Abstract
Idiopathic Parkinson’s disease (IPD) patients have abnormal visual evoked potentials (VEPs) and pattern electroretinograms (PERGs), attributed to dopaminergic transmission deficiency in visual pathway, probably the retina. VEP abnormalities are not reported in multiple system atrophy (MSA). The aim of this study was to investigate and compare chromatic (Ch) red-green (R-G) and blue-yellow (B-Y), and luminance yellow-black (Y-Bk) PERGs in patients with MSA and IPD. We investigated 6 MSA patients (mean age: 62±7.4 years) not undergoing any pharmacological treatment, as well as 12 early IPD patients (mean age: 60.1±8.3 years) and 12 age-matched normal observers. ChPERGs were recorded monocularly in response to full-field equiluminant R-G, B-Y and Y-Bk horizontal gratings. In MSA only responses to R-G stimuli showed minimal insignificant changes (slight but not significant amplitude reduction without any significant latency delay); no significant abnormality was detected for B-Y and luminance Y-Bk stimuli. By contrast, in IPD all responses were reduced in amplitude and delayed in latency, above all for B-Y stimuli. Present data indicate that both chromatic and achromatic PERGs are virtually unaffected in MSA, whereas in early IPD they are clearly impaired, suggesting different pathogenic retinal mechanisms and a useful simple tool for distinguishing MSA from IPD.
Keywords: Chromatic contrast, PERGs, Multiple system atrophy, Idiopathic Parkinson’s disease, Visual pathways subsystem, Parkinson and movement disorders
Introduction
Impairment of achromatic as well as chromatic vision in idiopathic Parkinson’s disease (IPD) has been extensively proven in recent years using clinical, psychophysiological and electrophysiological methods (electroretinograms, ERGs, and visual evoked potentials, VEPs) and attributed to dopaminergic deficiency at the retina level [1–8].
In multiple system atrophy (MSA), the visual system is believed to be spared and dopamine deficiency has been hypothesised to be less pronounced than in IPD [9], even though the data in the literature are scarce and not unanimous [10, 11] and nothing on retinal dopamine content has been reported. Little information is available on VEPs and pattern electroretinograms (PERGs) in MSA patients [9, 12]. Chromatic PERGs have not been previously employed to investigate possible dysfunction of the colour-opponent pathways at the retinal level in MSA. The main interest for studying responses elicited by pattern with pure chromatic contrast is that they allow recording of specific responses from colour-opponent pathways, anatomically and physiologically distinct from the achromatic ones at the retinal as well as the geniculate and cortical levels.
A differential diagnosis between MSA and IPD based on clinical criteria only is usually difficult, because up to 20% of patients diagnosed as IPD have another neurodegenerative disease [13]. In addition, both MSA and IPD patients exhibit rigidity and bradykinesia. Furthermore, up to 29% of MSA patients may be initially levodopa responsive [14]. Finally, the two conditions can erroneously be exchanged [9]. The differential diagnosis would benefit from a functional test able to distinguish MSA from IPD.
The aim of this study was to investigate chromatic PERG (ChPERGs) in response to equiluminant R-G and B-Y gratings in patients with MSA and IPD. ChPERGs were compared to achromatic PERG (LumPERGs) in response to yellow-black (Y-Bk) gratings to determine if there were differences between the colour-opponent pathways (parvocellular, koniocellular) and the luminance-opponent (magnocellular) pathway.
Materials and methods
Subjects
ChPERGs and LumPERGS were recorded in 18 patients (MSA=6, IPD=12). All were in the early stage of disease (IPD) or were of new or recent diagnosis (MSA), were tested before any therapy was started and had not been taking other drugs (sedatives, neuroleptic, anti-epileptic or others) for at least six months before inclusion in the study. The patients gave their informed consent after the aims and nature of the study had been fully explained. The experimental protocol had previously obtained the approval of the local ethics committee and followed the tenets of the Declaration of Helsinki. All subjects were preliminarily examined by an ophthalmologist and they satisfied the following ophthalmological criteria: visual acuity better than 6/10, clear ocular media, no evidence of retinal pathology or visual disorders (glaucoma, retinopathy, inherited disorders of colour vision). None of them had mental decline or other disease symptoms, like dementia (DSM III-R) or evidence of additional CNS pathology. Patients used their corrective glasses for near vision during all tests.
Multiple system atrophy
Six patients (4 men and 2 females) satisfying the clinical criteria of MSA [15] were enrolled in the study; their mean age was 62±7.4 years (range 51–70 years). Diagnosis was performed according to clinical criteria [16] and instrumental (blink-reflex habituation, anal sphincter electromyography, autonomic tests, MRI, CT scan) and pharmacological (l-dopa, apomorphine) tests.
Duration of illness ranged from 3 to 6 years (mean 4.7 (1.5 years). Parkinsonism was always present, associated with at least two of the following signs: autonomic failure, clinical signs of cerebellar or pyramidal tract involvement, cerebellar or brain stem atrophy on neuroimaging (MRI or CT scan). According to the above-mentioned classification they could be divided into two disease subtypes: 4 MSA of parkinsonian type (MSA-P) (striatonigral degeneration, SND) and 2 MSA of cerebellar type (MSA-C) (olivopontocerebellar, OPCA).
Idiopathic Parkinson’s disease
Twelve patients (6 men and 6 females) were studied. The diagnosis of IPD was established according to the United Kingdom Parkinson’s Disease Society Brain Bank Criteria [17]. Furthermore, instrumental (blink-reflex habituation, CT scan, MRI) and pharmacological (l-dopa, apomorphine) tests were employed. The mean age was 60.1±8.3 years (range: 46–74 years). All were IPD patients in the first stages of disease. Duration of illness ranged from 1 to 4 years (mean disease duration 2.1 (1.4 years).
The disease stage was 1.6±0.1 (range: 1–2) following the Hoehn-Yahr grading [18]; 8.6±1.0 according to sub-item II and 14.5±3.8 to sub-item III of UPDRS [19]. Exclusion criteria were adopted so that cases of other secondary parkinsonism were not included.
The main clinical visual features from ophthalmological examination, in PD as well as in MSA patients, are summarised in Table 1.
Table 1.
Summary of main clinical ophthalmological features of both groups of patients and normal observers; colour ratio was established by the method of heterochromatic flicker photometry [24]
| Groups | Decimal acuity (mean±1SD) | Chromatic perception | R-G ratio (mean±1SD) | B-Y ratio (mean±1SD) |
|---|---|---|---|---|
| MSA patients (n=6) | 9.2±0.8 | Normal in 6/6 | 49.2±1.3 | 49.4±2.2 |
| IPD patients (n=12) | 9.6±1.8 | Normal in 12/12 | 49.6±0.6 | 47.4±0.7 |
| Controls (n=12) | 9.2±1.1 | Normal in 12/12 | 51.1±0.5 | 49.7±0.6 |
Controls
A group of 12 age-matched normal volunteer observers, 6 men and 6 females: mean age 51.2±15.6 years (range: 41–74 years), served as control observers for both patient subtypes.
Visual stimuli
Visual stimuli were reversing horizontal sine-wave gratings generated by a widely used board and displayed on a colour monitor [20–22].
Chromatic visual stimuli were R-G and B-Y equiluminant horizontal sine-wave gratings; luminance achromatic ones were Y-Bk gratings [22].
For R-G and Y-Bk patterns, the display was viewed through yellow filters (Kodak Wratten 16) to attenuate wavelengths lower than 500 nm [23]; the filter also reduced the mean luminance. All tests were done at the same time between 14.00 and 17.00 p.m. in the same room and under constant mesopic illumination. The equiluminance was established psychophysically for every subject with a variant of the method of heterochromatic flicker photometry [24]. More details can be found elsewhere [8, 22].
PERG recordings
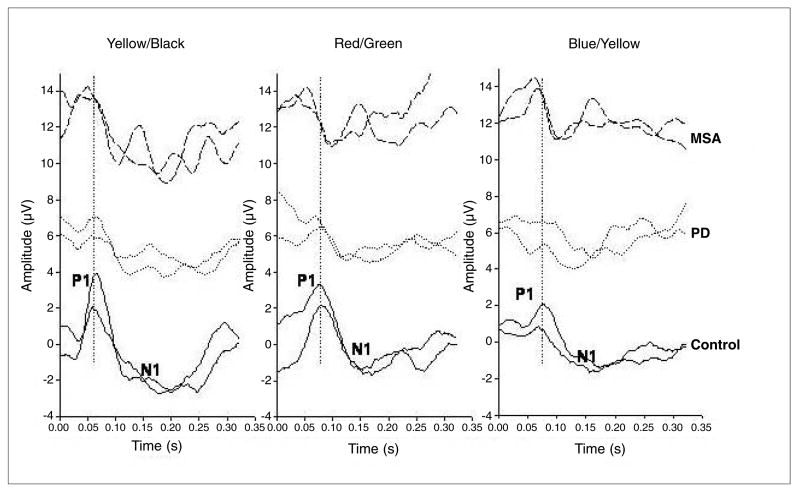
PERGs were recorded monocularly using common Ag/AgCl superficial cup electrodes, 9 mm in diameter (impedance <5 kΩ), the active taped in mid position over the inferior eyelid and the reference on the same position of the contralateral patched eye; the ground was located on the central forehead (Fz). Signals were filtered (0.3–100 Hz, –6 dB/oct), amplified (100 000 fold), digitised (2 kHz, 12-bit resolution) and on-line averaged by a Personal Computer (at least 100 sums) with rejection of signals exceeding a pre-established threshold voltage (80 mV). Two traces were obtained for each stimulating pattern to ensure response consistency (Fig. 1). PERGs showed a typical positive-negative waveform (P1-N1) [8]; latencies and amplitudes of different waves were measured using the cursor with the digital readout on the monitor: amplitudes from peak to peak, latencies at P1 peak. Sweep duration was 350 ms. Responses to patterns of zero contrast were frequently recorded to have a measure of residual noise. The subject under examination was seated in a semi-dark, acoustically isolated room in front of the display. Prior to the experiment, each subject was adapted to the ambient room light for 10 min. The diameter of undilated pupils was 4–5 mm in both control subjects and patients.
Fig. 1.
Examples of transient ChPERGs in a MSA patient (Z.E., m, 59 years; top traces), an IPD patient (C.G., m, 64 years; middle traces) and a control observer (D.R.C., f, 51 years; bottom traces) for all the three stimuli reported in a cascade way. Two traces are superimposed for each stimuli condition to ensure reliability. P1 and N1 components are labelled for each PERG waveform and type of stimulus. Note the evident amplitude loss and tendency to latency shift for B-Y gratings in the IPD cases
Statistical analysis
Comparison of amplitude and latency medians between control subjects and PD patients was performed by means of the non-parametric Mann-Whitney rank sum test. A p value of <0.05 was considered significant. Data were analysed with Sigma Stat 2.03 statistical package.
Results
Colour ratios (heterochromatic flicker photometry [24]) in MSA patients (mean value±SEM: R-G=49.2±1.3; B-Y=49.4±2.2) and IPD patients (mean value±SEM: R-G=49.6±0.6; B-Y=47.4±0.7) were not significantly different from those detected in age-matched controls (R-G=51.1±0.5; B-Y=49.7±0.6) (Table 1). This, in addition to yielding the necessary colour coordinates for generating equiluminant chromatic stimuli, provided evidence that patients were free from congenital or acquired colour vision abnormalities.
In MSA and IPD patients, as well as in control subjects, abrupt reversal of chromatic patterns evoked reproducible transient PERGs with the above cited typical positive-negative shape (P1-N1) for all stimulus conditions. As shown in the representative example of Figure 1, R-G, B-Y and Y-Bk PERGs differ in their relative amplitude and peak latency. Two responses are displayed superimposed to show reproducibility. An example of a typical waveshape recorded in a case of MSA, IPD and in a control is shown in Figure 1, from which it can clearly be seen that the latency and amplitude for all three stimuli changes in IPD, but not substantially in MSA, compared with controls.
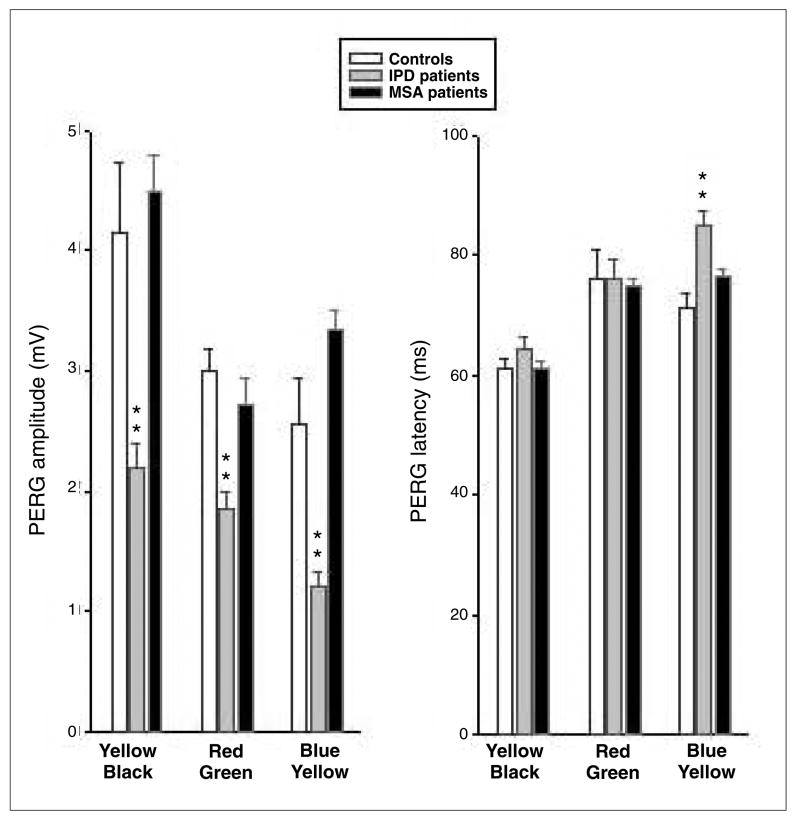
Mean ChPERG values of latency and amplitude obtained in both patient groups and controls are summarised in Figure 2. Median numerical data and Mann-Whitney rank sum test are summarised in Table 2. In MSA, the median PERG latencies were not significantly different from those of controls for the three stimulus conditions. In IPD, however, PERG latency was significantly delayed (about 15 ms) for B-Y stimuli as compared to controls. In MSA, the median amplitudes were not significantly different from those of controls. By contrast, in IPD a large loss (about 45%) was evident in all three conditions, more significantly for the B-Y system.
Fig. 2.
Mean (±Std. Err. ChPERGs) amplitude (mV) on the left and latency (ms) on the right histograms in controls (white columns), IPD (grey columns) and MSA (black columns) patients. Note that PERGs are substantially spared in MSA patients, whereas they are reduced and delayed in early IPD patients, especially for B-Y stimuli (**p<0.01)
Table 2.
Summary of non-parametric statistics performed by the Mann-Whitney rank sum test on PERG amplitude and latency in MSA, early IPD patients and control subjects (C). The reduction in amplitude in PD patients was highly significant for all stimulus conditions, whereas the latency increase was highly significant for the B-Y stimulus only
| Group | Median | 25% | 75% | Mann-Whitney (patients versus controls) | Mann-Whitney (MSA versus IPD patients) |
|---|---|---|---|---|---|
| Amplitude | |||||
| MSA Y-Bk | 4.5 | 2.4 | 6.39 | p=0.896 | p=0.002 |
| IPD Y-Bk | 2.2 | 1.3 | 2.7 | p≤0.001 | |
| C Y-Bk | 4.15 | 3.5 | 5.2 | ||
| MSA R-G | 2.72 | 2.7 | 3.1 | p=0.249 | p=0.358 |
| IPD R-G | 1.85 | 1.4 | 2.2 | p≤0.001 | |
| C R-G | 3 | 2.65 | 3.6 | ||
| MSA B-Y | 3.34 | 1.9 | 3.9 | p=0.216 | p≤0.001 |
| IPD B-Y | 1.2 | 0.95 | 1.55 | p≤0.001 | |
| C B-Y | 2.55 | 1.9 | 3 | ||
| Latency | |||||
| MSA Y-Bk | 61 | 61 | 66 | p=0.586 | p=0.461 |
| IPD Y-Bk | 64.5 | 58 | 73.5 | p=0.108 | |
| C Y-Bk | 61 | 56 | 68 | ||
| MSA R-G | 75 | 70 | 86 | p=0.955 | p=0.871 |
| IPD R-G | 76 | 68 | 85 | p=0.792 | |
| C R-G | 76 | 70.5 | 77 | ||
| MSA B-Y | 76.5 | 65 | 78 | p=0.508 | p=0.011 |
| IPD B-Y | 85 | 79.5 | 91 | p≤0.001 | |
| C B-Y | 71 | 70 | 75.5 | ||
By comparing latency and amplitude between MSA and IPD patients, a statistically significant difference was detected for B/Y latency (p=0.01) and amplitude (p=0.001) and Y/Bk amplitude (p=0.002).
In summary, MSA PERG latencies and amplitudes were substantially spared compared with controls for R-G, B-Y and luminance Y-Bk stimuli. In IPD patients PERG mean amplitude was reduced by about 45% for both chromatic and luminance stimuli; PERG latencies were delayed, especially significantly for B-Y.
Conclusion
R-G pathway originates from smaller (midget) ganglion cells relaying to the parvocellular layers of the lateral geniculate nucleus (LGN). The B-Y stems from bistratified ganglion cells (with dendritic arbours in both ON and OFF laminae of the inner plexiform layer) and projects to the interlaminar (koniocellular) neurons of the LGN. The achromatic stream instead originates from large (parasol) ganglion cells projecting to the magnocellular layers of the LGN [22, 25–32]. Moreover they have received increasing attention for studying basic mechanisms of vision and clinical application.
In this study the objective of recording responses to pure chromatic contrast was to isolate the activity of colour-opponent midget/parvocellular [25, 29] and bistratified/koniocellular [30] in an attempt to determine the retinal existence or not of chromatic visual losses in MSA compared with IPD.
Present results show that in IPD, both chromatic and achromatic PERGs are systematically reduced in amplitude across all stimulus conditions as compared to controls, and B-Y PERGs are also delayed in latency, remarkably for B-Y stimuli [9]. By contrast, the same responses are not significantly different from controls in our particular group of MSA patients. PERG abnormalities in IPD have been related to dopaminergic deficiency [33–36], as they are found in cases of neuroleptic-induced Parkinsonism and can be corrected by l-dopa administration [1, 37]. A retinal dopaminergic component for visual dysfunction in IPD has been proposed by several authors [3, 8, 34, 38]. Indeed, the retina contains both dopaminergic neurons [33] and dopaminergic receptors (D1 and D2) [3, 5, 39]. Dopaminergic deficiency at cortical level may occur as well in IPD [40, 41], as the human cortex has a widespread dopaminergic innervation [42]. The fact that in MSA chromatic and achromatic PERGs are in the normal range suggests that the function of parvocellular, koniocellular and magnocellular retinal ganglion cells is not significantly impaired in this disease.
These findings were very consistent among subjects and indicate that the results have not been biased by the small patient sample. Interpretation of our data is problematic due to the lack of other studies using chromatic stimuli in the literature. Only luminance PERGs have been tested in IPD and MSA and they were less impaired in the latter [43]. As far as concerns ChPERG, the visual deficits of the blue cone pathway in IPD are likely to be dopamine [8]. In the retina, dopamine is localised in the amacrine and interplexiform cells, playing a role in modulating the centre-surround organisation of the receptive field of retinal ganglion cells [44, 45]. In post-mortem studies of IPD patients a reduction in both dopamine and homovanillic acid in the retina has been reported [34, 46]. Furthermore PERG abnormalities were detected in MPTP-treated monkeys [47, 48], which cause dopamine depletion. The fact that MSA patients do not show PERG abnormalities compared with IPD patients suggests that the retinal dopaminergic deficiency is probably less pronounced or virtually absent compared to IPD.
In conclusion the different behaviour of chromatic and achromatic PERGs in IPD and MSA patients may suggest that different pathogenetic mechanisms, at least at the retinal level, underlie these two conditions. In addition, the different vulnerability of the PERG may provide an additional means to distinguish IPD from MSA, in particular before any treatment is initiated, even though the data deserve further confirmation in more extensive studies.
Acknowledgments
MIUR-PRIN 2003, NIH/NEI R01 EY14957, NIH centre grant P-30-EY014801, unrestricted grant to University of Miami from Research to Prevent Blindness
Contributor Information
F. Sartucci, Department of Neuroscience, Clinical Neurology, University of Pisa, Pisa, Italy, CNR - Institute of Neuroscience, Pisa, Italy
G. Orlandi, Department of Neuroscience, Clinical Neurology, University of Pisa, Pisa, Italy
U. Bonuccelli, Department of Neuroscience, Clinical Neurology, University of Pisa, Pisa, Italy
D. Borghetti, Department of Neuroscience, Clinical Neurology, University of Pisa, Pisa, Italy
L. Murri, Department of Neuroscience, Clinical Neurology, University of Pisa, Pisa, Italy
C. Orsini, CNR - Institute of Neuroscience, Pisa, Italy
L. Domenici, CNR - Institute of Neuroscience, Pisa, Italy, International School for Advanced Studies (I.S.A.S.-S.I.S.S.A.), Cognitive Neuroscience Sector, Trieste, Italy
V. Porciatti, CNR - Institute of Neuroscience, Pisa, Italy, Bascom Palmer Eye Institute, University of Miami, School of Medicine, Miami, FL, USA
References
- 1.Bodis-Wollner I, Yahr M. Measurement of visual evoked potentials in Parkinson’s disease. Brain. 1978;101:661–671. doi: 10.1093/brain/101.4.661. [DOI] [PubMed] [Google Scholar]
- 2.Bodis-Wollner I, Marx MS, Mitra S, et al. Visual dysfunction in Parkinson’s disease. Loss in spatiotemporal contrast sensitivity. Brain. 1987;110:1675–1698. doi: 10.1093/brain/110.6.1675. [DOI] [PubMed] [Google Scholar]
- 3.Stanzione P, Pierelli P, Peppe A. Pattern visual evoked potentials and electroretinogram abnormalities in Parkinson’s disease: effect of l-dopa therapy. Clin Vis Sci. 1989;4:115–127. [Google Scholar]
- 4.Peppe A, Stanzione P, Pierelli F, et al. Visual alterations in “de novo” Parkinson’s disease: pattern electroretinogram latencies are more delayed and more reversible by levodopa than are visual evoked potentials. Neurology. 1995;45:1144–1148. doi: 10.1212/wnl.45.6.1144. [DOI] [PubMed] [Google Scholar]
- 5.Stanzione P, Pierantozzi M, Semprini R, et al. Increasing doses of l-sulpiride reveal dose- and spatial frequency-dependent effects of D2 selective blockade in the human electroretinogram. Vision Res. 1995;35:2659–2664. doi: 10.1016/0042-6989(95)00037-z. [DOI] [PubMed] [Google Scholar]
- 6.Buttner T, Kuhn W, Muller T, et al. Distorted color discrimination in ‘de novo’ parkinsonian patients. Neurology. 1995;45:386–387. doi: 10.1212/wnl.45.2.386. [DOI] [PubMed] [Google Scholar]
- 7.Buttner T, Kuhn W, Muller T, et al. Chromatic and achromatic visual evoked potentials in Parkinson’s disease. Electroencephalogr Clin Neurophysiol. 1996;100:443–447. [PubMed] [Google Scholar]
- 8.Sartucci F, Orlandi G, Lucetti C, et al. Changes in pattern electroretinograms to equiluminant red-green and blue-yellow gratings in patients with early Parkinson’s disease. J Clin Neurophysiol. 2003;20:375–381. doi: 10.1097/00004691-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Delalande I, Hache J, Forzy G, et al. Do visual-evoked potentials and spatiotemporal contrast sensitivity help to distinguish idiopathic Parkinson’s disease and multiple system atrophy? Mov Disord. 1998;13:446–452. doi: 10.1002/mds.870130312. [DOI] [PubMed] [Google Scholar]
- 10.Ghaemi M, Hilker R, Rudolf J, et al. Differentiating multiple system atrophy from Parkinson’s disease: contribution of striatal and midbrain MRI volumetry and multi-tracer PET imaging. J Neurol Neurosurg Psychiatry. 2002;73:517–523. doi: 10.1136/jnnp.73.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scherfler C, Seppi K, Donnemiller E, et al. Voxel-wise analysis of [123I]beta-CIT SPECT differentiates the Parkinson variant of multiple system atrophy from idiopathic Parkinson’s disease. Brain. 2005;128:1605–1612. doi: 10.1093/brain/awh485. [DOI] [PubMed] [Google Scholar]
- 12.Arpa J, Lopez-Pajares R, Cruz-Martinez A, et al. Multimodal evoked potentials in multiple system and late onset cerebellar atrophies. Neurologia. 1995;10:288–296. [PubMed] [Google Scholar]
- 13.Quinn N. Multiple system atrophy. In: Marsden CD, Fahn S, editors. Movement disorders. Vol. 3. Butterworths; London: 1994. pp. 262–281. [Google Scholar]
- 14.Wenning GK, Ben Shlomo Y, Magalhaes M, et al. Clinical features and natural history of multiple system atrophy: an analysis of 100 cases. Brain. 1994;117:835–845. doi: 10.1093/brain/117.4.835. [DOI] [PubMed] [Google Scholar]
- 15.Quinn N. Multiple system atrophy – the nature of the beast. J Neurol Neurosurg Psychiatry. 1989;52(Spec Suppl):78–89. doi: 10.1136/jnnp.52.suppl.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilmann S, Low PA, Quinn N, et al. Consensus statement on the diagnosis of multiple system atrophy. J Auton Nerv Syst. 1998;74:189–192. [PubMed] [Google Scholar]
- 17.Gibb WRG, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 19.Fahn S, Elton RL . a.M.o.t.U.d. Committee. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent developments in Parkinson’s disease. Macmillan; New York: 1987. pp. 153–163. [Google Scholar]
- 20.Krauskopf J, Williams DR, Heeley DW. Cardinal directions of color space. Vision Res. 1982;22:1123–1131. doi: 10.1016/0042-6989(82)90077-3. [DOI] [PubMed] [Google Scholar]
- 21.Rabin J, Switkes E, Crognale M, et al. Visual evoked potentials in three-dimensional color space: correlates of spatio-chromatic processing. Vision Res. 1994;34:2657–2671. doi: 10.1016/0042-6989(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 22.Porciatti V, Sartucci F. Normative data for onset VEPs to chromatic contrast. Clin Neurophysiol. 1999;110:772–781. doi: 10.1016/s1388-2457(99)00007-3. [DOI] [PubMed] [Google Scholar]
- 23.Fiorentini A, Porciatti V, Morrone MC, Burr DC. Visual ageing: unspecific decline of the responses to luminance and colour. Vision Res. 1996;36:3557–3566. doi: 10.1016/0042-6989(96)00032-6. [DOI] [PubMed] [Google Scholar]
- 24.Mullen KT. The contrast sensitivity of human colour vision to red-green and blue-yellow chromatic gratings. J Physiol. 1985;359:381–400. doi: 10.1113/jphysiol.1985.sp015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derrington AM, Krauskopf J, Lennie P. Chromatic mechanisms in lateral geniculate of macaque. J Physiol. 1984;357:241–265. doi: 10.1113/jphysiol.1984.sp015499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lennie P, D’Zmura M. Mechanisms of color vision. Crit Rev Neurobiol. 1988;3:333–400. [PubMed] [Google Scholar]
- 27.Livingstone M, Hubel D. Segregation of form, color, movement and depth: anatomy, physiology, and perception. [Review] Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- 28.Lennie P, Krauskopf J, Sclar G. Chromatic mechanisms in striate cortex of macaque. J Neurosci. 1990;10:649–669. doi: 10.1523/JNEUROSCI.10-02-00649.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merigan WH, Maunsell JHR. How parallel are the primate visual pathways? Annu Rev Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- 30.Dacey DM, Lee BB. The ‘blue-on’ opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- 31.Tobimatsu S, Tomoda H, Kato M. Parvocellular and magnocellular contributions to visual evoked potentials in humans: stimulation with chromatic and achromatic gratings and apparent motion. J Neurol Sci. 1995;134:73–82. doi: 10.1016/0022-510x(95)00222-x. [DOI] [PubMed] [Google Scholar]
- 32.Engel S, Zhang X, Wandell B. Colour tuning in human visual cortex measured with functional magnetic resonance imaging. Nature. 1997;388:68–71. doi: 10.1038/40398. [DOI] [PubMed] [Google Scholar]
- 33.Frederick JM, Rayborn ME, Laties AM, et al. Dopaminergic neurons in the human retina. J Comp Neurol. 1982;210:65–79. doi: 10.1002/cne.902100108. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen-Legros J. Functional neuroarchitecture of the retina: hypothesis on the dysfunction of retinal dopaminergic circuitry in Parkinson’s disease. Surg Radiol Anat. 1988;10:137–144. doi: 10.1007/BF02307822. [DOI] [PubMed] [Google Scholar]
- 35.Denis P, Fardin V, Nordmann JP. Localisation and characterization of substance P binding sites in rat and rabbit eyes. Invest Ophthalmol Vis Sci. 1991;32:1894–1902. [PubMed] [Google Scholar]
- 36.Domenici L, Trimarchi C, Piccolino M, et al. Dopaminergic drugs improve human visual contrast sensitivity. Human Neurobiol. 1985;4:195–197. [PubMed] [Google Scholar]
- 37.Buttner T, Kuhn W, Patzold T, Przuntek H. L-Dopa improves colour vision in Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1994;7:13–19. doi: 10.1007/BF02252659. [DOI] [PubMed] [Google Scholar]
- 38.Tagliati M, Bodis-Wollner I, Yahr MD. The pattern electroretinogram in Parkinson’s disease reveals lack of retinal spatial tuning. Electroencephalogr Clin Neurophysiol. 1996;100:1–11. doi: 10.1016/0168-5597(95)00169-7. [DOI] [PubMed] [Google Scholar]
- 39.Denis P, Elena PP, Nordmann JP, et al. Autoradiographic localization of D1 and D2 dopamine binding sites in the human retina. Neurosci Lett. 1990;116:81–86. doi: 10.1016/0304-3940(90)90390-u. [DOI] [PubMed] [Google Scholar]
- 40.Garnett ES, Nahmias C, Firnau C. Central dopaminergic pathways in hemiparkinsonism examined by positron emission tomography. Can J Neurol Sci. 1984;11(Suppl 1):174–179. doi: 10.1017/s0317167100046369. [DOI] [PubMed] [Google Scholar]
- 41.Bulens C, Meerwaldt JD, Van der Wildt GJ. Effect of stimulus orientation on contrast sensitivity in Parkinson’s disease. Neurology. 1988;38:76–81. doi: 10.1212/wnl.38.1.76. [DOI] [PubMed] [Google Scholar]
- 42.De Keyser J, Ebinger G, Vauquelin G. Evidence for a widespread dopaminergic innervation of the human cerebral cortex. Neurosci Lett. 1989;104:281–285. doi: 10.1016/0304-3940(89)90589-2. [DOI] [PubMed] [Google Scholar]
- 43.Langheinrich T, Tebartz van Elst L, Lagreze WA, et al. Visual contrast response functions in Parkinson’s disease: evidence from electroretinograms, visually evoked potentials and psychophysics. Clin Neurophysiol. 2000;111:66–74. doi: 10.1016/s1388-2457(99)00223-0. [DOI] [PubMed] [Google Scholar]
- 44.Dowling J. Functional and pharmacological organization of the retina: dopamine, interplexiform cells, and neuro-modulation. Res Publ Assoc Res Nerv Ment Dis. 1990;67:1–18. [PubMed] [Google Scholar]
- 45.Bodis-Wollner I, Tzelepi A. The push-pull action of dopamine on spatial tuning of the monkey retina: the effects of dopaminergic deficiency and selective D1 and D2 receptor ligands on the pattern electroretinogram. Vision Res. 1998;38:1479–1487. doi: 10.1016/s0042-6989(98)00028-5. [DOI] [PubMed] [Google Scholar]
- 46.Bodis-Wollner I. Visual deficits related to dopamine deficiency in experimental animals and Parkinson’s disease patients. Trends Neurosci. 1990;13:296–302. doi: 10.1016/0166-2236(90)90113-o. [DOI] [PubMed] [Google Scholar]
- 47.Ghilardi MF, Bodis-Wollner I, Onofrj MC, et al. Spatial frequency-dependent abnormalities of the pattern electroretinogram and visual evoked potentials in a parkinsonian monkey model. Brain. 1988;111:131–149. doi: 10.1093/brain/111.1.131. [DOI] [PubMed] [Google Scholar]
- 48.Bodis-Wollner I, Antal A. On the functional significance of primate retinal dopamine receptors. J Neural Transm. 1995;45:67–74. [PubMed] [Google Scholar]