeA were viewed with a microprojector equipped with a 4× objective (Fig. 1). Beginning rostrally, when the MeAad first appears adjacent to the bed nucleus of the accessory olfactory tract (coordinates in Paxinos, 1998: interaural, +7.2 mm; medial / lateral, 3 mm), each subnucleus was completely traced in the right and left hemispheres. The area occupied by each tracing was measured with NIH Image, and subnucleus regional volume was estimated by multiplying the total area of each traced subnucleus by the section thickness.
Figure 1.
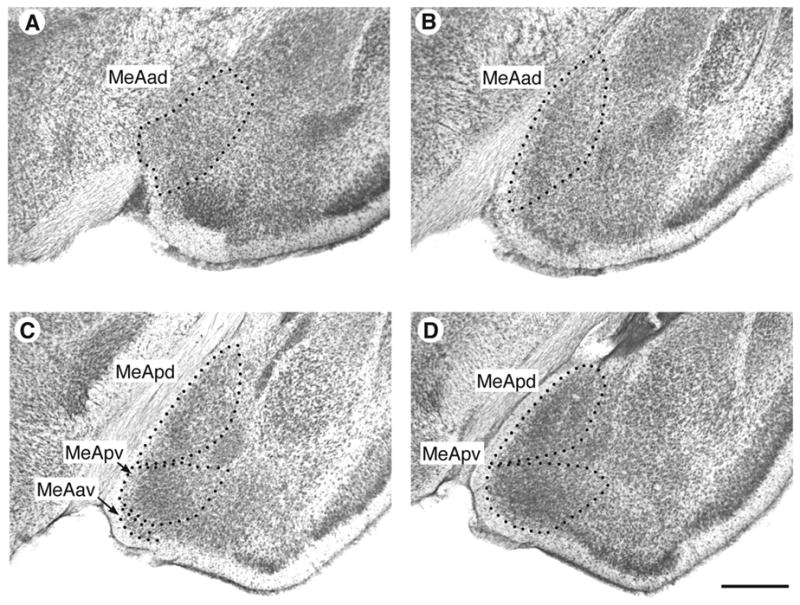
Photomicrographs of the MeA in P. sungorus showing the typical appearance and boundaries of the subnuclei. A–B. The rostral MeA contains the MeAad. C. A section containing the MeApd, the MeApv, and the MeAav. D. Caudal portion containing the MeApd and MeApv. Bar = 0.5 mm
Twenty regions within each MeA subnucleus, distributed across the entire rostrocaudal extent, were systematic-randomly selected for soma size measurements in the 30 μm-thick sections. Two well-isolated neurons from each region were selected for soma size measurements. Neuronal somata were viewed and traced with a microscope equipped with a 63x objective and a drawing tube. Neurons were recognized by their lightly stained nucleus surrounded by a rim of cytoplasm. The average soma size per subnucleus per hemisphere was then estimated by measuring each soma with NIH Image and dividing the total measured area by the number of traced neurons.
Neurons were counted in the MeApd using a systematic-random procedure. A grid was laid over a randomly selected tracing of the left or right MeApd, and 20 grid squares within the subnucleus were then systematically selected for counts, starting at a randomly selected point. In each selected region, a 1,600 μm2 counting frame was superimposed over a live video image of the MeApd at 100x. The focus was advanced in 0.7 μm steps from the top of the 30 μm-thick section to the bottom. Neurons were counted once as the focus was advanced downward and again as the focus was advanced upward. The average of the two counts was then used. The mean number of cells counted per hemisphere per animal was 105. Neuronal density was calculated by dividing the total number of cells by the volume of MeApd analyzed by the optical disector; this value was then multiplied by MeApd regional volume to estimate total neuron number.
Unless stated otherwise, data for each subnucleus were analyzed with a mixed design two-way analysis of variance, using photoperiod as an independent variable and hemispheric laterality as a repeated measure. Significant main effects prompted post-hoc analysis within each hemisphere with Tukey tests.
Results
As described in an earlier report (Hegstrom and Breedlove, 1998), LD hamsters had summer-like pelage, and significantly heavier body and paired testes weights than SD hamsters, which had winter-like pelage. Together, these data indicate that SD housing delayed the onset of puberty. In accordance with the report of Hoffman (1978), the paired testes weights of the LD hamsters were approximately 600 times greater than those raised in SD (Hegstrom and Breedlove, 1998) indicating that LD hamsters had adult-like levels of circulating androgen.
Brain weights did not significantly differ between the two groups (SD: 0.54 g ± 0.02; LD: 0.54 ± 0.01), indicating that reproductive maturation did not influence gross brain weight. However, in two of the four MeA subnuclei, LD hamsters had greater regional volumes than SD hamsters (Fig. 2). The MeApd was 25% larger in hamsters from LD than SD (p = 0.006; Fig. 2C, E–F). The left and right hemispheres were equivalent in volume and responded equally to the photoperiod (main effect of laterality and interaction, ps > 0.05), indicating that the MeApd is not lateralized nor lateralized in its response to puberty. Puberty also influenced the regional volume of the MeApv, which was 22% larger in LD males than in SD males (p = 0.008; Fig. 2D). Interestingly, the right MeApv was larger than the left (p = 0.003), but this laterality did not interact with either hemisphere’s responsiveness to photoperiod (p = 0.4).
Figure 2.
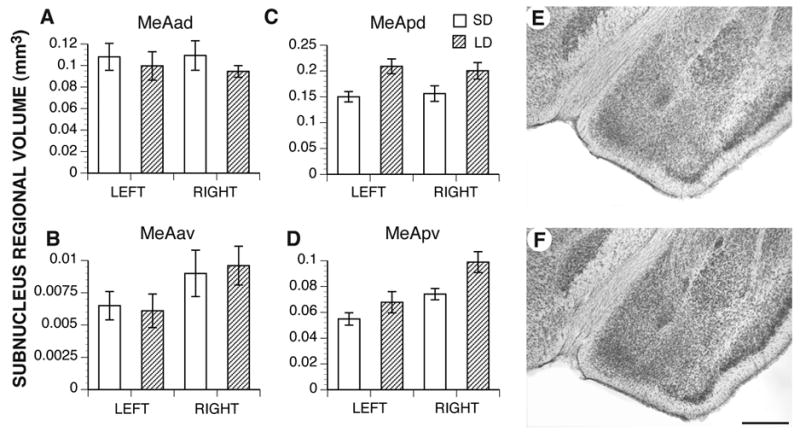
Mean regional volume of the four MeA subnuclei in the left and right hemispheres. In both hemispheres, rearing in long day (LD) photoperiods associated with gonadal maturation resulted in greater regional volumes only in the posterior nuclei (MeApd, C, and MeApv, D) compared to rearing in short days (SD; both ps < 0.01). Photoperiod had no effect on the anterior subnuclei. The right MeApv was significantly larger than the left in both groups of males (p < 0.01). E. MeApd from a SD hamster representing the mean regional volume for that group. F. MeApd from an LD hamster representing the mean regional volume for that group. Bar = 0.5 mm
The anterior subnuclei of the MeA, in contrast, were not affected by the photoperiod manipulation (Fig. 2A–B; MeAad, p = 0.3; MeAav, p = 0.9). Although the mean regional volume of the MeAav was larger in the right hemisphere than in the left, this was only marginally significant (p = 0.05).
Measurements of MeApd neuronal density indicated that LD males had slightly, albeit insignificantly, lower neuronal density than SD males (LD neuronal density: 62,385 ± 5,000 mm−3; SD neuronal density: 68,603 ± 3,100 mm−3, p = 0.3), with no effect of laterality (p = 0.9; Fig. 3A). Having estimated both MeApd regional volume and neuron density, neuron number could then be calculated. Since neuron density and regional volume were inversely related to each other in the LD and SD hamsters, this operation resulted in no differences in MeApd neuronal number. In LD hamsters, total bilateral MeApd neuron number was 11, 603 ± 900, whereas in SD hamsters, total MeApd neuron number was 10,301 ± 712 (t-test, p = 0.3).
Figure 3.
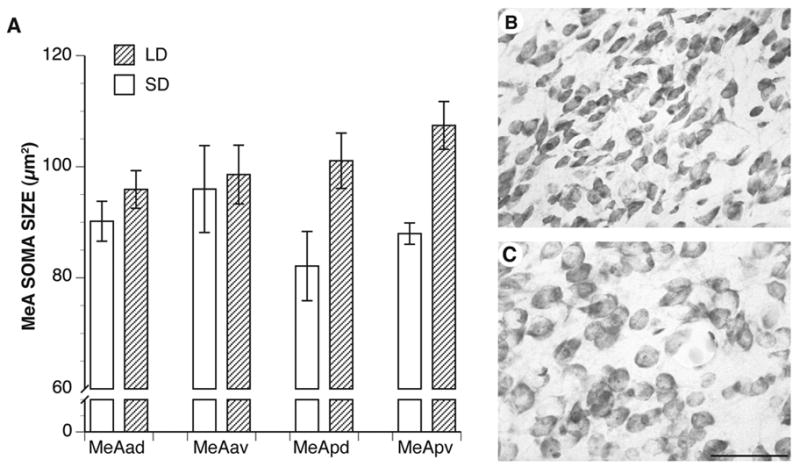
A. Mean bilateral neuronal soma sizes in the four MeA subnuclei. Male hamsters reared in LD had larger neuronal somata in the MeApd and MeApv subnuclei than males raised in SD (both ps ≤ 0.01). The anterior subnuclei were not affected by photoperiod. B. Photomicrograph of MeApd somata from an SD hamster. C. Photomicrograph of MeApd somata from an LD hamster. Bar = 5 μm.
In parallel with the volumetric growth seen in the posterior MeA, puberty stimulated growth in posterior MeA neuronal somata (Fig. 3A–C). That is, within the MeApd and MeApv, LD hamsters had significantly larger neuronal somata than SD hamsters (both ps ≤ 0.01). In contrast, neuronal somata within the MeAad and MeAav did not respond to the photoperiod manipulation (both ps ≥ 0.3). Although neuronal somata in the posterior subnuclei of the MeA responded to photoperiod, the response was equivalent across the hemispheres (p = 0.7), indicating that neuronal soma responsiveness is not lateralized.
Discussion
Hormone-dependent morphological plasticity in the MeA was originally demonstrated with artificial manipulations of circulating androgen in adult male and female rats. These studies showed that androgen levels characteristic of adult males maintain a male-like phenotype in the regional volume and mean soma size in the MeApd, but not in the anterior subnuclei (Cooke et al., 2003; Cooke et al., 1999). Experiments utilizing photoperiodic manipulations of adult P. sungorus revealed that photoperiod-dependent changes in pelage color and testis weight are associated with MeApd morphological plasticity (Cooke et al., 2002b), and that SD prevent male hamsters’ MeApds from responding to androgen (Cooke et al., 2002a). Here, we manipulated photoperiod to delay the onset of puberty in P. sungorus. This resulted in heavier body weights and darker pelage in the LD hamsters, as well as a massive difference in testes weight between the LD and SD hamsters. Previous research has shown that circulating androgen level is roughly proportional to paired testes weight (Yellon and Goldman, 1984). Thus, this result suggests that the LD hamsters had drastically higher levels of circulating androgen than the SD hamsters. The regional volume of the MeApd and MeApv in LD hamsters was also greater than in SD hamsters. Likewise, the mean soma size within the MeApd and MeApv was greater in the LD hamsters. Taken together, these findings indicate that gonadal maturation, and the concomitant rise in circulating androgen, is associated with growth in the regional volume and neuronal size of the posterior MeA in this species.
These effects of puberty on brain morphology appear to be specific: overall brain weight was unaffected by photoperiod and even within the MeA the anterior nuclei were unaffected. Presumably the structural growth in the posterior MeA at puberty reflects functional changes that are important for the transition to reproductive competence, including behavior.
Similar to the results reported here, the MeApd in reproductively mature Syrian hamsters has a greater cross-sectional area than in younger prepubertal hamsters (Romeo and Sisk, 2001). However, the underlying cellular basis of the volumetric plasticity in this study remains undetermined. Unpublished observations by Cooke indicate that neuronal somata account for less than 10% of the total regional volume of the rat MeApd. Assuming the composition of the MeApd in P. sungorus is similar to that of the rat, the 18% difference between LD and SD hamsters’ somata cannot explain the 25% difference in regional volume. Moreover, previous work in rats shows that cell size can be increased by non-aromatizable androgens without concomitant increases in MeApd regional volume (Cooke et al., 2003). Neuron counts in this study indicate that puberty in Siberian hamsters is associated with an insignificant reduction in neuronal density, and no change in total neuron number. The lack of a difference in neuron number between pre- and postpubertal hamsters suggests that growth in the extant neuropil, including dendrites, glial processes, or axons, is likely to account for the volumetric plasticity observed here.
MeApd neurons are capable of responding directly to testosterone or its metabolites with dendritic growth, as demonstrated with primary cell cultures of MeA neurons, which indicated that estrogen (Lorenzo, et al., 1992) or a single pulse of testosterone (Cooke and Woolley, 2005) increases overall dendritic length. The ability of embryonic MeA neurons to grow in response to hormone is consistent with gonadal hormones in adulthood modifying dendritic length, and consequently modifying MeApd volume. In support of this hypothesis, Gomez and Newman (1991) reported that gonadectomy reduces, and estradiol replacement maintains, the mean soma size and dendritic branching of posterior MeA neurons in Syrian hamsters. Thus, the pubertal rise in androgen may likewise increase the dendritic branching of P. sungorus MeA neurons, leading to an increase in regional volume.
Although there is precedent for gonadal hormones in adulthood to maintain high levels of dendritic branching, Zehr, et al., (2006) reported that the number of primary dendrites is reduced in reproductively mature Syrian hamsters compared to younger, prepubertal hamsters. Barring the possibility of a species difference, it is possible, then, that while the number of primary dendrites is reduced, the number of excitatory or inhibitory afferents is increased in reproductively mature P. sungorus, which would increase regional volume. Cooke and Woolley (unpublished observations) have recorded a greater frequency of spontaneous miniature excitatory postsynaptic currents in the MeApd of gonadally intact, reproductively mature male rats, as compared to adult, prepubertally gonadectomized males. This observation suggests that gonadal maturation results in greater functional connectivity, which could be due to an increase in the number of excitatory inputs to MeApd neurons.
Regional volume of the MeApv was lateralized in P. sungorus, being larger in the right hemisphere than the left. The mean regional volume of the MeAav was slightly greater in the right than in the left hemisphere as well. However, there was no correlation between the lateralized regional volume and soma sizes, which were equivalent between the hemispheres. These results also suggest that neuronal elements other than soma size must underlie the volumetric differences. The underlying basis of hemispheric laterality may rest on multiple factors, including neuron number, and the extent of dendritic and/or glial processes (Cooke, et al., 2007). Further study may determine which of these processes is responsible for the hemispheric laterality in the MeApv of P. sungorus.
Our finding that the photoperiodic delay of puberty in these males is accompanied by a delay in the development of the MeA indicates that this system is normally “activated” by testicular secretions at puberty. The growth in the regional volume of the MeApd without a corresponding increase in neuron number suggests that the rise in pubertal androgen and/or the resultant behavioral changes that accompany puberty, modify the volume occupied by extant neurons. Changes in regional volume may therefore reflect changes in neuropil, including the extent of dendritic branching from those neurons. The synaptic changes implied by this morphological plasticity may be related to the initiation of socio-sexual behaviors that are mediated in part by the MeA.
Acknowledgments
This research was supported by the National Institutes of Health (MH58703) and the generous contributions of Carol D. Hegstrom.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.


