Abstract
Oxidative stress alters cellular metabolic processes including protein synthesis. The eukaryotic initiation factor, eIF4E, acts in the rate-limiting steps of initiation and promotes nuclear export. Phosphorylation of eIF4E by mitogen activated protein kinase signal-integrating kinases 1 and 2 (Mnk) influences the affinity of eIF4E for the 5'-mRNA cap and fosters nuclear export activity. Although phosphorylation of eIF4E on Ser209 is observed following oxidant exposure, the contribution of Mnk isoforms and the significance of phosphorylation remain elusive. Using a Mnk inhibitor and fibroblasts derived from Mnk knockout mice, we demonstrate that that H2O2 enhances eIF4E phosphorylation in cells containing Mnk1. In contrast, cells containing only Mnk2 show little change or a decrease in eIF4E phosphorylation in response to H2O2. H2O2 also shifted eIF4GI protein from the nucleus to the cytoplasm suggesting that the increases in eIF4E phosphorylation may reflect enhanced substrate availability to cytoplasmic Mnk1. In Mnk1+/+ cells, H2O2 also enhanced eIF4E phosphorylation in the nucleus to a greater degree than in the cytoplasm, an effect not observed in cells containing Mnk2. In response to H2O2, all MEFs showed increased eIF4E:4E-BP1 and 4E-BP2:eIF4E binding and reduced eIF4E:eIF4GI binding. We also observed a dramatic increase in the amount of Mnk1 associated with eIF4E following affinity chromatography. These changes coincided with a smaller reduction in global protein synthesis in response to H2O2 in the DKO cells. These findings suggest that changes in eIF4GI distribution may enhance eIF4E phosphorylation and that the presence of either Mnk1 or 2 or any degree of eIF4E phosphorylation negatively regulates global protein synthesis in response to oxidant stress.
Keywords: translation, oxidant stress, signal transduction, mitogen-activate protein kinase signal-integrating kinase, eIF4E
Introduction
Reactive oxygen species (ROS) play a central role in the pathogenesis of numerous chronic diseases including atherosclerosis, chronic obstructive pulmonary disease, and bronchopulmonary dysplasia. The increased oxidant stress generated in these diseases has the capacity to modify basic cellular metabolic processes including protein synthesis. Alterations in the translational machinery and regulatory pathways is known to influence cell proliferation, survival, and phenotype, any or all of which may lead to aberrant tissue repair and remodeling (Richter & Sonenberg, 2005). Recent reports from our laboratory and others indicate that reactive oxygen species directly modulate translation at the level of initiation (Alirezaei, Marin, Nairn, Glowinski, & Premont, 2001; Duncan, Peterson, & Sevanian, 2005; Shenberger, Adams, & Zimmer, 2002; Shenberger, Myers, Zimmer, Powell, & Barchowsky, 2005). Under basal conditions, translation is principally regulated by a complex of peptide factors that join the mRNA with the ribosome (Gingras, Raught, & Sonenberg, 1999). Each eukaryotic cytoplasmic mRNA contains a 7-methyl-guanosine triphosphate (m7-GTP) moiety at the 5' end. Eukaryotic initiation factor 4E (eIF4E) binds this 5'-cap structure and with the subsequent binding of eIF4G and eIF4A, forms the eIF4F complex. The scaffold protein, eIF4G, contains binding sites both for eIF4E and poly (A)-binding protein in the N terminal region and for eIF3, eIF4A, and mitogen-activated protein kinase (MAPK) signal-integrating kinases (Mnk) in the C terminus (Prevot, Darlix, & Ohlmann, 2003). Assembly of these factors facilitates binding of the mRNA to the small ribosomal subunit, thereby completing formation of the 48S preinitiation complex (Gingras, Raught, & Sonenberg, 1999).
Generation of active eIF4F complexes is tightly regulated by a group of inhibitory proteins known as 4E-binding proteins (4E-BP), which compete for the eIF4G-binding site. Hyper-phosphorylation of 4E-BPs following stimulation with growth factors, insulin, and serum leads to the dissociation eIF4E from 4E-BP, thereby freeing eIF4E to join with eIF4G to promote cap-dependent translation. The affinity of eIF4E for the 5'-cap is modulated through the phosphorylation of eIF4E at Ser209 located near the C-terminus (Flynn & Proud, 1995). Presently, four human Mnk isoforms (1a, 1b, 2a, and 2b) and two mouse Mnk isoforms (1 and 2) have been reported which directly phosphorylate eIF4E (Fukunaga & Hunter, 1997; O'Loghlen, Gonzalez, Pineiro, Perez-Morgado, Salinas, & Martin, 2004; Scheper, Morrice, Kleijn, & Proud, 2001; Scheper et al., 2003; Waskiewicz, Johnson, Penn, Mahalingam, Kimball, & Cooper, 1999). Mnk1a is activated by factors that stimulate either the classical extracellular regulated kinase (ERK) or the stress- and cytokine-activated p38 MAPK pathways, while the Mnk1b variant, which lacks a MAPK binding domain, is constitutively active (O'Loghlen, Gonzalez, Pineiro, Perez-Morgado, Salinas, & Martin, 2004). The Mnk2 isoforms also possess high basal activity secondary to their insensitivity to the upstream MAPKs (Scheper, Morrice, Kleijn, & Proud, 2001). In addition, Mnk1b and Mnk2b lack the C-terminal nuclear export sequence resulting in distribution to both nuclear and cytoplasmic pools while the “a” variants remain predominantly cytoplasmic (O'Loghlen, Gonzalez, Pineiro, Perez-Morgado, Salinas, & Martin, 2004; Parra-Palau, Scheper, Wilson, & Proud, 2003; Scheper et al., 2003).
Because eIF4E phosphorylation is induced by growth-promoting factors, it has been postulated that phosphorylation enhances translational efficiency (Scheper & Proud, 2002). In support of this view, a point mutation within the eIF4E phosphorylation site of Drosophila reduces viability and body size of the larvae (Lachance, Miron, Raught, Sonenberg, & Lasko, 2002). Similarly, inhibition of eIF4E phosphorylation with the Mnk inhibitor, CGP57380, inhibits angiotensin II-mediated protein synthesis and hypertrophy in rat vascular smooth muscle cells (Ishida et al., 2003). Other reports, however, do not support a stimulatory role of eIF4E phosphorylation in translational regulation. In vitro analysis of m7-GTP binding kinetics demonstrates that phosphorylation of eIF4E at Ser209 actually reduces cap-affinity by electrostatic repulsion, thereby increasing the rate of dissociation of eIF4E from the 5'-cap (Slepenkov, Darzynkiewicz, & Rhoads, ; Zuberek et al., 2003). In addition, mice lacking Mnk develop normally and display no particular phenotype for up to 12 months (Ueda, Watanabe-Fukunaga, Fukuyama, Nagata, & Fukunaga, 2004). Inhibition of eIF4E phosphorylation with CGP57380 also fails to hinder de novo translation initiation during recovery from hypertonic stress (Morley & Naegele, 2002) .
Within the nucleus, eIF4E resides in nuclear bodies combined with the inhibitory proteins, promyelocytic leukemia protein (PML) and proline-rich homeodomain protein (Topisirovic, Capili, & Borden, 2002; Topisirovic, Culjkovic, Cohen, Perez, Skrabanek, & Borden, 2003). As in the cytoplasm, nuclear eIF4E must bind the mRNA cap to be functional, a process that requires the release of eIF4E from these binding partners. Whereas the regulatory pathways leading to nuclear eIF4E-mRNA binding are not well defined, it is clear that mRNA binding selectively promotes the export of cyclin D1 transcripts, but not GAPDH or actin transcripts (Topisirovic, Culjkovic, Cohen, Perez, Skrabanek, & Borden, 2003; Rousseau, Kaspar, Rosenwald, Gehrke, & Sonenberg, 1996). The recent demonstration that inhibition of eIF4E phosphorylation impairs cyclin D1 export indicates that phosphorylation is an important regulator of the nucleo-cytoplasmic transport activity of eIF4E (Topisirovic, Ruiz-Gutierrez, & Borden, 2004).
Although it has been clearly demonstrated that ROS enhance eIF4E phosphorylation, the contribution of the individual Mnk isoforms, as well as the significance of phosphorylation to altered translation kinetics, has remained largely unexplored (Alirezaei, Marin, Nairn, Glowinski, & Premont, 2001; Duncan, Peterson, Hagedorn, & Sevanian, 2003; Shenberger, Adams, & Zimmer, 2002; Shenberger, Myers, Zimmer, Powell, & Barchowsky, 2005). For this reason, we sought to characterize the contributions of Mnk1 and Mnk2 to oxidant-mediated changes in eIF4E phosphorylation and to assess the relationship between Mnk expression, eIF4F formation, and the nuclear eIF4E content. We show that oxidant-mediated increases in eIF4E phosphorylation involve Mnk1, but not Mnk2, and report evidence that increased availability of eIF4E and eIF4G to Mnk1 in the cytoplasm contributes to the apparent increase in Mnk1 “activity”. Furthermore, we show that the presence of either Mnk1 or Mnk2 contributes to oxidant-mediated reductions in protein synthesis, possibly by reducing eIF4F complex formation.
Materials and methods
Reagents and Supplies.
Antibodies were purchased from the corresponding suppliers: 4E-BP1, 4E-BP2, eIF2α, 4E-T, phospho (Thr197/202)-Mnk1, phospho (Thr180/Tyr 182)-p38 MAPK, ERK 1/2, c-jun, and p38 MAPK from Cell Signaling Technologies (Beverly, MA); eIF4E and eIF4E:FITC from BD/Transduction Laboratories (San Diego, CA); active-ERK 1/2 from Promega (Madison, WI); anti-rabbit Cy-2 from (Jackson ImmnoResearch Laboratories, Inc, West Grove, PA); and phospho (Ser209)-eIF4E, eIF4GI, RNA polymerase II (Pol II), Mnk1, and Mnk2 from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) and from Dr. Rikiro Fukunaga. Tritiated phenylalanine, anti-mouse and rabbit HRP-IgG, and m7-GTP Sepharose beads were obtained from GE Healthcare Bio-Science (Piscataway, NJ). Pre-cast gels were purchased from Invitrogen Corp. (Carlsbad, CA) and chemiluminescence detection was received from Pierce (Rockford, IL). CGP57380 was a kind gift from Dr. Hermann Gram at Novartis Institutes for Biomedical Research, Switzerland. Leptomycin was purchased from LC Laboratories (Woburn, MA). The remaining reagents and chemicals were obtained from Sigma Chemical (St. Louis, MO) unless otherwise stated in the text.
Cell culture and conditions.
The establishment of mouse embryonic fibroblasts from wild-type (WT), Mnk1 (1KO), Mnk2 (2KO), and Mnk1/2 (DKO) double knockout mice has been previously described (Ueda, Watanabe-Fukunaga, Fukuyama, Nagata, & Fukunaga, 2004). These cells were grown in DMEM supplemented with 10% FBS, 50 IU/ml of penicillin, 50 μg/ml streptomycin, and 2 mM glutamine. A549 cells (American Type Culture Collection, Manassas, VA) were propagated in Advanced Dulbeco's Minimal Essential Medium (ADMEM; Gibco, Grand Island, NY) supplemented with 5% FBS, 50 IU/ml of penicillin, 50 μg/ml streptomycin, and 2 mM glutamine.
Protein synthesis.
Protein synthesis was determined by measuring the incorporation of [2,3,4,5-3H]phenylalanine (2 μCi/ml, final concentration) into protein after a 1-hour labeling period. Following incubation, monolayers were rinsed with ice-cold PBS prior to precipitation with 10% trichloroacetic acid for 15 minutes. Precipitated proteins were then dissolved in 0.5 N NaOH and the corresponding radioactivity measured by liquid scintillation counting.
Immunoblotting.
Cell monolayers were rinsed with PBS, trypsinized, and lysed in western lysis buffer [20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 0.5 mM PMSF (final concentrations)]. Lysates were either passed through a 28-gauge needle three times or flash frozen with liquid N2 and thawed. Supernatants were collected following centrifugation at 1000 x g and total protein determined by the bicinchoninic acid assay. Equal amounts of protein were resolved on either 12% Bis-Tris or 3−8% Tris-acetate gels. Resolved proteins were transferred to PVDF membranes and incubated overnight at 4°C with primary antibodies [all at 1:1000, except 4E-BP1 (1:500), α-tubulin (1:2500), active-ERK 1/2 (1:2000) and β-actin (1:5000)] in 5% non-fat dry milk blocking buffer as previously described (Shenberger, Myers, Zimmer, Powell, & Barchowsky, 2005). Following rinsing, membranes were incubated with the corresponding HRP-conjugated secondary antibody (1:5000) and the signal detected by chemiluminescence. Values were normalized to β-actin expression.
m7-GTP affinity chromatography.
Affinity chromatography was performed as previously described (Shenberger, Myers, Zimmer, Powell, & Barchowsky, 2005). Briefly, equal amounts of protein lysates were added to 35 μl of m7-GTP-Sepharose beads in 250 μl of buffer A (20 mM Tris-HCl, pH 7.4, 0.2 mM EDTA, 100 mM KCl, 1 mM PMSF, 7 mM β-mercaptoethanol) and incubated for 2 hours at 4°C with agitation. The beads were pelleted, washed, and bound proteins removed by boiling in 20 μl of buffer B (50 mM Tris-HCl, pH 6.8, 100 mM DTT, 2% SDS, 0.1% bromophenol blue, 10% glycerol). Eluted proteins were then resolved by electrophoresis. The presence of eIF4GI, eIF4E, Mnk1, 4E-T, 4E-BP1, and 4E-BP2 were identified by immunoblotting. Band density ratios of eIF4E to 4E-BP1, 4E-BP2, Mnk1, 4E-T or eIF4GI were the determined by densitometry.
Cell fractionation.
Cells grown in 6-well plates were trypsinized, combined, and washed twice in cold-PBS. Cells were resuspended in 500 μl of ice-cold, hypotonic buffer [10 mM HEPES, pH 7.9, 0.5 mM DTT, 0.5 mM PMSF, 1 mM 4-(2-aminoethyl)-bezenesulfonylfluoride hydrochloride (AEBSF), 0.8 μM aprotinin, 50 μM bestatin, 15 μM E-64, 20 μM leupeptin, 10 μM pepstatin A (final concentrations)] and maintained on ice for 15 minutes. Following addition of 20 μl of 10% NP-40, lysates were centrifuged at 800 x g for 1 minute to pellet the nuclei. The supernatants were saved on ice as the cytoplasmic fraction. Pelleted nuclei were washed once with 500 μl of hypotonic buffer containing 2.5% NP-40 and then extracted with 100 μl of hypertonic buffer [20 mM HEPES, pH 7.9, 25% glycerol, 0.42 M NaCl, 0.2 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, 1mM AEBSF, 0.8 μM aprotinin, 50 μM bestatin, 15 μM E-64, 20 μM leupeptin, 10 μM pepstatin A (final concentrations)] on ice for 30 minutes. Fractions were then stored at −80°C until analysis by immunoblotting.
Immunofluorescent staining.
Cells grown on slides were fixed as previously described (Shenberger, Myers, Zimmer, Powell, & Barchowsky, 2005). Slides were incubated overnight at 4°C with anit-eIF4E:FITC antibody diluted 1:100 in PBS. Slides were then thoroughly rinsed with PBS, mounted with 30% glycerol, and immediately examined using a Nikon Diaphot-TMD microscope (Tokyo, Japan) equipped with epifluorescence optics and appropriate filters. Digital images were obtained using a QColor3 camera and QCapture software (QImaging, Burnaby, British Columbia).
Statistics.
All studies were performed a minimum of 3 times, with the exception of the leptomycin dose-response which was performed in duplicate on 3 different concentrations. Data were analyzed using analysis of variance with Fisher's LSD testing to determine individual differences when appropriate or via Student's t tests. Data are listed as mean ± standard error and the level of significance set at p<0.05.
Results
H2O2-induced eIF4E phosphorylation requires Mnk1.
Our laboratory previously reported that 4-hour incubations of A549 cells in the presence of H2O2 lead to increased eIF4E phosphorylation within 24 hours (Shenberger, Adams, & Zimmer, 2002). The current study sought to determine the relevance of the phosphorylation event to the acute alterations in translation kinetics induced by oxidants. As an initial measure, we established that treatment of A549 cells with graded concentrations of H2O2 stimulated eIF4E phosphorylation at Ser209. Responses became apparent between 15−30 minutes and reached maximal levels, 10-fold above baseline, at 60 minutes using 400 μM H2O2 (Fig 1A and B). At 1200 μM, phosphorylation of eIF4E diminished, but remained significantly elevated compared to baseline.
Figure 1.
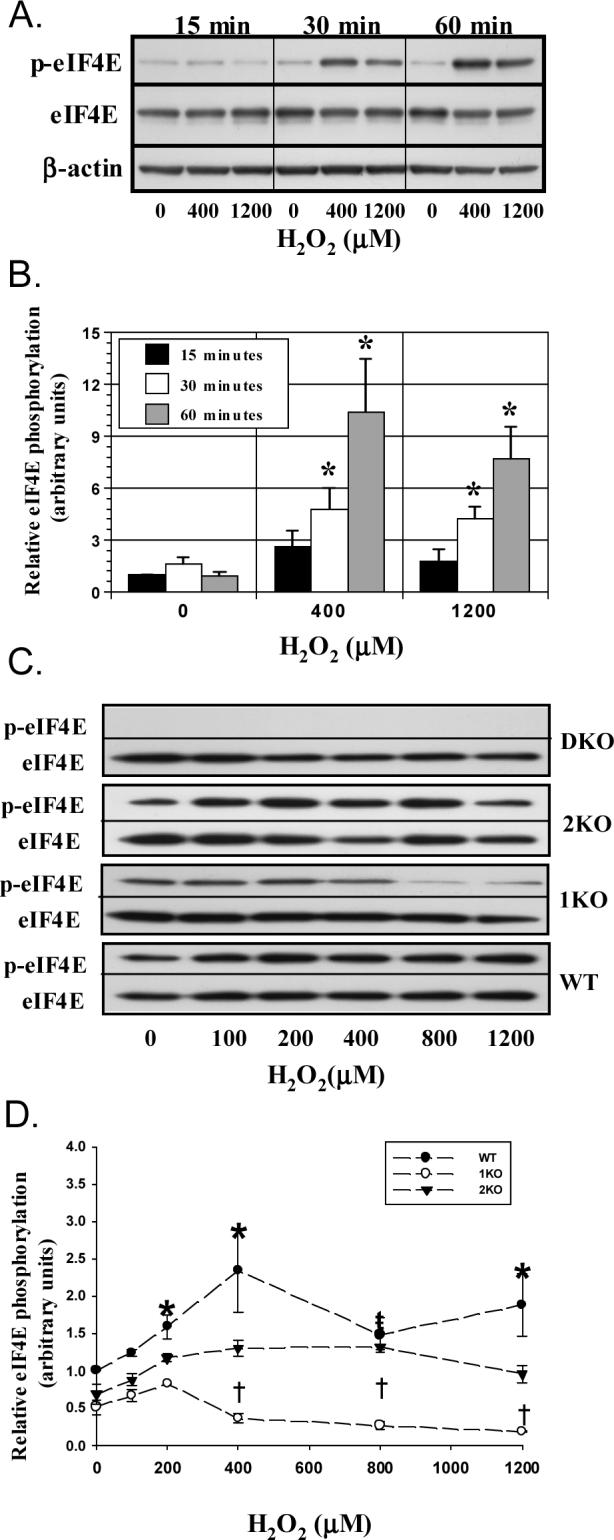
H2O2 induces eIF4E phosphorylation. A549 and MEF cells were treated with H2O2 for 15−60 min, lysed, and extracts immunoblotted for eIF4E, phosphorylated eIF4E, and β-actin. A. Figure displays representative time-course immunoblots in A549 cells. B. Histogram quantitating the relative eIF4E phosphorylation (p4E/4E) at various H2O2 concentrations in A549 cells. “*” denotes p<0.05 versus 0 μM H2O2 at 15 min (n=5). C. Representative immunoblots from MEFs treated with 100−1200 μM H2O2 for 60 min. D. Measurement of relative eIF4E phosphorylation (p4E/4E) in MEF cells. Each point represents mean of 4−8 trials ± SEM. “*” denotes difference between WT and 1KO/2KO at given concentration; “ŧ” a difference between WT and 1KO cells at given concentration; and “†” a difference between 2KO and 1KO cells at a given concentration. Columns represent mean and bars SEM.
We next studied the effects of H2O2 on MEFs lacking Mnk1, Mnk2, or both to determine the contributions of Mnk isoforms to oxidant-mediated eIF4E phosphorylation. Each MEF type displayed a somewhat different morphology and proliferation rate. Specifically, the 2KO cells were larger and slower to proliferate than WT and 1KO cells, while the DKO and WT cells were more stellate in appearance than either 2KO or 1KO cells. Despite these variations, the cells possessed uniform rates of phenylalanine incorporation while growing in 10% serum, although there was a tendency for a somewhat higher global protein synthetic rate in the DKO cells (WT: 239±37; 1KO: 266±26; 2KO: 265±35; DKO: 288±31 cpm/mg/min, NS, summation of 3 trials). To further define differences among the cell types, we quantitated the basal expression of factors involved in eIF4E phosphorylation. MEF cells expressed similar levels of eIF4GI, 4E-T, eIF4E, and 4E-BP1 proteins (n=3 trials). The expression of 4E-BP2, however, was greater in 1KO and 2KO cells than in WT and DKO cells (WT: 0.93±0.13; 1KO: 1.50±.08*; 2KO: 1.49±0.14*; DKO: 0.81±0.13, *p<0.05 vs. WT and DKO, n=3). The lack of a commercially available antibody prevented us from quantifying eIF4GII expression. Whether these specific differences in the MEF cells contributed to their unique morphologies is uncertain.
When treated with H2O2, MEFs generally elicited a less robust eIF4E phosphorylation response than was observed in A549 cells. As in A549 cells, treatment of WT and 2KO cells increased phosphorylation at concentrations greater 100 μM with maximal responses being reached at 60 minutes with 400 μM. Mnk1-deficient fibroblasts showed little change in eIF4E phosphorylation at concentrations ≤ 200 μM while higher concentrations reduced phosphorylation compared to WT and 2KO cells (Fig 1C and D). Not surprisingly, DKO cells were unable to phosphorylate eIF4E in response to H2O2 at all concentrations. These observations illustrate that Mnk isoforms may possess unique sensitivities to oxidants.
To ensure that changes in eIF4E phosphorylation were not secondary to alterations in upstream signaling pathways, we studied the activity of the Mnk kinases, p38 MAPK and ERK1/2. Treatment of all cell types with H2O2 increased the phosphorylation of p38 MAPK and ERK, although activation of p38 MAPK was consistently less in cells lacking Mnk2. As displayed in Figure 2A, p38 MAPK and ERK activation coincided with a dramatic increase in phosphorylation of Mnk1 in WT and 2KO cells (WT: 31±10; 2KO: 22±8 fold in arbitrary units, 400 vs. 0 μM H2O2, p<0.01), but not in 1KO or DKO cells. Our preliminary studies showed that CGP57380 dose-dependently reduced eIF4E phosphorylation with maximal effects achieved at 10−20 μM in both A549 and WT cells. Treatment of A549 cells with 20 μM CGP57380 reduced H2O2-stimulated eIF4E phosphorylation without altering ERK or p38 phosphorylation (Fig. 2B). We also found that the inhibitor enhanced Mnk1 phosphorylation at Thr197/202 in A549 and WT cells (Fig 2B and C). At concentrations of 10 and 20 μM, CGP57380 completely abolished Mnk2-mediated eIF4E phosphorylation, but only partially reduced phosphorylation in Mnk1-containing cells (Fig 2D). These findings confirm that H2O2 activates upstream MAP kinases in all knockout cell lines and further support Mnk1 as the kinase responsible for enhanced, oxidant-mediated, eIF4E phosphorylation.
Figure 2.
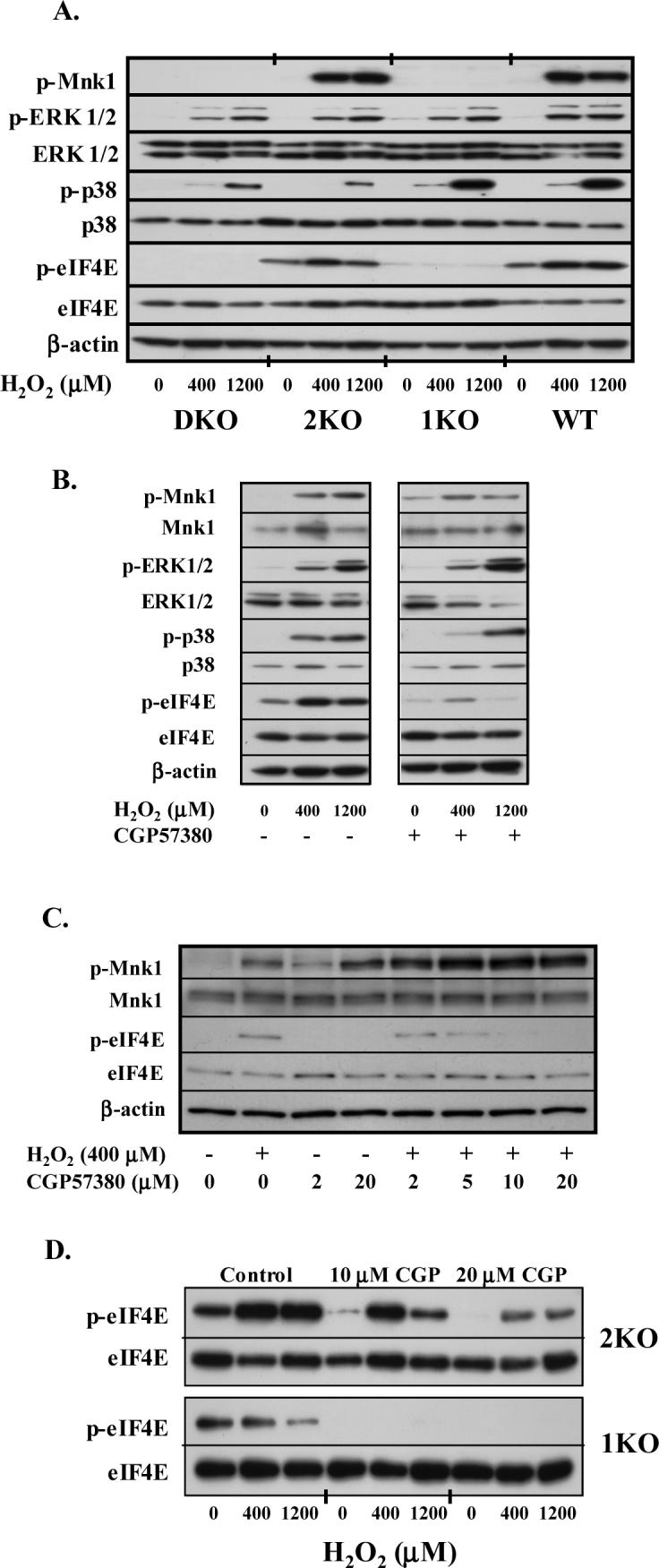
Kinase activation and signaling intermediates involved in eIF4E phosphorylation. A. Immunoblots obtained from MEFs treated with H2O2 for 60 min. Cells were prepared as described under MATERIALS AND METHODS, separated by electrophoresis, blotted onto PVDF, and incubated with respective kinase antibodies. B. Representative immunoblots of A549 cells incubated with varying concentrations of H2O2 for 60 min ± CGP57380 (20 μM) added 1 hour prior to H2O2. C. Immunoblot of A549 cells treated with varying concentrations of CGP57380 for 60 min prior to a 1-hr incubation with 400 μM H2O2. D. Representative immunoblot from 2KO and 1KO cells treated with CGP57380 prior to treatment with H2O2.
H2O2 reduces eIF4F complex formation.
In affinity purified extracts, H2O2 increased eIF4E:4E-BP1 binding and decreased eIF4E:eIF4GI binding in A549 cells (Fig 3A). Despite the 32−74% reduction in eIF4E phosphorylation in CGP57380-treated A549 cells, the binding ratios of eIF4E with 4E-BP1 and eIF4GI remained unchanged. Given the isoform-dependent nature of the eIF4E phosphorylation response, we also performed affinity purification of eIF4E from WT and Mnk knockout cells (Fig 3B). Affinity purification resulted in pull-down of equal amounts of eIF4E from all cell types. Calculation of eIF4GI:eIF4E and 4E-BP1:eIF4E binding ratios revealed that H2O2 reduced the binding of eIF4GI with eIF4E and increased the sequestration of eIF4E with 4E-BP1 (Fig. 3C and D: ANOVA, p<0.05). Post-hoc analysis found no isolated differences in eIF4GI:eIF4E binding, but a smaller reduction in 4E-BP1:eIF4E binding in DKO cells compared to the other MEFs. A separate set of affinity purifications was performed to assess the effect of H2O2 on 4E-BP2:eIF4E binding. As with 4E-BP1, we found that peroxide increased 4E-BP2:eIF4E binding (Fig. 4A and B).
Figure 3.
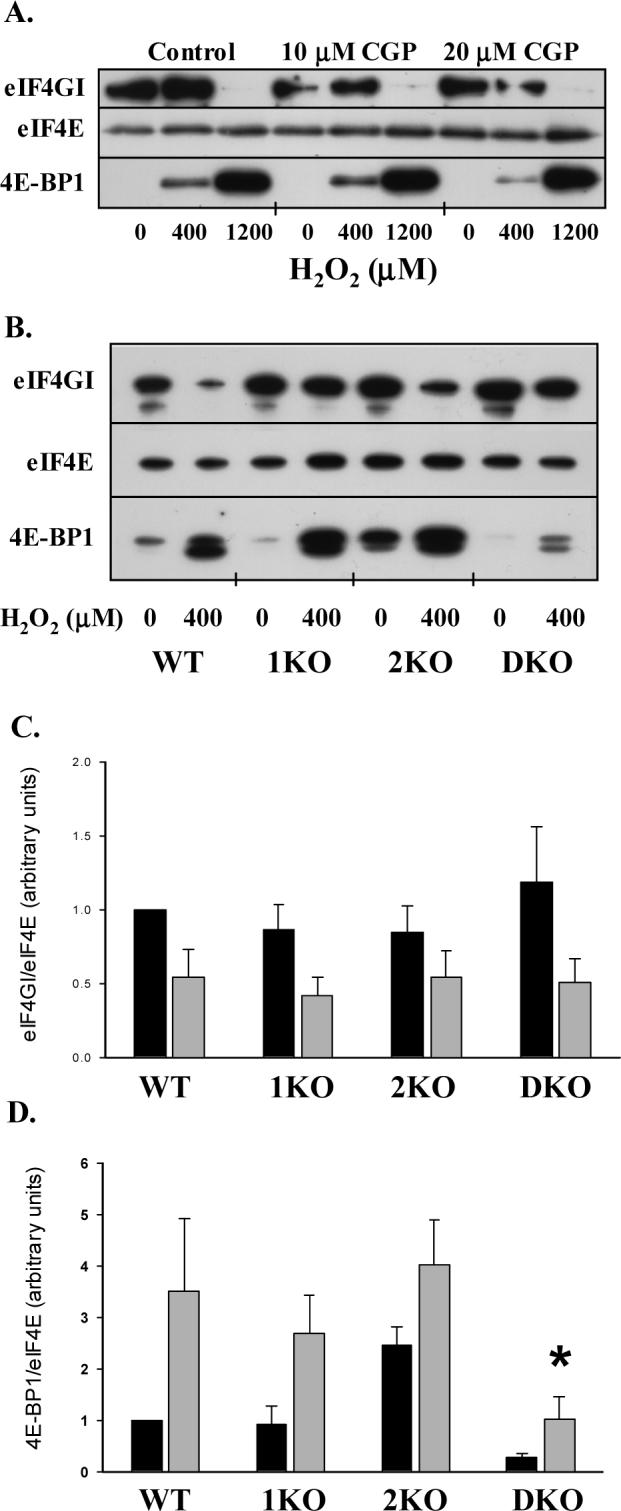
Role of Mnk1 and 2 in eIF4F complex formation during oxidant stress. A. Representative immunoblots of A549 cells treated with CGP57380 for 60 min prior to the addition of H2O2 for 60 min. Cells were lysed and eIF4E affinity-purified with m7-GTP Sepharose as described in MATERIALS AND METHODS. Following electrophoretic separation, blots were incubated with anti-eIF4E, 4E-BP1, and eIF4G antibodies. B. Representative immunoblots from affinity-purified extracts of MEFs incubated with and without 400 μM H2O2. C and D. Graphical presentation of eIF4G:eIF4E (C) and 4E-BP1:eIF4E (D) binding ratios calculated by densitometry following affinity purification of eIF4E. Columns represent mean (n=4−5) and bars SEM. “*” denotes p<0.05 vs. WT, 1KO, and 2KO cells following treatment with H2O2.
Figure 4.
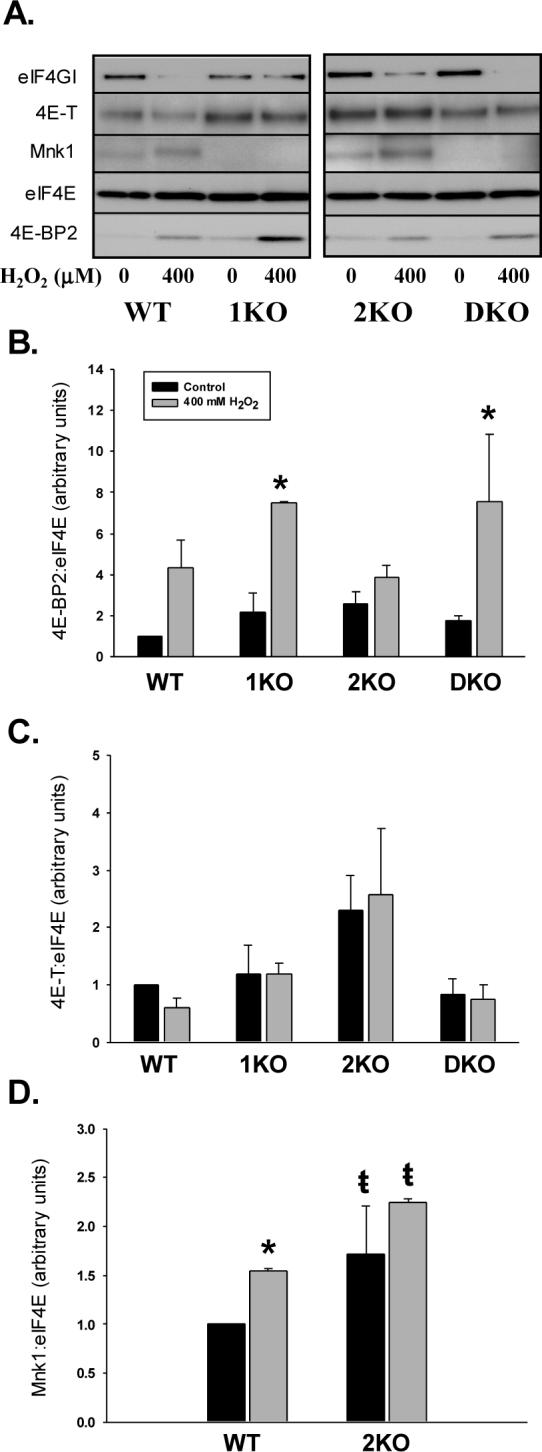
Effect of H2O2 on the association of 4E-BP2, 4E-T, and Mnk1 with eIF4E. A. Representative immunoblots from affinity purified MEF lysates blotted for eIF4GI, 4E-T, Mnk1, eIF4E, and 4E-BP2. B, C, D. Histograms illustrating the impact of H2O2 on 4E-BP2:eIF4E and 4E-T:eIF4E binding and the amount of Mnk1 associated with eIF4E, respectively. Columns represent means of 3 trials and bars SEM. “*” denotes p<0.05 difference with and with H2O2. “ŧ” denotes p<0.05 difference between 2KO cells treated with H2O2 and WT cells at baseline and following H2O2 treatment.
Peroxide increases Mnk1 co-purifying with eIF4E.
Using affinity chromatography, we also analyzed the amount of Mnk1 associated with eIF4E. Figure 4A illustrates that H2O2 increased the amount of Mnk1 co-purified with eIF4E. Correction of Mnk1 for eIF4E found no differences in the quantity of Mnk1 purified in WT and 2KO cells at baseline, but a greater increase in Mnk1 in 2KO after treatment with 400 μM H2O2 (Fig. 4D). Lack of a functional Mnk2 antibody precluded us from performing similar experiments in WT and 1KO cells. Lastly, we investigated peroxide-induced alterations in 4E-T:eIF4E binding. In the 3 trials conducted, we found no differences in basal or H2O2-stimulated 4E-T:eIF4E binding among the various MEFs (ANOVA, P=0.18; Fig. 4C).
Mnk1 enhances the nuclear content of phosphorylated eIF4E.
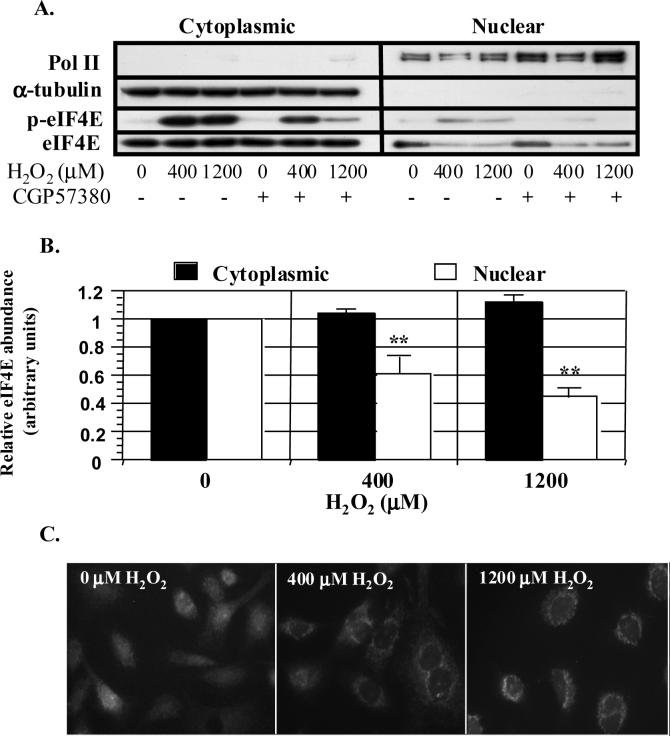
Aside from the well-defined role in the regulation of translation, eIF4E also serves as a nucleo-cytoplasmic shuttling factor, aiding in the transfer of specific mRNA species (Topisirovic, Capili, & Borden, 2002; Topisirovic, Culjkovic, Cohen, Perez, Skrabanek, & Borden, 2003; Topisirovic, Ruiz-Gutierrez, & Borden, 2004). Conceptually, phosphorylation could modify the cellular distribution of eIF4E thereby influencing the availability of eIF4E to shuttle nuclear mRNA. To determine if eIF4E phosphorylation impacts the nucleo-cytoplasmic distribution of eIF4E, we employed cell fractionation to separate eIF4E into nuclear and cytoplasmic compartments. Purity of compartment pools was indicated by the lack of α-tubulin and Pol II or c-jun proteins within the nuclear and cytoplasmic fractions, respectively. As shown in Figures 5A and B, exposure of the A549 cells to H2O2 decreased nuclear eIF4E content (**p<0.01) and tended to increase cytoplasmic eIF4E compared to baseline (p=0.072). The shift in eIF4E compartmentalization was also evident with fluorescent microscopy. As seen in Figure 4C, H2O2 treatment lead to a loss of eIF4E staining within the nucleus and more intense staining at the perinuclear region compared to control cells. Examination of both the nuclear and cytoplasmic fractions for phosphorylated eIF4E content revealed that H2O2 increased phosphorylation in both compartments with the greatest effect observed at 400 μM. Time course experiments revealed that phosphorylation increased within 15 minutes in the cytoplasm, but not until 30 minutes in the nucleus (not shown). Pretreatment of A549 cells with CGP57380 failed to alter the distribution of eIF4E, but reduced eIF4E phosphorylation in the cytoplasmic fraction by 37 and 76% for 400 and 1200 μM H2O2, respectively (p<0.05). Similar percentile reductions in eIF4E phosphorylation were seen in the nuclear fraction (Fig 5A).
Figure 5.
H2O2 reduces nuclear eIF4E content. Cells were pre-incubated with CGP57380 60 min prior to the addition of H2O2. Cells were then fractionated into cytoplasmic and nuclear compartments as described under MATERIALS AND METHODS and separated by SDS-PAGE. A. Representative immunoblots demonstrating regionalization of eIF4E. Purity of fractions was determined using the cytoplasmic marker, α− tubulin, and the nuclear marker, Pol II. Concentration of CGP57380 is 20 μM. B. Histograms of relative eIF4E abundance (arbitrary units) in nuclear and cytoplasmic fractions following H2O2 treatment. Columns represent mean change (n=3) relative to 0 μM H2O2 and bars indicate standard error. “**” denotes p<0.05 vs. 0 μM H2O2 in nuclear fraction. C. Photomicrograph illustrating H2O2-induced alterations in eIF4E distribution. Cells were fixed in paraformaldehyde and methanol and incubated overnight with anti-eIF4E:FITC. Fluorescent images were captured using a digital CCD camera.
Fractional analysis also detected total and phosphorylated Mnk1 only within the cytoplasmic fraction. The inability to identify either phosphorylated or total Mnk1 in nuclear fractions, suggests that phosphorylation of eIF4E occurs in the cytoplasm with subsequent nuclear transport. On the other hand, because Mnk1 is actively shuttled, it is possible that H2O2-mediated changes in Mnk1 distribution could influence eIF4E phosphorylation (McKendrick, Thompson, Ferreira, Morley, & Lewis, 2001). To aid in this determination, we pre-treated cells with the CRM1-/exportin 1 inhibitor, leptomycin, and investigated the effect of 400 μM H2O2 of eIF4E phosphorylation (Kudo et al., 1998). Duplicate experiments revealed that 1−10 ng/ml of leptomycin had no effect on basal or H2O2-stimulated eIF4E phosphorylation. Although these results do not preclude alterations in the distribution of phosphorylation eIF4E, they suggest that alterations in the distribution of Mnk1 are unlikely to modulate total cellular eIF4E phosphorylation.
Because our initial studies revealed that the MEFs display unique eIF4E phosphorylation responses to H2O2, we fractionated the knockout cells in an attempt to clarify these differences. Consistent with the results obtained in A549 cells, H2O2 reduced the nuclear content of eIF4E and tended to increase the quantity of cytoplasmic eIF4E in WT cells (Fig 6A and B). These effects were not influenced by either Mnk1 or Mnk2. Peroxide increased the phosphorylation of eIF4E in both cytoplasmic and nuclear compartments of Mnk1-containing cells (WT and 2KO). The relative increase in phosphorylated eIF4E in the nuclear compartment, however, was 2-fold greater than in the cytoplasmic compartment in 2KO cells. Cells deficient in Mnk1, on the other hand, displayed similar reductions in eIF4E phosphorylation in both compartments.
Figure 6.
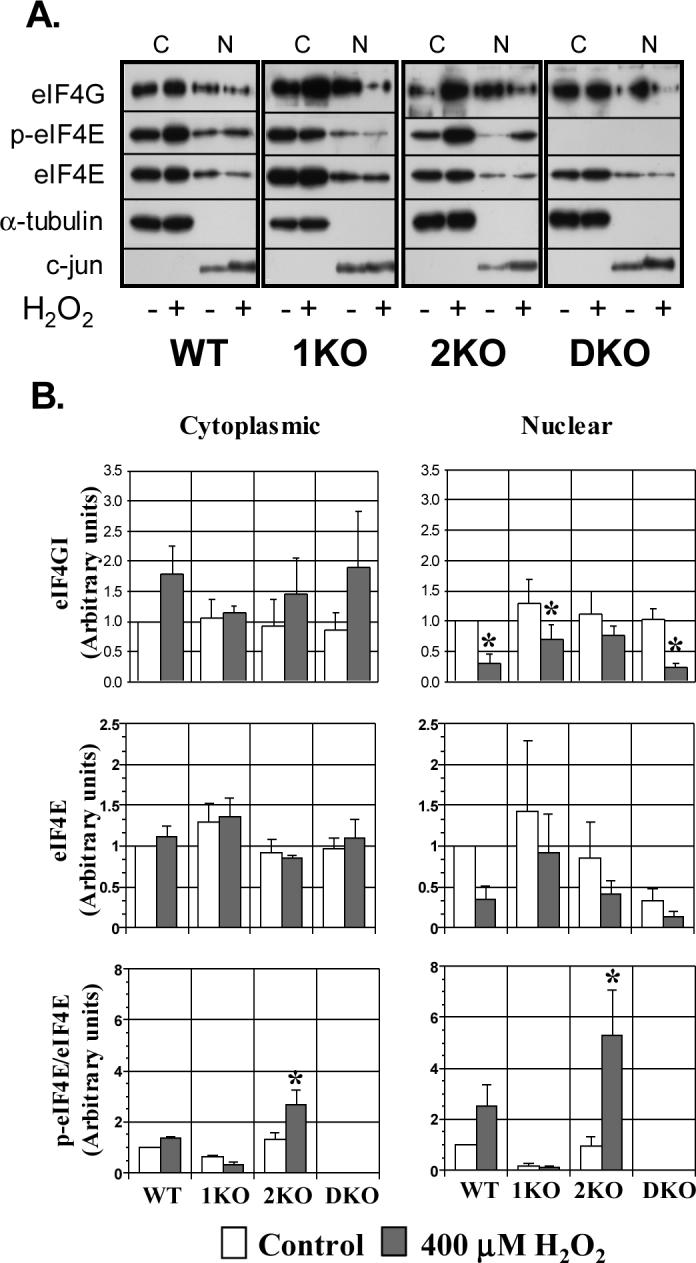
Role of Mnk isoforms in nuclear localization of eIF4E and phosphorylated eIF4E during oxidant stress. A. Representative immunoblots of fractionated cell lysates from MEFs following treatment with 400 μM H2O2 for 60 min. α-tubulin and c-jun were used as cytoplasmic and nuclear fraction markers. C=cytoplasmic; N=nuclear. B. Histograms portraying the relative expression of eIF4GI, eIF4E, and p-(phosphorylated) eIF4E relative to WT cells without H2O2. p-eIF4E was normalized to total eIF4E obtained from the same cell lysates and immunoblot. Columns represent mean of 4 experiments and bars indicate SEM. “*” denotes p<0.05 vs. control (no H2O2) of the same cell type.
The distribution of phosphorylated eIF4E might be explained by differences in the cellular location of Mnk isoforms or eIF4GI, the scaffold protein containing binding domains for both eIF4E and Mnk. In current study, we found total and phosphorylated Mnk1 exclusively within the cytoplasmic fraction. We were unable to detect a specific band corresponding to either Mnk2 in any cell type using commercially available antibodies or the antibody we prepared (Ueda, Watanabe-Fukunaga, Fukuyama, Nagata, & Fukunaga, 2004). eIF4GI was isolated from both cellular compartments and demonstrated a modest reduction in the nuclear fraction and an increase in the cytoplasmic expression upon stimulation with H2O2 (Fig 6B. p<0.05, ANOVA). Post-hoc analysis identified differences in only the nuclear fractions of WT, 1KO, and DKO cells before and after H2O2. Peroxide also decreased the nuclear content of eIF4E in MEFs (ANOVA, p<0.05), though cell-specific differences were note detected on post-hoc analysis. Treatment with H2O2 did not increase cytoplasmic eIF4E perhaps owing to the greater abundance in the cytoplasm offsetting these minor enhancements. In summary, the findings from this group of experiments suggest that oxidant mediated changes in eIF4E and eIF4GI distribution may contribute to an “apparent” increase in Mnk1 activity.
Mnk contributes to H2O2-mediated reductions in protein synthesis.
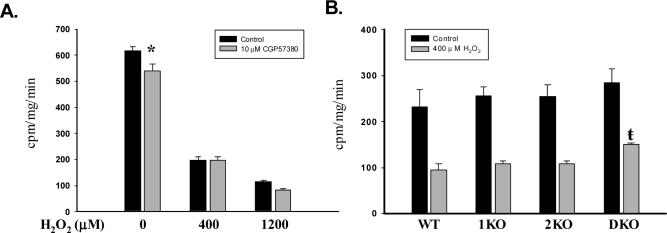
We next sought to determine if oxidant-mediated reductions in protein synthesis correlate with changes in eIF4E phosphorylation. Treatment of A549 cells with 400 and 1200 μM H2O2 reduced the incorporation of phenylalanine into protein by 68 and 81%, respectively. Blockade of Mnk activity with 20 μM CGP57380 prior to treatment with H2O2 decreased the incorporation of phenylalanine into protein in both control and H2O2 groups. This effect was not secondary to cytotoxicity as cell viability assessed by trypan blue exclusion and Annexin V staining was unchanged 24 hours after CGP57380 treatment (not shown). At 10 μM, CGP57380 diminished global protein synthesis by 12% at baseline (p<0.05), but not after H2O2 treatment (Fig 7A). To clarify the specificity of CGP57380 for Mnk, we next studied the impact of various concentrations of CGP57380 on phenylalanine incorporation in DKO cells. As illustrated in Figure 7B, CGP57380 decreased protein synthesis in DKO cells at 10 and 20 μM, but not at 5 μM, a concentration which modestly reduced, but did not eliminate eIF4E phosphorylation in A549 cells (Fig 2C).
Figure 7.
Role of Mnk isoforms in oxidant-mediated suppression of global protein synthesis. A. Representative experiment (n=3) showing effect of H2O2 on [3H]phenylalanine incorporation in A549 cells. Columns represent mean of 6 replicates and bars SEM. “*” p<0.05 vs Control at same H2O2 concentration. B. Histogram documenting the effect of 400 μM H2O2 on phenylalanine incorporation in MEFs. Columns represent mean of averaged values from 4 trials (n=4, per trial) while bars represent SEM. “ŧ” signifies p<0.05 vs. WT, 1KO, and DKO cells following 400 μM H2O2 treatment.
Although these results imply that eIF4E phosphorylation does not modulate oxidant-mediated translational repression, the methodology does not permit isolation of the effects of each isoform nor does it guarantee complete abrogation of Mnk activity. Accordingly, we determined phenylalanine incorporation in knockout MEFs in the presence and absence of 400 μM H2O2. Figure 7B illustrates that global protein synthetic rates were similar between the cells at baseline (Control). Treatment with H2O2 reduced the protein synthesis in each cell line, by 46−60% (ANOVA, p<0.01). Absolute incorporation, however, was greater in DKO cells following H2O2 than in WT, 1KO, and 2KO cells (p<0.05).
Discussion
Despite being a consistent finding during periods of stress, the physiologic significance of eIF4E phosphorylation remains undefined. In 48S preinitiation complexes, the majority of eIF4E resides in the phosphorylated form (Joshi-Barve, Rychlik, & Rhoads, 1990). This led to the concept that eIF4E phosphorylation increases cap-affinity, a theory supported by original crystallographic observations showing that phosphorylation at Ser209 promotes the formation of a lysine salt bridge between eIF4E and the 5'-terminus of mRNA (Minich, Balasta, Goss, & Rhoads, 1994). More recent analysis of stoichiometrically phosphorylated eIF4E found that phosphorylation at Ser209 actually decreased cap-analogue and capped-mRNA affinity 10-fold (Scheper, van Kollenburg, Hu, Luo, Goss, & Proud, 2002). In support of the current in vitro data, over-expression of Mnk2 in HEK 293 cells reduced overall protein synthetic rates while constitutively active Mnk1 mutants diminished cap-dependent translation (Knauf, Tschopp, & Gram, 2001). Given the conflicting data surrounding the function of eIF4E phosphorylation, it is not surprising that oxidant-mediated alterations in eIF4E phosphorylation have been correlated with both the promotion and impairment of translation (Duncan, Peterson, Hagedorn, & Sevanian, 2003; O'Loghlen, Perez-Morgado, Salinas, & Martin, 2006; Patel, McLeod, Vries, Flynn, Wang, & Proud, 2002; Shenberger, Adams, & Zimmer, 2002; Wang, Campbell, Miller, & Proud, 1998).
In a previous report, our laboratory demonstrated that exposure of actively proliferating A549 cells to H2O2 augments eIF4E phosphorylation by 24 hours (Shenberger, Adams, & Zimmer, 2002). The present study illustrates that exposure of A549 cells and MEFs to translation-inhibiting concentrations of H2O2 induces eIF4E phosphorylation within 15−30 minutes. The MAPK activated protein kinase family members, Mnk1 and 2, are the only known enzymes to directly phosphorylated eIF4E on Ser209 (Scheper, Morrice, Kleijn, & Proud, 2001; Waskiewicz, Johnson, Penn, Mahalingam, Kimball, & Cooper, 1999). Mnk1 has low basal activity but contains a MAPK-binding motif permitting activation by either ERK or p38 MAPK (Fukunaga & Hunter, 1997; Scheper, Morrice, Kleijn, & Proud, 2001; Waskiewicz, Flynn, Proud, & Cooper, 1997). Mnk2, on the other hand, possesses high basal activity that changes little upon MAPK stimulation (Scheper, Morrice, Kleijn, & Proud, 2001). By utilizing knockout cells, we show for the first time, that moderate concentrations of oxidants stimulate phosphorylation of eIF4E at Ser209 in Mnk1-containing cells, but not in cells containing only Mnk2. These isoform-specific changes in eIF4E phosphorylation are not secondary to altered upstream signaling of Mnk, as H2O2 stimulated ERK and p38 MAPK in all knockout cells. Oxidant-mediated activation of ERK and p38 MAPK also coincided with the phosphorylation of Mnk1 at Thr197/202, sites deemed essential for Mnk1 to phosphorylate eIF4E.
Although the consistent phosphorylation of the upstream kinases implicates the differential activation of Mnk isoforms as the source of the unique eIF4E phosphorylation dose-response curves in 1KO and 2KO cells, additional mechanisms are also likely to be involved. Endpoint phosphorylation is the net result of enzyme activity, substrate availability, and phosphatase activity. Phosphorylation of eIF4E principally occurs when eIF4E and Mnk are bound to eIF4G within the eIF4F complex. Mnk can phosphorylate eIF4E in the absence of eIF4G, but the efficiency of the process is low (Pyronnet, 2000). To bind eIF4GI/II, eIF4E must be free from competitive inhibition from hypophosphorylated 4E-BP1 and 4E-BP2 and potentially from 4E-T, which interacts with eIF4E through a conserved eIF4E recognition motif also found in eIF4G and 4E-BP (Dostie, Ferraiuolo, Pause, Adam, & Sonenberg, 2000). Oxidant stress illustrates the complexity of this interaction. Our findings indicate that peroxide increases the binding of eIF4E with 4E-BP1 and diminishes eIF4E binding to eIF4GI, effects which should decrease the capacity for Mnk to phosphorylate eIF4E. We also observed a small increase in cytoplasmic eIF4GI content following H2O2 treatment and an increase in the amount of Mnk1 co-purifying with eIF4E in WT and 1KO cells. The lower Mnk1:eIF4E ratio in WT cells may reflect competition between Mnk1 and Mnk2 for eIF4GI binding. Because we were unable to detect Mnk1 in eIF4GI immunoprecipitates due to interference from IgG heavy chain, we cannot definitively conclude that the Mnk1 co-purifying with eIF4E was bound to eIF4GI. Nonetheless, our results suggest that oxidant-mediated eIF4E phosphorylation involves enhanced access of eIF4E to Mnk1. Theoretically, peroxide might also inhibit protein phosphatase 2A (PP2A) activity, the putative eIF4E phosphatase, leading to a reduction in eIF4E phosphorylation despite stable or increasing Mnk activities (Peterson, Desai, Hardwick, & Schreiber, 1999). While H2O2 has not been found to modulate PP2A activity, inhibition of PP2A with okadaic acid has been reported to block H2O2-mediated reductions in 4E-BP1 phosphorylation (O'Loghlen, Perez-Morgado, Salinas, & Martin, 2003).
As an initial step in deciphering the physiologic significance of oxidant-induced eIF4E phosphorylation, we treated cells with the cell-permeable, Mnk inhibitor, CGP57380. Preliminary studies revealed that 10−20 μM CGP57380 markedly reduced H2O2-induced eIF4E phosphorylation in the absence of appreciable changes in the phosphorylation of ERK, p38 MAPK, Akt, or 4E-BP1. Surprisingly, CGP57380 increased Mnk1 phosphorylation at Thr197/202 both in A549 cells and MEFs. Enhanced phosphorylation of Mnk1 could contribute to the inhibitory effects of CGP57380 by reducing Mnk1:eIF4G binding (Parra, Buxade, & Proud, 2005). Treatment of the Mnk knockout cells with CGP57830 suppressed Mnk2-dependent eIF4E phosphorylation to a greater degree than Mnk1-dependent phosphorylation, demonstrating that CGP57380 is not specific for Mnk1 as is commonly reported. Peroxide treatment also appeared to suggest that Mnk2 is more sensitive to inhibition than Mnk1. Given that H2O2 modulates eIF4G:eIF4E binding, eIF4E phosphorylation by Mnk1 is likely to be less influenced secondary to temporizing effect of increased kinase activity.
Agents that commonly enhance eIF4E phosphorylation also tend to promote eIF4F formation, as noted in phenylephrine-treated cardiac myocytes (Rolfe, McLeod, Pratt, & Proud, 2005). Exposure to ROS, however, augments eIF4E phosphorylation while reducing eIF4F formation, suggesting that phosphorylation may reduce cap-affinity according to the electrostatic repulsion model proposed by Zuberek and colleagues (Zuberek et al., 2003). Nevertheless, inhibition of Mnk activity with CGP57380 and omission of either or both Mnk isoforms failed to alter H2O2-mediated reductions in eIF4E:eIF4G binding. These results signify that neither Mnk nor eIF4E phosphorylation modulates the reduction in eIF4F complex formation induced by oxidant stress.
Nuclear eIF4E also plays an important role in the regulation of translation by enhancing the transport of a restricted subset of growth-promoting mRNAs such as cyclin D1 and ornithine decarboxylase (Rousseau, Kaspar, Rosenwald, Gehrke, & Sonenberg, 1996). Stress induced by γ interferon and cadmium modulates eIF4E-dependent mRNA transport via a PML-dependent process (Topisirovic, Capili, & Borden, 2002). We found that oxidant stress reduced nuclear eIF4E in both A549 and MEFs, with a concomitant trend toward increased cytoplasmic eIF4E in A549 cells. The inability to identify reciprocal changes in cytoplasmic eIF4E could mean that changes were “diluted” in the cytoplasmic pool secondary to the much greater proportion of eIF4E within this compartment. Nonetheless, these findings depict the dynamic nature of eIF4E shuttling. Since growth arrest at the G1/S interface has been reported at these concentrations of H2O2, diminution of nuclear eIF4E could contribute indirectly to checkpoint function by reducing translation of growth-regulatory proteins (Deshpande et al., 2002; Shenberger, Adams, & Zimmer, 2002).
The combination of decreasing nuclear eIF4E protein and increasing phosphorylation raises speculation that phosphorylation modifies eIF4E translocation across the nuclear membrane by altering the affinity of eIF4E nuclear transporters (Topisirovic, Capili, & Borden, 2002; Topisirovic, Culjkovic, Cohen, Perez, Skrabanek, & Borden, 2003). Although phosphorylation has not been reported to alter the nucleo-cytoplasmic distribution of eIF4E, it has been shown to enhance eIF4E shuttling activity by enhancing nuclear export of cyclin D1 mRNA (Topisirovic, Ruiz-Gutierrez, & Borden, 2004). Blockade of eIF4E phosphorylation with CGP57380 reduced H2O2-mediated phosphorylation of eIF4E in both cellular compartments, but produced no net effect on eIF4E distribution. A similar observation was made in NIH3T3 cells treated with the Mnk inhibitor or transfected with a non-phosphorylatable mutant of eIF4E (Topisirovic, Ruiz-Gutierrez, & Borden, 2004). Likewise, loss of either Mnk1 or Mnk2 had no effect on the subcellular localization of eIF4E in MEFs. Thus it appears that phosphorylation plays little role in the subcellular distribution of total eIF4E. By comparison, phosphorylated eIF4E did show a distinct localization pattern in MEFs in response to H2O2. We found that Mnk1 stimulation enhanced the nuclear proportion of phosphorylated/total eIF4E to a greater extent than in the cytoplasm. Despite containing a nuclear localization motif in its N terminus, Mnk1 has not been found in the nucleus under stress and non-stress conditions (Parra-Palau, Scheper, Wilson, & Proud, 2003). Yet, rapid shuttling of Mnk1 could account for the peroxide-induced increase in eIF4E phosphorylation (McKendrick, Thompson, Ferreira, Morley, & Lewis, 2001). While we found no change in total eIF4E phosphorylation in A549 cells following leptomycin treatment, the distribution of phosphorylated eIF4E may have been altered. The eIF4E binding protein, 4E-T, which modulates the import of eIF4E into the nucleus could also account for fractional differences in phosphorylated eIF4E distribution (Ferraiuolo, Basak, Dostie, Murray, Schoenberg, & Sonenberg, 2005). In the knockout fibroblasts, we found little change in 4E-T:eIF4E binding in affinity purified extracts in response to H2O2. Although not proof-in-principle, these findings do not support active shuttling as a major factor regulating the distribution of phosphorylated eIF4E during oxidant stress.
Since H2O2-induced eIF4E phosphorylation failed to influence eIF4F formation or eIF4E distribution, we anticipated that global protein synthesis would not be influenced as well. We first sought to determine the impact of kinase inhibition on oxidant-mediated reductions in protein synthesis using CGP57380. To our surprise, 10 μM CGP57380 inhibited basal phenylalanine incorporation in A549 cells at baseline, but not following treatment with H2O2. Because A549 cells are a malignant cell line with multiple documented deficiencies, we tested CGP57380 on DKO cells. Here too CGP57380 inhibited global protein synthesis at concentrations of 10 μM and above, demonstrating that the compound possesses translational inhibitory properties unrelated to Mnk. Nevertheless, use of the knockout cells validated the accuracy of our original premise except when all Mnk activity was abolished by eliminating Mnk1 and 2. Under these conditions, complete loss of Mnk partially “protected” cells from oxidant-mediated translational repression. Indeed, assessment of eIF4F complex formation shows that DKO cells showed less 4E-BP1:eIF4E binding than other MEFs in response to H2O2.
Finally, the extrapolation of the findings reported in this study to other oxidants or cell systems must be done with some caution. As presented, the knockout cells displayed unique, unexplained morphologies and proliferative rates. In fact, 1KO and 2KO cells were noted to possess greater levels of 4E-BP2 protein, despite possessing similar protein synthetic rates Furthermore, our studies on eIF4F complex formation and cellular fractionation did not include measurement of eIF4GII nor Mnk2, elements likely to have significant impact on the overall protein synthetic response. In addition, H2O2 is known to stimulate multiple translational regulatory pathways. Although we found no differences in eIF2α and eEF2 levels or phosphorylation among the knockout cells (duplicate trials, data not shown), we cannot exclude the existence of other dispersant regulatory mechanisms.
In summary, this study illustrates that oxidant-mediated stimulation of eIF4E phosphorylation is likely to involve the enhanced availability of Mnk1 to eIF4E in addition to previously documented changes in kinase activity. Furthermore, the reductions in eIF4F complex formation and nuclear eIF4E and eIF4GI content following oxidative stress do not appear to be mediated by Mnk1/2 or eIF4E phosphorylation. Nonetheless, the presence of either Mnk isoform supports the suppression of protein synthesis during oxidative stress through an unknown mechanism. Future investigations will be required to clarify these findings and to explore the potential roles of eIF4GII and Mnk2 in these processes.
Acknowledgments
The authors would like to thank Dr. Leonard S. Jefferson and Dr. Scot Kimball for the wisdom and insight provided during the preparation and revision of this manuscript.
* This research was supported by NIH Grant 5K08HL71905 (to J.S.S.)
Glossary
List of Abbreviations
- Mnk
mitogen activated protein kinase signal-integrating kinase
- 1KO
Mnk1 knockout
- 2KO
Mnk2 knockout
- DKO
Mnk1/2 knockout
- WT
wild-type
- eIF
eukaryotic initiation factor
- ROS
reactive oxygen species
- m7-GTP
7-methyl-guanosine triphosphate
- MAPK
mitogen activated protein kinase
- ERK
extracellular regulated kinase
- PML
promyelocytic leukemia protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alirezaei M, Marin P, Nairn AC, Glowinski J, Premont J. Inhibition of protein synthesis in cortical neurons during exposure to hydrogen peroxide. J Neurochem. 2001;76:1080–8. doi: 10.1046/j.1471-4159.2001.00105.x. [DOI] [PubMed] [Google Scholar]
- Deshpande NN, Sorescu D, Seshiah P, Ushio-Fukai M, Akers M, Yin Q, et al. Mechanism of hydrogen peroxide-induced cell cycle arrest in vascular smooth muscle. Antioxid Redox Signal. 2002;4:845–54. doi: 10.1089/152308602760599007. [DOI] [PubMed] [Google Scholar]
- Dostie J, Ferraiuolo M, Pause A, Adam SA, Sonenberg N. A novel shuttling protein, 4E-T, mediates the nuclear import of the mRNA 5′ cap-binding protein, eIF4E. Embo J. 2000;19:3142–56. doi: 10.1093/emboj/19.12.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan RF, Peterson H, Hagedorn CH, Sevanian A. Oxidative stress increases eukaryotic initiation factor 4E phosphorylation in vascular cells. Biochem J. 2003;369:213–25. doi: 10.1042/BJ20020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan RF, Peterson H, Sevanian A. Signal transduction pathways leading to increased eIF4E phosphorylation caused by oxidative stress. Free Radic Biol Med. 2005;38:631–643. doi: 10.1016/j.freeradbiomed.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Ferraiuolo MA, Basak S, Dostie J, Murray EL, Schoenberg DR, Sonenberg N. A role for the eIF4E-binding protein 4E-T in P-body formation and mRNA decay. J Cell Biol. 2005;170:913–24. doi: 10.1083/jcb.200504039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn A, Proud CG. Serine 209, not serine 53, is the major site of phosphorylation in initiation factor eIF-4E in serum-treated Chinese hamster ovary cells. J Biol Chem. 1995;270:21684–8. doi: 10.1074/jbc.270.37.21684. [DOI] [PubMed] [Google Scholar]
- Fukunaga R, Hunter T. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 1997;16:1921–33. doi: 10.1093/emboj/16.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–63. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- Ishida M, Ishida T, Nakashima H, Miho N, Miyagawa K, Chayama K, et al. Mnk1 is required for angiotensin II-induced protein synthesis in vascular smooth muscle cells. Circ Res. 2003;93:1218–24. doi: 10.1161/01.RES.0000105570.34585.F2. [DOI] [PubMed] [Google Scholar]
- Joshi-Barve S, Rychlik W, Rhoads RE. Alteration of the major phosphorylation site of eukaryotic protein synthesis initiation factor 4E prevents its association with the 48 S initiation complex. J Biol Chem. 1990;265:2979–83. [PubMed] [Google Scholar]
- Knauf U, Tschopp C, Gram H. Negative regulation of protein translation by mitogen-activated protein kinase-interacting kinases 1 and 2. Mol Cell Biol. 2001;21:5500–11. doi: 10.1128/MCB.21.16.5500-5511.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Wolff B, Sekimoto T, Schreiner EP, Yoneda Y, Yanagida M, et al. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp Cell Res. 1998;242:540–7. doi: 10.1006/excr.1998.4136. [DOI] [PubMed] [Google Scholar]
- Lachance PE, Miron M, Raught B, Sonenberg N, Lasko P. Phosphorylation of eukaryotic translation initiation factor 4E is critical for growth. Mol Cell Biol. 2002;22:1656–63. doi: 10.1128/MCB.22.6.1656-1663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendrick L, Thompson E, Ferreira J, Morley SJ, Lewis JD. Interaction of eukaryotic translation initiation factor 4G with the nuclear cap-binding complex provides a link between nuclear and cytoplasmic functions of the m(7) guanosine cap. Mol Cell Biol. 2001;21:3632–41. doi: 10.1128/MCB.21.11.3632-3641.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minich WB, Balasta ML, Goss DJ, Rhoads RE. Chromatographic resolution of in vivo phosphorylated and nonphosphorylated eukaryotic translation initiation factor eIF-4E: increased cap affinity of the phosphorylated form. Proc Natl Acad Sci U S A. 1994;91:7668–72. doi: 10.1073/pnas.91.16.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley SJ, Naegele S. Phosphorylation of eukaryotic initiation factor (eIF) 4E is not required for de novo protein synthesis following recovery from hypertonic stress in human kidney cells. J Biol Chem. 2002;277:32855–9. doi: 10.1074/jbc.C200376200. [DOI] [PubMed] [Google Scholar]
- O'Loghlen A, Gonzalez VM, Pineiro D, Perez-Morgado MI, Salinas M, Martin ME. Identification and molecular characterization of Mnk1b, a splice variant of human MAP kinase-interacting kinase Mnk1. Exp Cell Res. 2004;299:343–55. doi: 10.1016/j.yexcr.2004.06.006. [DOI] [PubMed] [Google Scholar]
- O'Loghlen A, Perez-Morgado MI, Salinas M, Martin ME. Reversible inhibition of the protein phosphatase 1 by hydrogen peroxide. Potential regulation of eIF2 alpha phosphorylation in differentiated PC12 cells. Arch Biochem Biophys. 2003;417:194–202. doi: 10.1016/s0003-9861(03)00368-0. [DOI] [PubMed] [Google Scholar]
- O'Loghlen A, Perez-Morgado MI, Salinas M, Martin ME. N-acetylcysteine abolishes hydrogen peroxide-induced modification of eukaryotic initiation factor 4F activity via distinct signalling pathways. Cell Signal. 2006;18:21–31. doi: 10.1016/j.cellsig.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Parra-Palau JL, Scheper GC, Wilson ML, Proud CG. Features in the N and C termini of the MAPK-interacting kinase Mnk1 mediate its nucleocytoplasmic shuttling. J Biol Chem. 2003;278:44197–204. doi: 10.1074/jbc.M302398200. [DOI] [PubMed] [Google Scholar]
- Parra JL, Buxade M, Proud CG. Features of the catalytic domains and C-termini of the MAP kinase signal-integrating kinases Mnk1 and Mnk2 determine their differing activities and regulatory properties. J Biol Chem. 2005 doi: 10.1074/jbc.M508356200. [DOI] [PubMed] [Google Scholar]
- Patel J, McLeod LE, Vries RG, Flynn A, Wang X, Proud CG. Cellular stresses profoundly inhibit protein synthesis and modulate the states of phosphorylation of multiple translation factors. Eur J Biochem. 2002;269:3076–85. doi: 10.1046/j.1432-1033.2002.02992.x. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Desai BN, Hardwick JS, Schreiber SL. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycinassociated protein. Proc Natl Acad Sci U S A. 1999;96:4438–42. doi: 10.1073/pnas.96.8.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevot D, Darlix JL, Ohlmann T. Conducting the initiation of protein synthesis: the role of eIF4G. Biol Cell. 2003;95:141–56. doi: 10.1016/s0248-4900(03)00031-5. [DOI] [PubMed] [Google Scholar]
- Pyronnet S. Phosphorylation of the cap-binding protein eIF4E by the MAPK activated protein kinase Mnk1. Biochem Pharmacol. 2000;60:1237–43. doi: 10.1016/s0006-2952(00)00429-9. [DOI] [PubMed] [Google Scholar]
- Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433:477–80. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- Rolfe M, McLeod LE, Pratt PF, Proud CG. Activation of protein synthesis in cardiomyocytes by the hypertrophic agent phenylephrine requires the activation of ERK and involves phosphorylation of tuberous sclerosis complex 2 (TSC2). Biochem J. 2005;388:973–84. doi: 10.1042/BJ20041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau D, Kaspar R, Rosenwald I, Gehrke L, Sonenberg N. Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc Natl Acad Sci U S A. 1996;93:1065–70. doi: 10.1073/pnas.93.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper GC, Morrice NA, Kleijn M, Proud CG. The mitogen-activated protein kinase signal-integrating kinase Mnk2 is a eukaryotic initiation factor 4E kinase with high levels of basal activity in mammalian cells. Mol Cell Biol. 2001;21:743–54. doi: 10.1128/MCB.21.3.743-754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper GC, Parra JL, Wilson M, Van Kollenburg B, Vertegaal AC, Han ZG, et al. The N and C termini of the splice variants of the human mitogen-activated protein kinase-interacting kinase Mnk2 determine activity and localization. Mol Cell Biol. 2003;23:5692–705. doi: 10.1128/MCB.23.16.5692-5705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper GC, Proud CG. Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur J Biochem. 2002;269:5350–9. doi: 10.1046/j.1432-1033.2002.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper GC, van Kollenburg B, Hu J, Luo Y, Goss DJ, Proud CG. Phosphorylation of eukaryotic initiation factor 4E markedly reduces its affinity for capped mRNA. J Biol Chem. 2002;277:3303–9. doi: 10.1074/jbc.M103607200. [DOI] [PubMed] [Google Scholar]
- Shenberger JS, Adams MH, Zimmer SG. Oxidant-induced hypertrophy of A549 cells is accompanied by alterations in eukaryotic translation initiation factor 4E and 4E-binding protein-1. Am J Respir Cell Mol Biol. 2002;27:250–6. doi: 10.1165/ajrcmb.27.2.4785. [DOI] [PubMed] [Google Scholar]
- Shenberger JS, Myers JL, Zimmer SG, Powell RJ, Barchowsky A. Hyperoxia alters the expression and phosphorylation of multiple factors regulating translation initiation. Am J Physiol Lung Cell Mol Physiol. 2005;288:L442–9. doi: 10.1152/ajplung.00127.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepenkov SV, Darzynkiewicz E, Rhoads RE. Stopped-flow kinetic analysis of eIF4E and phosphorylated eIF4E binding to cap analogs and capped oligoribonucleotides: evidence for a one-step binding mechanism. J Biol Chem. 2006;281:14927–38. doi: 10.1074/jbc.M601653200. [DOI] [PubMed] [Google Scholar]
- Topisirovic I, Capili AD, Borden KL. Gamma interferon and cadmium treatments modulate eukaryotic initiation factor 4E-dependent mRNA transport of cyclin D1 in a PML-dependent manner. Mol Cell Biol. 2002;22:6183–98. doi: 10.1128/MCB.22.17.6183-6198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topisirovic I, Culjkovic B, Cohen N, Perez JM, Skrabanek L, Borden KL. The proline-rich homeodomain protein, PRH, is a tissue-specific inhibitor of eIF4E-dependent cyclin D1 mRNA transport and growth. Embo J. 2003;22:689–703. doi: 10.1093/emboj/cdg069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topisirovic I, Ruiz-Gutierrez M, Borden KL. Phosphorylation of the eukaryotic translation initiation factor eIF4E contributes to its transformation and mRNA transport activities. Cancer Res. 2004;64:8639–42. doi: 10.1158/0008-5472.CAN-04-2677. [DOI] [PubMed] [Google Scholar]
- Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol. 2004;24:6539–49. doi: 10.1128/MCB.24.15.6539-6549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Campbell LE, Miller CM, Proud CG. Amino acid availability regulates p70 S6 kinase and multiple translation factors. Biochem J. 1998;334(Pt 1):261–7. doi: 10.1042/bj3340261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. Embo J. 1997;16:1909–20. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz AJ, Johnson JC, Penn B, Mahalingam M, Kimball SR, Cooper JA. Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol Cell Biol. 1999;19:1871–80. doi: 10.1128/mcb.19.3.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberek J, Wyslouch-Cieszynska A, Niedzwiecka A, Dadlez M, Stepinski J, Augustyniak W, et al. Phosphorylation of eIF4E attenuates its interaction with mRNA 5′ cap analogs by electrostatic repulsion: intein-mediated protein ligation strategy to obtain phosphorylated protein. Rna. 2003;9:52–61. doi: 10.1261/rna.2133403. [DOI] [PMC free article] [PubMed] [Google Scholar]