Abstract
Parturition at term, the end stage of a successful pregnancy occurs as a result of powerful, co-ordinated and periodic contractions of uterine smooth muscle (myometrium). To occur in a propitious manner, a high degree of control over the activation of a myometrial cell is required. We review the molecular mechanisms and structural composition of myometrial cells that may contribute to their increased contractile capacity at term. We focus attention on pathways that lead to the activation of filamentous networks traditionally labeled ‘contractile’ or ‘cytoskeletal’ yet draw attention to the fact that functional discrimination between these systems is not absolute.
Keywords: myometrium, myofilaments, cytoskeleton, dense plaques, lipid rafts
1. Introduction
Smooth muscle tissue is incredibly adaptable to changes in its environment. Thus, smooth muscle is not in a terminally differentiated state but can accommodate myriad stimuli such as mechanical stretch, increased systemic (circulatory) or local stimulants or inflammatory insult by altering its phenotype [1]. This could be, for example, changes in intracellular signaling molecule expression, cell growth, proliferation or contraction. The stimuli for these changes would most commonly be associated with some pathophysiology e.g. hypertension or atherosclerosis of blood vessels, asthma, bladder obstruction etc. However, the uterus, and in particular the smooth muscle of the uterus, the myometrium, has to adapt to all such changing stimuli during the entirely physiological process of pregnancy – increased intrauterine volume with the growing fetus and placenta, increased endocrine/paracrine signaling from placental/uterine tissues during gestation and proinflammatory cytokine production from leukocyte invasion at term to name but a few [2–4]. There are two interlinked purposes to such myometrial adaptations: Firstly, to enable the fetus to grow and develop in the womb without prematurely activating contraction of the uterus. Secondly, to facilitate just that myometrial activation but in a timely manner (i.e. at term) that also allows for a discrete determination of the strength, periodicity and co-ordination of contractile effort.
In this review we will consider the molecular, structural and functional aspects of the myometrial cell at the end of pregnancy that enable it to be such a powerful contractile unit. Our attention will focus on known and emerging mechanisms of contractile filament activation interspersed with consideration of how ultrastructural aspects of the myometrial cell relate to function.
2. The contractile apparatus and cytoskeleton
Traditionally, the intracellular filamentous systems of smooth muscle cells have been labeled in terms of contractile filaments and the cytoskeleton. The former comprises thick myosin-containing filaments with thin actin-containing filaments and their interaction governs the extent of smooth muscle contraction. The cytoskeleton, on the other hand, is a term given to plasmalemmal and cytoplasmic structures whose role was thought largely to be in regulation of cell shape and motility. Three filamentous structures contribute to the cytoplasmic cytoskeleton – actin thin filaments (~6nm diameter), intermediate filaments (~10nm) and microtubules (~20–25nm) [5]. We will consider the role of the contractile and cytoskeletal filaments in turn.
2.1 Myosin-containing (thick) filaments
A principal determinant of contractile force is the level of intracellular calcium ([Ca2+]i). Stimulation of the myometrial cell across all animal species, whether this be by a spontaneous action potential, uterotonic agonist or perhaps mechanical stretch, elicits a rapid rise in [Ca2+]i) that arises from entry across the plasmalemma (primarily via voltage-gated Ca2+ channels but receptor-operated and store-operated Ca2+ channels may contribute too [6–7]) and/or release from the intracellular sarcoplasmic reticulum [8–9]. Detailed information on the regulatory pathways of Ca2+ homeostasis contributing to myometrial activation are described elsewhere in this series (Sanborn 2007 and Wray 2007, this issue).
The thick filaments are considered to be the major site of action of [Ca2+]i regulatory influences precipitating changes in contractile force yet there is accruing evidence that suggests dynamic alteration of thin filament proteins may also alter contractility. Myosin (thick) filaments consist of two heavy chains (MHC) that form a coiled rod-like structure together with a globular head domain, two regulatory light chains (MLC20) and two essential light chains (MLC17). One of each type of light chain is associated with the head domain of MHC. Regulation of each myosin subunit, whether that be in terms of expression or post-translational modification, may well participate in myometrial contractile adaptations with gestation.
An elevation of Ca2+ results in the co-operative binding to the calcium binding protein calmodulin (CaM) and subsequent activation by Ca2+-(CaM)4 of the intracellular enzyme myosin light chain kinase (MLCK). Ca2+-(CaM)4–MLCK, in turn, acts to increase the serine/threonine phosphorylation of the regulatory light chains of myosin (MLC20). A matching of the elevation of [Ca2+]i to phosphorylation of MLC20 has been reported in uterine smooth muscle of many species and in response to diverse contractile stimuli [10–16]. In many cases, there is a direct association of [Ca2+]i, MLC20 phosphorylation and force at least in the rising phases of each parameter. In the continued presence of external stimuli (e.g. high K+ depolarization or prostaglandin stimulation), force may be maintained with levels of global [Ca2+]i and/or MLC20 phosphorylation that have decreased from initial maximum but that remain suprabasal until the stimulus ceases [10,16]. These are indicative of the formation of slowly cycling, ADP-bound cross-bridges. Moreover, pharmacological inhibition of MLCK results in dissociation of this relationship such that elevations in [Ca2+]i persist in the presence of MLCK inhibition but force is markedly inhibited [17]. The link between [Ca2+]i, MLCK activation, MLC20 phosphorylation and force is even evidenced in phasic contractions of the myometrium illustrating its importance to normal physiological events associated with term laboring contractions [12].
However, this seemingly straightforward situation is actually more complex. In myometrial tissues taken at term, the rate and extent of MLC20 phosphorylation upon agonist stimulation is actually less than that of tissues from before term [11]. Yet the contractile capacity of myometrial tissue from several species is actually increased [11, 18–22]. Myometrial contractile initiation near term would appear to still be associated with phosphorylation of MLC20 but some mechanistic shift occurs that renders the dynamics and magnitude of contraction to be less dependent upon on MLC20 phosphorylation. There could be several explanations for these findings including:
A reduced activation of MLCK. A number of signaling pathways, including those involving cyclic nucleotides and Ca2+(CaM)4 kinase II, result in phosphorylation and blunted activation of MLCK [23]. Such pathways may be anticipated to contribute to relative myometrial quiescence during gestation and, indeed, are likely to be down-regulated near-term [24–25] (see also Lopez Bernal 2007, this issue). Conceptually, therefore, it does not seem likely that reduced MLCK activity near term would be responsible for the observed change in MLC20 phosphorylation profile; indeed, MLCK activities in homogenates from uteri of non-pregnant versus pregnant women were reported to be similar [11].
Increased co-operative activation of actomyosin interactions for a given level of MLC20 phosphorylation. In other smooth muscles, co-operative cross-bridge binding has been suggested as a means of promoting and maintaining substantial contractile output for lower energetic cost [26–27]. It remains unknown if this occurs in uterine smooth muscle but, in any case, is likely to be driven by the kinetics of MgADP binding to, and release from, myosin [27]. Prominent in tonic smooth muscle, the affinity of MgADP for myosin in phasic smooth muscle is thought to be significantly less. It could be envisaged, although it remains speculative, that late pregnancy may favor a switch towards a more tonic, MgADP-dependent latch-like state for each phasic contraction. Would a lesser reliance upon MLC20 phosphorylation encourage this? We do not know for sure but in biophysical studies of other permeabilized smooth muscles, driving the phosphorylation status of MLC20 towards maximum actually enhances the release of MgADP from cross-bridges suggesting that lower, but suprabasal, MLC20 phosphorylation towards term in myometrium may favor strongly bound MgADP to cross-bridges [27].
Altered expression of MLC20. This may be the most likely explanation as towards term in both mouse and human myometrium, there is a marked reduction in the expression of MLC20 [20, 28].
Altered expression of other myosin filament subunits may also impact upon myometrial contractile dynamics. Initially, two MHC isoforms (SM1 of 204kDa and SM2 of 200kDa) were described and suggested to vary with pregnancy and/or estrogen treatment of ovariectomized animals and correlation of SM1:SM2 content with unloaded shortening velocity suggested [29–31]. It is now known that non-muscle myosin heavy chain (NM-MHC) is also present in smooth muscles including the myometrium [32]. These MHC variants arise from alternative splicing of a single gene and it is now known that further splice events result in the possible generation of two variants of SM1 (SM1A, SM1B) or SM2 (SM2A, SM2B) [33]. SM1B and SM2B contain an insert that in other tissues has been correlated to an elevated maximal shortening velocity. It is unclear if this exists in uteri from late pregnancy or what the role of myometrial NM-MHC may be. Rather provocatively, Moreno suggests that the latch-like state of lowered [Ca2+]i, MLC20 phosphorylation and Vmax achieved following initial rapid increases of each parameter may indicate a contribution of NM-MHC to contractile dynamics [34]. However, two MLC17 isoforms can also arise from alternative splicing and correlations of myometrial Vmax with increased expression of MLC17a over MLC17b have also been proposed [32].
2.2 Actin-containing (thin) filaments
Although less often considered than thick filaments, the direct regulation of thin filament protein components by Ca2+ or other effectors (again via changes in expression or post-translational modification) may also impact upon the contractile potential of a myometrial cell. Contractile thin filaments consist mainly of an alpha helical coil of actin filaments and associated proteins tropomyosin and caldesmon (and possibly calponin) [35]. Smooth muscle actin, however, has been suggested to exist as part of both a contractile domain directly involved in force-generating events and a cytoskeletal domain important for structural integrity [36]. The latter may also contain the actin-binding protein calponin. Both filamentous systems one may imagine to be important to the myometrial cell as it undergoes hypertrophic shape change with advancing gestation and requires an increased contractile output at term. In myometrium at term, the intracellular filamentous arrangement observed by electron microscopy (EM) suggests a predominance of a contractile domain (see Figures 1–2) – the myofilaments are densely packed running in parallel to the longitudinal axis of the cell and enveloping a centrally located network of organelles comprising the nucleus, SR and mitochondria. This myofilament lattice is usually associated with expression of the smooth muscle α-actin isoform. Nonetheless, all of the three main actin isoforms (α, β and γ type) are expressed in myometrial cells; α- and β-actin have been suggested to be invariant with respect to total protein during pregnancy whereas γ-actin has recently been suggested to exhibit an increased expression and altered localization towards term [20, 28, 37–38]. Specific roles for each myometrial actin isoform remain to be established but a distinct incorporation of isoform-specific actin-containing thin filaments into contractile or cytoskeletal domains would appear not to be straightforward and may be an over-simplification. As indicated below, signaling pathways oft-described as impacting upon the cytoskeleton actually affect contractile function.
Figure 1. Intracellular geometry of uterine smooth muscle.
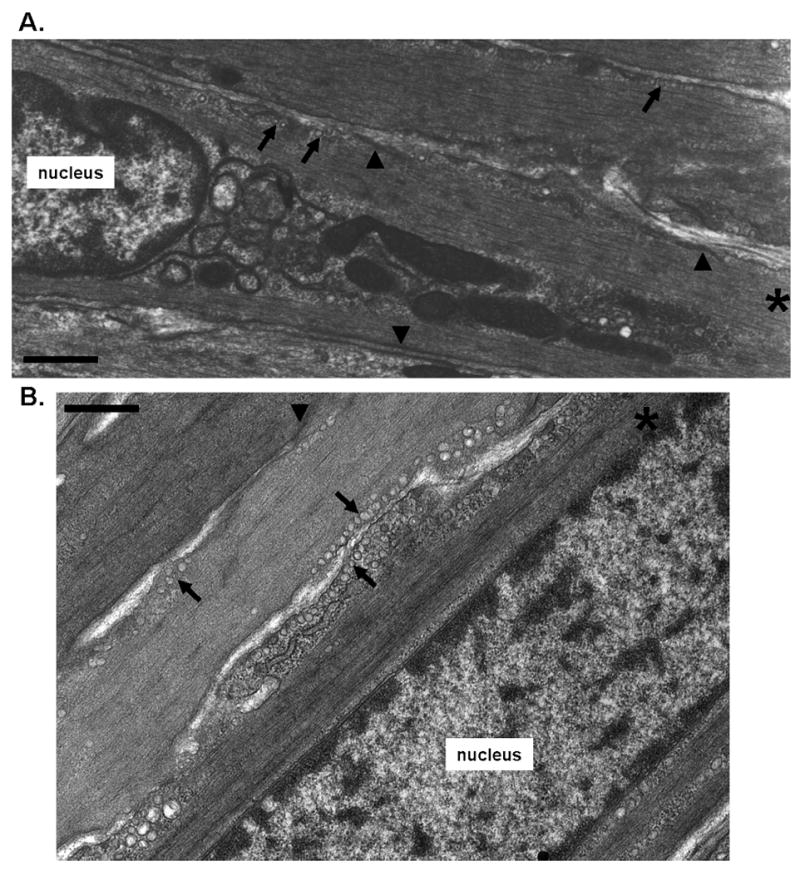
Electron microscopy of late pregnant mouse longitudinal myometrium reveals the myofilament lattice to run in parallel to the plasmalemma. Some regions of the plasma membrane consist of caveolae (singular or in rows, indicated by arrows) interspersed with electron denser regions indicatyive of dense plaques (arrowheads). A, B, in cells marked * the myofibrils are observed to envelope centrally packed organelles such as the nucleus, mitochondria and ER. Dense plaque regions of the plasma membrane marked by arrowheads are interspersed with regions of caveolae marked by the arrows. Scale bars A = 500nm, B = 400nm.
Figure 2. Myofilament lattice in uterine smooth muscle.
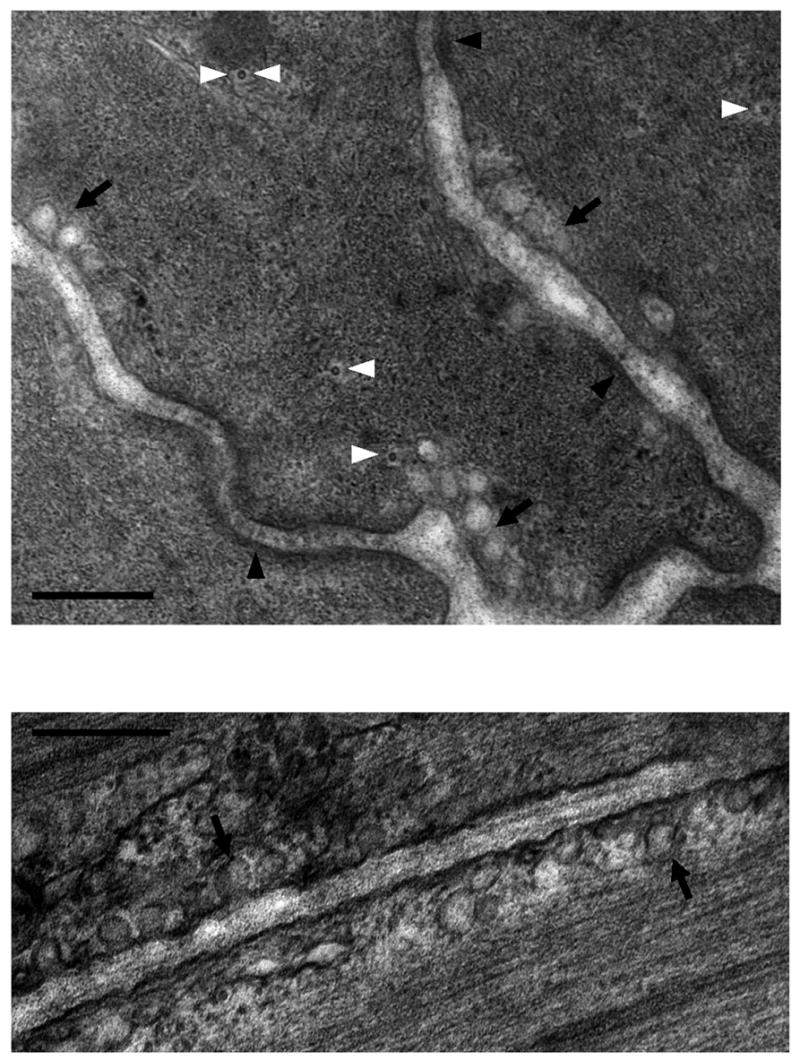
Electron microscopy of late pregnant mouse longitudinal myometrium in transverse section. A, Again revealed are the regions of dense plaques (arrowheads) interspersed with caveolae (arrows). Microtubules are denoted by the white arrowheads; one microtubule, denoted by two white arroheads, resides next to a mitochondrion. B, the myofilaments run in close proximity to caveolae occasionally grazing over them. Scale bars = 200nm.
Thin filaments extracted from smooth muscle, including those of uterus, have been shown to activate myosin ATPase activity in a Ca2+-dependent manner indicating an alternative pathway to MLC20 phosphorylation by which Ca2+ may regulate myometrial activation [39]. h-Caldesmon we now know to be the actin-binding protein that inhibits actin-activated myosin ATPase activity in the absence of Ca2+ and it is emerging as an unexpectedly important player in the regulation of myometrial myofilament activation [40]. The levels of h-caldesmon, the smooth muscle-specific isoform, is elevated during pregnancy in animal and human myometrium [11, 22, 28] which, given its tendency to limit actomyosin activity, places h-caldesmon as mediator of the relative myometrial quiescence during gestation. In vitro, the h-caldesmon inhibition of actomyosin MgATPase can be relieved not only Ca-CaM binding but also by direct phosphorylation by the intracellular kinase Erk1/2. Erk1/2 is itself activated by phosphorylation and in rat myometrium basal ERK1/2 phosphorylation is elevated at term and, further, is activated by stimulation with uterotones [41–42]. Coincident with an elevated basal Erk1/2 activation during pregnancy was an increase in h-caldesmon phosphorylation at an Erk1/2 site [22]. Finally, in rats treated with the progesterone antagonist RU-486, the precipitated preterm labor was associated with an increase in both Erk1/2 phosphorylation and Erk1/2-specific h-caldesmon phosphorylation, effects prevented by pre-administration of a pharmacological agent preventing Erik activation (U-0126) [41].
Consideration has also been given to the possibility that dynamic reorganization of actin filaments – an increase in the ratio of filamentous F-actin to monomeric globular G-actin - may participate in the contractile response of other smooth muscles to external stimulants [43]. Support for this occurring in uterine smooth muscle too arises from investigations of agonist-dependent constrictions in myometrium of non-pregnant rats. Agents that inhibit actin polymerization by two distinct processes both markedly reduce agonist-mediated constrictions whilst leaving global [Ca2+]i changes largely unaltered [44].
2.3 Intermediate filaments and microtubules
Cytoskeletal support is also thought to be given to smooth muscle cells by intermediate (~10nm) and microtubular (~25nm) networks [45–46]. In many smooth muscles the intermediate filaments (~10nm) are thought to insert into both cytoplasmic dense bodies and plasmalemmal dense plaques and are resistant to detergent procedures that extract the myofilament lattices. It is suggested, therefore, that they have a main role in regulation of cell shape [36, 45–46]. However, the precise arrangement of intermediate filaments in native myometrium, and their functional role, remains rather elusive [47]. In cultured myometrial cells, which admittedly may exhibit different growth and functional phenotypes from cells in native tissue, the protein components of both intermediate filaments (cytokeratin) and microtubules (tubulin) are present [5]. Intriguingly, recent gene array studies indicate that term is associated with up-regulation of genes encoding intermediate filament-associated proteins (human myometrium [48]) and genes encoding proteins associated with microtubule polymerization (guinea-pig, [49]) lending credence to the possibility at least that these structures are not only important for hypertrophic shape change but also for readying the myometrial cell architecture for contractile effort with labor. In airway smooth muscle [50] and native myometrium (Figure 2), microtubules have been noted in close proximity to the centrally congregated mitochondria. This is suggestive of a role for these filaments in maintaining organellar registration with respect to each other and the enveloping myofilaments.
2.4 Filament anchorage sites
Structural integrity to the filament lattices in smooth muscle arises from dense bodies in the cytoplasm and dense plaques at the plasmalemma from which intermediate and thin actin filaments are thought to emanate [36, 45–46]. Both cytoplasmic dense bodies and plasmalemmal dense plaques are prominent features of myometrial cells too and, when viewed in longitudinal or transverse cross section appear to assist in the registration of filament lattices roughly in parallel to the long axis of the cell (Figure 1–2). This arrangement implies that thin/intermediate filaments congregating at plasmalemmal dense plaques in the middle portions of the plasmalemma are likely to do so at a rather shallow angle.
It is not known if dynamic regulation of protein interactions with cytoplasmic dense bodies participate in myometrial contraction per se. However, plasmalemmal dense plaques are sites of focal adhesion complexes that, via a series of protein linkages involving molecules including integrins, focal adhesion kinase (FAK), paxillin, c-Src and Erk1/2, link intracellular actin (and possibly intermediate) filaments with the extracellular matrix thereby facilitating transduction of mechanical strains. The expression of several putative myometrial dense plaque proteins - α5 integrin, FAK, paxillin, Heat shock protein27 and ERK1/2, [22, 51–53] - are regulated with gestation. Moreover, phosphorylation-dependent activation of focal adhesion proteins occurs with acute stretch and above we reported that Erk1/2 activation participated in uterine contractility of preterm labor [41–42]; further evidence that so-called cytoskeletal events can actually directly affect contractile function.
3. Ca2+-sensitisation of myofilaments
In addition to altering Ca2+, many uterotones have the potential to influence myometrial contractility by altering the sensitivity of the myofilaments to that activating Ca2+. This process of Ca2+-sensitisation of force, although well-studied in many smooth muscles, is perhaps overlooked as a regulatory mechanism because of the phasic nature of laboring contractions. Yet, if agonist-mediated Ca2+-sensitisation occurs in this setting it may result in prolongation of each phasic contraction by 20–30%. Over the period of laboring contractions lasting several hours, this would contribute a substantial amount parturient contractile effort.
Agonist-mediated Ca2+-sensitisation has been observed in permeabilized myometrial preparations – where myofilament activating Ca2+can be clamped at sub-maximal levels - of rat, guinea-pig, and human [15, 54–57]. The phenomenon is also evident in intact rat, guinea-pig and human myometrium where the temporal relationship of simultaneous Ca2+ and force measurements, including hysteresis profiles, have been made [9, 58–60].
In recent years the focus of molecular mechanisms mediating Ca2+-sensitisation of smooth muscle contractility has centred around signaling pathways that impair myosin phosphatase (MLCP) activity, thereby elevating MLC20 phosphorylation and force. The two most studied pathways involve receptor-coupled activation of rho-associated kinase (ROK) by GTP-rhoA and CPI-17 by DAG-PKC [61]. MLCP is a trimeric protein consisting of a myosin binding subunit (MBS or MYPT, first cloned in uterine smooth muscle [62], a catalytic subunit (PP1c) and a MBS binding subunit (M20) of unresolved function [61]. Activation of ROK can result in the phosphorylation of two possible inhibitory sites on MBS (human Thr 696 and Thr953) although the precise involvement of ROK-mediated MBS phosphorylation may vary between smooth muscle tissues and particular stimuli [63–65]. On the other hand, PKC-dependent phosphorylation of a smooth muscle-specific intracellular effector, CPI-17 (Thr38), increases the potency of the protein to bind to PP1c and inhibit MLCP activity [21, 66]. CPI-17 may also be phosphorylated by ROK or the Rho effector molecule protein kinase N (PKN1) [67]. PKN1 expression is increased in human pregnancy near term as is CPI-17 phosphorylation [68] although it is unknown if the latter reflects an actual elevation of CPI-17 expression as this has been reported in rat myometrium in late pregnancy [21].
In rodent myometrium, the expression of ROKI and ROKII is increased with gestation [20, 69], yet ROKII has been reported to be invariant (like ROKI) or down-regulated in late term human myometrium [28, 70]. Pharmacological inhibition of ROK has also been associated with reduction of agonist-mediated myometrial contractions in permeabilized and intact preparations [15, 20, 69, 71–74]; the effect of ROK inhibition is greater in pregnancy than non-pregnancy [20, 69] and, in a rodent model of preterm labor administration of a ROK inhibitor reduced the incidence of premature delivery [74]. Although detailed data are limited, it is emerging that the ROK pathway is also open to modulation by:
The small constitutively active G-protein Rnd molecules that have been suggested to selectively inhibit ROK-mediated Ca-sensitization [75]. However, the reported up-regulation of Rnd3 with late pregnancy in human myometrium [76–77] when contractile activation is enhanced is difficult to reconcile with this mechanism.
Other rho proteins (rhoB, rhoD) and their effectors (DIAPH1 and DIAPH2). Human myometrial DIAPH1 expression, for example, is increased with labor onset [68].
Recently, alternative pathways of Ca2+-sensitisation have come to light in other smooth muscles that may converge on ROK- or CPI-17-mediated events including those involving the β-integrin binding protein integrin linked kinase (ILK) or CPI-17 homologue PHI-1 [78–79]. However, we presently know nothing about their presence or role in myometrium. Clearly, the process of myometrial Ca2+-sensitisation may involve the participation of any number of the myriad pathways above and is oft-mentioned as an attractive mechanism(s) for targeting of new tocolytic approaches for preterm labor. However, before such a possibility can be realized, there remains a pressing need to fill in many gaps in our knowledge of this mechanism(s) in myometrium: for example, to establish the temporal sequence of phosphorylation and activation cascades that may be activated by specific uterotones especially in human myometrium.
4. Lipid raft microdomains
Of final consideration is what signaling processes may occur at the plasmalemma, which are not part of the cytoskeletal framework – that is, the non-dense plaque regions of the membrane. Within these regions there often appear rows of omega-shaped invaginations known as caveolae (Figure 1–3). These structures are enriched in cholesterol and sphingolipids as well as the integral protein caveolin of which three main isoforms exist – caveolins1–3 [80–81] Of the many roles postulated for caveolae, two are key to their likely participation in events regulating myometrial contraction. Firstly, in other smooth muscles, the immunoelectron microscopy localization of proteins involved in Ca2+ homeostasis, coupled to a close apposition of peripherally located SR to caveolae structures, suggested a role of caveolae in determining the flux of Ca2+ across the plasmalemma [82–83]. If also so in the myometrium, then these structures may be implicated in determining myometrial excitability and contractile status. Indeed, recent evidence suggests the localization of small conductance K+ channels to myometrial caveolae [84]. Secondly, caveolin proteins themselves are not simply structural determinants of the -shaped morphology of caveolae; they also contain a 20 amino acid N-terminal peptide region that binds to a wide range of intracellular kinase molecules and, potentially, dynamically modulates intracellular signaling pathways [81, 85]. In myometrial cells, this is known to involve both PKC and RhoA with contractile consequences [56, 86]. The positioning of caveolae in very close proximity to the myofilaments running parallel to the long axis of the cell (Figure 2) indicates that either of these localized signaling events – Ca2+ plasmalemmal fluxes or excitatory signaling molecule congregations – may impact upon the activation of the underlying myofilament lattice. Indeed, chemical extraction of cholesterol, albeit a crude method of reducing caveolae number and appearance, results in altered excitability and contractility of both human and rodent myometrium [87–88, see Figure 3]. Rather surprisingly given the reliance of caveolae/ins upon the local lipid environment which may be anticipated to change with gestation, the expression levels of caveolin protein appear invariant in rodent and human myometria with pregnancy [20, 28, 89].
Figure 3. Effects of cholesterol depletion on uterine smooth muscle function and caveolae abundance.

A, treatment of human myometrium isolated from a late pregnant woman with methyl-β-cyclodextrin results in an initial increased flurry of spontaneous contractile activity followed by maintenance of steady-state tone above basal. Electron microscopic examination of untreated (B) or cholesterol-depleted myometrium (C) also showed that methyl-β-cyclodextrin resulted in ablation or flattening of caveolae (denoted by arrows). Scale bars = 300nm. ECM = extracellular matrix between two cells. D, Comparison of caveolae abundance relative to total myometrial plasmalemmal area revealed the number of caveolae to be significantly reduced by 86+4% (n=5) with cholesterol depletion.
5. Conclusions
It is clear from all of the above that the integration of events leading to myometrial contraction is incredibly complex. Even at the level of a single myometrial cell there is much we still do not understand. In particular, we are largely ignorant of how spatially discrete events at the plasmalemma – for example, mechanical sensing at dense plaques or Ca2+ ion movements at caveolae – effect a reorganization of cytoskeletal and myofilament lattices some distance inside the cell; far less again how these may interact with the regulation of energy provision and transcriptional activation/suppression in the myofilament-engulfed mitochondrial and nuclear domains. All these pathways must interact to ensure appropriate remodeling of the myometrial cell with gestation and efficient contractility at term. It is likely that although many of the signaling processes may originate in spatially segmented regions of the plasmalemma, they share some common aspects of second messenger information transfer and/or eventual site of functional impact. If so, perhaps there is some modularity to the function of the myometrial cell; in which case, our future advances in understanding the complexity of myometrial excitation–contraction coupling will be assisted by unraveling three issues: (i) the possibility of spatial discrimination to signaling pathways originating at the plasmalemma; (ii) in concert, the temporal nature of any such processes; (iii) yet more important than the minutiae of any signaling discrimination, how these processes may be integrated to determine the spatiotemporal nature of myometrial excitability at the level of a single cell. Then we will be better placed to comprehend the mechanisms of intercellular communication – electrical, mechanical, chemical – that lend laboring uterus at term the capability to act like a functional syncytium.
Acknowledgments
The authors work towards this topic is supported by the Wellcome Trust (UK), Action Medical Research (UK) and NIH grant HD43054 (USA). We thank Dr Carolyn Jones (University of Manchester) for sharing her expertise of electron microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael J Taggart, Maternal & Fetal Health Research Centre & Cardiovascular Research Group, University of Manchester, St Mary’s Hospital, Hathersage Road, Manchester, M130JH, UK, Tel: +44 161 276 5469, Fax: +44 161 276 6134, E mail: Michael.j.taggart@manchester.ac.uk.
Kathleen G Morgan, Health Sciences Department, Sargent College, Boston University, 635 Commonwealth Avenue, Boston MA 02215, USA, Tel: 617-353-7464, Fax: 617-353-7567, E-mail: kmorgan@bu.edu.
References
- 1.Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circ Res. 2005;96:280–291. doi: 10.1161/01.RES.0000155951.62152.2e. [DOI] [PubMed] [Google Scholar]
- 2.Gibb W, Challis JR. Mechanisms of term and preterm birth. J Obstet Gynaecol Can. 2002;24:855–860. doi: 10.1016/s1701-2163(16)31044-1. [DOI] [PubMed] [Google Scholar]
- 3.Lye SJ, Mitchell J, Nashman N, Oldenhof A, Ou R, Shynlova O, Langille L. Role of mechanical signals in the onset of term and preterm labor. Front Horm Res. 2001;27:165–178. doi: 10.1159/000061025. [DOI] [PubMed] [Google Scholar]
- 4.Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labor at term. Mol Hum Reprod. 2003;9:41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 5.Yu JT, Lopez Bernal A. The cytoskeleton of human myometrial cells. J Reprod Fertil. 1998;112:185–198. doi: 10.1530/jrf.0.1120185. [DOI] [PubMed] [Google Scholar]
- 6.Dalrymple A, Slater DM, Poston L, Tribe RM. Physiological induction of transient receptor potential canonical proteins, calcium entry channels, in human myometrium: influence of pregnancy, labor, and interleukin-1 beta. J Clin Endocrinol Metab. 2004;89:1291–1300. doi: 10.1210/jc.2003-031428. [DOI] [PubMed] [Google Scholar]
- 7.Babich LG, Ku CY, Young HW, Huang H, Blackburn MR, Sanborn BM. Expression of capacitative calcium TrpC proteins in rat myometrium during pregnancy. Biol Reprod. 2004;70:919–924. doi: 10.1095/biolreprod.103.023325. [DOI] [PubMed] [Google Scholar]
- 8.Shymgol A, Wray S. Functional architecture of the SR calcium store in uterine smooth muscle. Cell Calcium. 2004;35:501–508. doi: 10.1016/j.ceca.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Taggart MJ, Wray S. Contribution of sarcoplasmic reticular calcium to smooth muscle contractile activation: gestational-dependence in isolated rat uterus. J Physiol. 1998;511:133–144. doi: 10.1111/j.1469-7793.1998.133bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taggart MJ, Menice CB, Morgan KG, Wray S. Effect of metabolic inhibition on intracellular Ca2+, phosphorylation of myosin regulatory light chain and force in isolated rat smooth muscle. J Physiol. 1997;499:485–496. doi: 10.1113/jphysiol.1997.sp021943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Word RA, Stull JT, Casey L, Kamm KE. Contractile elements and myosin light chain phosphorylation in myometrial tissue from nonpregnant and pregnant women. J Clin Invest. 1993;92:29–37. doi: 10.1172/JCI116564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Word RA, Tang D-C, Kamm KE. Activation properties of myosin light chain kinase during contraction/relaxation cycles of tonic and phasic smooth muscles. J Biol Chem. 1994;269:21596–21602. [PubMed] [Google Scholar]
- 13.Shojo H, Kaneko Y. Oxytocin-induced phosphorylation of myosin light chain is meduated by extracellular calcium influx in pregnant rat myometrium. J Mol Recog. 2001;14:401–405. doi: 10.1002/jmr.551. [DOI] [PubMed] [Google Scholar]
- 14.Kim B-k, Ozaki H, Hori M, Takahashi K, Karaki H. Increased contractility of rat uterine smooth muscle at the end of pregnancy. Compar Biochem Physiol. 1998;121:165–173. doi: 10.1016/s1095-6433(98)10118-6. [DOI] [PubMed] [Google Scholar]
- 15.Oh JH, You SK, Hwang MK, Ahn DS, Kwon SC, Taggart MJ, Lee YH. Inhibition of Rho-Associated Kinase Reduces MLC20 Phosphorylation and Contractility of Intact Myometrium and Attenuates Agonist-Induced Ca2+ Sensitization of Force of Permeabilized Rat Myometrium. J Vet Med Sci. 2003;65:43–50. doi: 10.1292/jvms.65.43. [DOI] [PubMed] [Google Scholar]
- 16.Haeberle JR, Hott JW, Hathaway DR. Regulation of isometric force and isotonic shortening velocity by phosphorylation of the 20,000 dalton myosin light chain of rat uterine smooth muscle. Plugers Arch. 1985;403:215–219. doi: 10.1007/BF00584103. [DOI] [PubMed] [Google Scholar]
- 17.Longbottom ER, Luckas MJ, Kupittayanant S, Badrick E, Shmygol A, Wray S. The effects of inhibiting myosin light chain kinase on contraction and calcium signaling in human and rat myometrium. Pflugers Arch. 2000;440:315–321. doi: 10.1007/s004240000305. [DOI] [PubMed] [Google Scholar]
- 18.Izumi H, Byam-Smith M, Garfield RE. Gestational changes in oxytocin- and endothelin-1-induced contractility of pregnant rat myometrium. Eur J Pharmacol. 1995;278:187–194. doi: 10.1016/0014-2999(95)00089-4. [DOI] [PubMed] [Google Scholar]
- 19.Bai X. PhD thesis. University of Manchester: 2005. Investigation of potassium channels in the human uteroplacental unit. [Google Scholar]
- 20.Riley M, Wu X, Baker PN, Taggart MJ. Gestational-Dependent changes in the expression of signal transduction and contractile filament-associated proteins in mouse myometrium. J Soc Gynecol Invest. 2005;12:e33–e43. doi: 10.1016/j.jsgi.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozaki H, Yasuda K, Kim Y-S, Egawa M, Kanzaki H, Nakazawa H, Hori M, Seto M, Karaki H. Possible role of the protein kinase C/CPI-17 pathway in the augmented contraction of human myometrium after gestation. Brit J Pharmacol. 2003;140:1303–1312. doi: 10.1038/sj.bjp.0705552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Je HD, Malek S, Morgan KG. ERK1/2-mediated phosphorylation of caldemson during pregnancy and labor. Am J Physiol. 2003;284:R192–R199. doi: 10.1152/ajpregu.00290.2002. [DOI] [PubMed] [Google Scholar]
- 23.Word RA. Myosin phosphorylation and the control of myometrial contraction/relaxation. Semin Perinatol. 1995;19:3–14. doi: 10.1016/s0146-0005(95)80043-3. [DOI] [PubMed] [Google Scholar]
- 24.Cornwell TL, Li J, Sellak H, Miller RT, Word RA. Reorganisation of myofilament proteins and decreased cGMP-dependent protein kinase in the human uterus during pregnancy. J Clin Endocrinol Metab. 2001;86:3981–3988. doi: 10.1210/jcem.86.8.7727. [DOI] [PubMed] [Google Scholar]
- 25.MacDougall MW, Europe-Finner GN, Robson SC. Human myometrial quiescence and activation during gestation and parturition involve dramatic changes in expression and activity of particulate type II (RII alpha) protein kinase A holoenzyme. J Clin Endocrin Metabol. 2003;88:2194–2205. doi: 10.1210/jc.2002-021862. [DOI] [PubMed] [Google Scholar]
- 26.Khromov A, Somlyo AV, Trentham DR, Zimmermann B, Somlyo AP. The role of MgADP in force maintenance by dephosphorylated cross bridges in smooth muscle. Biophys J. 1995;69:2611–2622. doi: 10.1016/S0006-3495(95)80132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Somlyo AV, Khromov AS, Webb MR, Ferenczi MA, Trentham DR, He Z-H, Sheng S, Shao Z, Somlyo AP. Smooth muscle myosin: regulation and properties. Phil Trans R Soc B. 2004;359:1921–1930. doi: 10.1098/rstb.2004.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riley M, Baker PN, Tribe RM, Taggart MJ. Expression of Scaffolding, Signalling and Contractile-Filament Proteins in Human Myometria: Effects of Pregnancy and Labor. J Cell Mol Med. 2005;9:122–134. doi: 10.1111/j.1582-4934.2005.tb00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capriani A, Chiavegato A, Franch R, Azzarello G, Vinante O, Sartore S. Oestrogen-dependent expression of the SM2 smooth muscle-type myosin isform in rabbit myometrium. J Muscle Res Cell Motil. 1997;18:413–427. doi: 10.1023/a:1018642713934. [DOI] [PubMed] [Google Scholar]
- 30.Hewett TE, Martin AF, Paul RJ. Correlations between myosin heavy chain isoforms and mechanical parameters in rat myometrium. J Physiol. 1993;460:351–364. doi: 10.1113/jphysiol.1993.sp019475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sparrow M, Mohammad MA, Arner A, Hellstrand P, Ruegg JC. Myosin composition and functional properties of smooth muscle from the uterus of pregnant and non-pregnant rats. Pflugers Arch. 1988;412:624–633. doi: 10.1007/BF00583764. [DOI] [PubMed] [Google Scholar]
- 32.Morano I, Erb G, Sogl B. Expression of myosin heavy and light chains changes during pregnancy in the rat uterus. Pflugers Arch. 1993;423:434–441. doi: 10.1007/BF00374938. [DOI] [PubMed] [Google Scholar]
- 33.Arner A, Lofgren M, Morano I. Smooth, slow and smart motors. J Muscle Res Cell Motil. 2003;24:165–173. doi: 10.1023/a:1026001513928. [DOI] [PubMed] [Google Scholar]
- 34.Morano I. Tuning smooth muscle contraction by molecular motors. J Mol Med. 2003;81:481–487. doi: 10.1007/s00109-003-0451-x. [DOI] [PubMed] [Google Scholar]
- 35.Marston SB, Redwood CS. The molecular anatomy of caldesmon. Biochem J. 1991;279:1–16. doi: 10.1042/bj2790001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Small JV, Gimona M. The cytoskeleton of the vertebrate smooth muscle cell. Acta Physiol Scand. 1998;164:341–348. doi: 10.1046/j.1365-201X.1998.00441.x. [DOI] [PubMed] [Google Scholar]
- 37.Bai X, Greenwood SL, Glazier JD, Baker PN, Sibley CP, Taggart MJ, Fyfe GK. Expression of TASK and TREK, two-pore domain K+ channels, in human myometrium. Reproduction. 2005;129:525–530. doi: 10.1530/rep.1.00442. [DOI] [PubMed] [Google Scholar]
- 38.Shynlova O, Tsui P, Dorogin A, Chow M, Lye SJ. Expression and localization of alpha-smooth muscle and gamma-actins in the pregnant rat myometrium. Biol Reprod. 2005;73:773–780. doi: 10.1095/biolreprod.105.040006. [DOI] [PubMed] [Google Scholar]
- 39.Marston S. Calcium ion-dependent regulation of uterine smooth muscle thin filaments by caldesmon. Am J Obstet Gynecol. 1989;160:252–257. doi: 10.1016/0002-9378(89)90131-2. [DOI] [PubMed] [Google Scholar]
- 40.Morgan KG, Gangopadhyay SS. Cross-bridge regulation by thin filament associated proteins. J Appl Physiol. 2001;91:953–962. doi: 10.1152/jappl.2001.91.2.953. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Je H-D, Malek S, Morgan KG. Role of ERK1/2 in uterine contractility and preterm labor in rats. Am J Physiol. 2004;287:R328–R335. doi: 10.1152/ajpregu.00042.2004. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Gallant C, Malek S, Morgan KG. Focal adhesion signaling is required for myometrial ERK activation and contractile phenotype switch before labor. J Cell Biochem. 2007;100:129–40. doi: 10.1002/jcb.21033. [DOI] [PubMed] [Google Scholar]
- 43.Gunst SJ, Tang DD, Opazo Saez A. Cytoskeletal remodeling of the airway smooth muscle cell: a mechanism for adaptation to mechanical forces in the lung. Respir Physiol Neurobiol. 2003;137:151–168. doi: 10.1016/s1569-9048(03)00144-7. [DOI] [PubMed] [Google Scholar]
- 44.Shaw L, Sweeney M, Jones CJP, O’Neill SC, Austin C, Taggart MJ. Caveolae and sarcoplasmic reticular coupling in smooth muscle cells of pressurised arteries: the relevance for Ca2+ oscillations and tone. Cardiovasc Res. 2006;69:825–835. doi: 10.1016/j.cardiores.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 45.Gabella G. Structural apparatus for force transmission in smooth muscles. Physiol Rev. 1984;64:455–477. doi: 10.1152/physrev.1984.64.2.455. [DOI] [PubMed] [Google Scholar]
- 46.Somlyo AP, Somlyo AV. Smooth Muscle Structure and Fucntion. In: Fozzard HA, editor. The Heart and Cardiovascular System. New York: Raven press; 1992. pp. 1295–1324. [Google Scholar]
- 47.Eyden BP, Hale RJ, Richmond I, Buckley CH. Cytoskeletal filaments in the smooth muscle cells of uterine leiomata and myometrium. Virchows Arch A Pathol Anat Histopathol. 1992;420:51–58. doi: 10.1007/BF01605984. [DOI] [PubMed] [Google Scholar]
- 48.Salomonis N, Cotte N, Zambon AC, Pollard KS, Vranizan K, Doniger SW, Dolganov G, Conklin BR. Identifying gene networks underlying myometrial transition to labor. Genome Biology. 2005;6:R12. doi: 10.1186/gb-2005-6-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mason CW, Swaan PW, Weiner CP. Identification of interaction gene networks: a novel approach in gene array profiling of myometrial events during guinea pig pregnancy. Am J Obstet Gynecol. 2006;194:1513–1523. doi: 10.1016/j.ajog.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 50.Kuo K-H, Seow CY. Contractile filament architecture and force transmission in swine airway smooth muscle. J Cell Sci. 2003;117:1503–1511. doi: 10.1242/jcs.00996. [DOI] [PubMed] [Google Scholar]
- 51.Williams SJ, White B, Macphee DJ. Expression of α5 Integrin (Itga5) is elevated in the rat myometrium during late pregnancy and labor: Implications for development of a mechanical syncytium. Biol Reprod. 2005;72:1114–1124. doi: 10.1095/biolreprod.104.035626. [DOI] [PubMed] [Google Scholar]
- 52.Macphee DJ, Lye SJ. Focal adhesion signaling in rat myometrium is abruptly terminated with the onset of labor. Endocrinology. 2000;141:274–283. doi: 10.1210/endo.141.1.7264. [DOI] [PubMed] [Google Scholar]
- 53.White BG, Williams SJ, Highmore K, Macphee DJ. Small heat shock protein 27 (Hsp27) expression is highly induced in rat myometrium during late pregnancy and labor. Reproduction. 2005;129:15–26. doi: 10.1530/rep.1.00426. [DOI] [PubMed] [Google Scholar]
- 54.Izumi H, Garfield RE, Morishita F, Shirikawa K. Some mechanical properties of skinned fibres of pregnant human myometrium. Eur J Obstet Gynecol Reprod Biol. 1994;56:55–62. doi: 10.1016/0028-2243(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 55.Izumi H, Bian K, Bukoski RD, Garfield RE. Agonists increase the sensitivity of contractile elements for Ca in pregnant rat myometrium. Am J Obstet Gynecol. 1996;175:199–206. doi: 10.1016/s0002-9378(96)70275-2. [DOI] [PubMed] [Google Scholar]
- 56.Lee Y-H, Hwang M-K, Morgan KG, Taggart MJ. Receptor-coupled contractility of uterine smooth muscle: from membrane to myofilaments. Exp Physiol. 2001;86:283–288. doi: 10.1113/eph8602184. [DOI] [PubMed] [Google Scholar]
- 57.Somlyo AP, Somlyo AV. From pharmacomechanical coupling to G-proteins and myosin phosphatase. Acta Physiol Scand. 1998;164:437–448. doi: 10.1046/j.1365-201X.1998.00454.x. [DOI] [PubMed] [Google Scholar]
- 58.McKillen K, Thornton S, Taylor CW. Oxytocin increases the Ca sensitivity of human myometrium during the falling phase of phasic contractions. Am J Physiol. 1999;276:E345–E351. doi: 10.1152/ajpendo.1999.276.2.e345. [DOI] [PubMed] [Google Scholar]
- 59.Woodcock NA, Taylor CW, Thornton S. Prostaglandin F2α increases the sensitivity of the contractile proteins to Ca in human myometrium. Am J Obstet Gynecol. 2006;195:1404–1406. doi: 10.1016/j.ajog.2006.03.099. [DOI] [PubMed] [Google Scholar]
- 60.Coleman HA, Hart JD, Tonta MA, Parkington HC. Changes in the mechanisms involved in uterine contractions during pregnancy in guinea-pigs. J Physiol. 2000;523:785–798. doi: 10.1111/j.1469-7793.2000.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arthur P, Taggart MJ, Mitchel BF. Oxytocin and parturition. Front In Biosci. 2007;12:619–633. doi: 10.2741/2087. [DOI] [PubMed] [Google Scholar]
- 62.Johnson D, Cohen P, Chen MX, Chen YH, Cohen P. Identification of the regions on the M11o subunit of the protein phosphatase 1M that interact with the M21 subunit and with myosin. Eur J Biochem. 1997;244:931–939. doi: 10.1111/j.1432-1033.1997.00931.x. [DOI] [PubMed] [Google Scholar]
- 63.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–8. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 64.Somlyo AP, Somlyo AV. Ca sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 65.Sward K, Mita M, Wilson DP, Deng JT, Susnjar M, Walsh MP. The role of RhoA and Rho-associated kinase in vascular smooth muscle contraction. Curr Hypertens Rep. 2003;5:66–72. doi: 10.1007/s11906-003-0013-1. [DOI] [PubMed] [Google Scholar]
- 66.Kitazawa T, Eto M, Woodsome TP, Khalequzzaman M. Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca sensitization in rabbit smooth muscle. J Physiol. 2003;546:879–89. doi: 10.1113/jphysiol.2002.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamaguchi T, Ito M, Feng J, Seko T, Koyama M, Machida H, Takase K, Amano M, Kaibuchi K, Hartshorne DJ, Nakano T. Phosphorylation of CPI-17, an inhibitor of myosin phosphatase, by Proteine kinase N. Biochem Biophys, Res Comm. 2000;274:825–830. doi: 10.1006/bbrc.2000.3225. [DOI] [PubMed] [Google Scholar]
- 68.Lartey J, Smith M, Pawade J, Strachan B, Mellor H, Lopez Bernal A. Up-regulation of myometrial Rho effector proteins (PKN1 And DIAPH1) and CPI-17 (PP1R4A) phosphorylatyion in human pregnancy is associated with increased GTP-RHOA in spontaneous preterm labor. Biol Reprod. 2007 doi: 10.1095/biolreprod.106.058982. In Press. [DOI] [PubMed] [Google Scholar]
- 69.Tahara M, Morishige K-I, Sawada K, Ikebuchi Y, Kawagishi R, Tasaka K, Murata Y. RhoA/Rho-kinase cascade is involved in oxytocin-induced rat uterine contraction. Endocrinology. 2002;143:920–929. doi: 10.1210/endo.143.3.8696. [DOI] [PubMed] [Google Scholar]
- 70.Friel AM, Curley M, Ravikumar N, Smith TJ, Morrison JJ. RhoA/Rho kinase mRNA and protein levels in human myometrium during pregnancy and labor. J Soc Gynecol Invest. 2005;12:20–27. doi: 10.1016/j.jsgi.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 71.Woodcock NA, Taylor CW, Thornton S. Effect of an oxytocin receptor antagonist and rho kinase inhibitor on the Ca sensitivity of human myometrium. Am J Obstet Gyncecol. 2004;190:222–228. doi: 10.1016/s0002-9378(03)00925-6. [DOI] [PubMed] [Google Scholar]
- 72.Moran CJ, Friel AM, Smith TJ, Cairns M, Morrison JJ. Expression and modulation of Rho kinase in human pregnant myometrium. Mol Hum Reprod. 2002;8:196–200. doi: 10.1093/molehr/8.2.196. [DOI] [PubMed] [Google Scholar]
- 73.Kupittayanant S, Burdyga T, Wray S. The effects of inhibiting Rho-associated kinase with Y-27632 on force and intracellular calcium in human myometrium. Pflugers Arch. 2001;443:112–114. doi: 10.1007/s004240100668. [DOI] [PubMed] [Google Scholar]
- 74.Tocolytic effect of a Rho-kinase inhibitor in a mouse model of lipopolysaccharide-induced preterm delivery. Am J Obstet Gynecol. 2005 Mar;192(3):903–8. doi: 10.1016/j.ajog.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 75.Cario-Toumaniantz C, Reillaudoux G, Sauzeau V, Heutte F, Vaillant N, Finet M, Chardin P. Modulation of RhoA-Rho kinase-mediated Ca sensitization of rabbit myometrium during pregnancy – role of Rnd3. J Physiol. 2003;552:403–413. doi: 10.1113/jphysiol.2003.047738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lartey J, Gampel A, Pawade J, Mellor H, Bernal AL. Expression of RND proteins in human myometrium. Biol Reprod. 2006;75:452–461. doi: 10.1095/biolreprod.105.049130. [DOI] [PubMed] [Google Scholar]
- 77.Riley M, Tribe RM, Baker PN, Taggart MJ. The expression of rnd3, a constitutively active GTP-binding rho family protein, in myometria isolated from non-pregnant and pregnant humans. J Physiol. 2004;565P:PC174. [Google Scholar]
- 78.Wilson DP, Sutherland C, Borman MA, Deng JT, Macdonald JA, Walsh MP. Integrin-linked kinase is responsible for Ca-independent myosin diphosphorylation and contraction of vascular smooth muscle. Biochem J. 2005;392:641–8. doi: 10.1042/BJ20051173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deng JT, Sutherland C, Brautigan DL, Eto M, Walsh MP. Phosphorylation of the myosin phosphatase inhibitors, CPI-17 and PHI-1, by integrin-linked kinase. Biochem J. 2002;367:517–24. doi: 10.1042/BJ20020522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taggart MJ. Smooth mucle excitation-contraction coupling: a role for caveolae and caveolins? News Physiol Sci. 2001;16:61–65. doi: 10.1152/physiologyonline.2001.16.2.61. [DOI] [PubMed] [Google Scholar]
- 81.Parton RG. Caveolae- from ultrastructure to molecular mechanisms. Nat Rev Mol Cell Biol. 2003;4:162–167. doi: 10.1038/nrm1017. [DOI] [PubMed] [Google Scholar]
- 82.Fujimoto T, Nakade S, Miyawaki A, Mikoshiba K, Ogawa K. Localisation of inositol 1,4,5-triphosphate receptor-like protein in plasmalemmal caveolae. J Cell Biol. 1992;119:1507–1513. doi: 10.1083/jcb.119.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fujimoto T. Calcium pump of the plasma membrane is localized in caveolae. J Cell Biol. 1993;120:1147–1157. doi: 10.1083/jcb.120.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brainard AM, Miller AJ, Martens JR, England SK. Maxi-K channels localize to caveolae in human myometrium: a role for an actin-channel-caveolin complex in the regulation of myometrial smooth muscle K current. Am J Physiol. 2005;289:C49–C59. doi: 10.1152/ajpcell.00399.2004. [DOI] [PubMed] [Google Scholar]
- 85.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 86.Taggart MJ, Leavis P, Feron O, Morgan KG. Inhibition of PKC and rhoA translocation in differentiated smooth muscle by a caveolin scaffolding domain peptide. Exp Cell Res. 2000;258:72–81. doi: 10.1006/excr.2000.4891. [DOI] [PubMed] [Google Scholar]
- 87.Riley M, Baker PN, Taggart MJ. Effects of methyl-β-cyclodextrin on spontaneous and oxytocin-induced contractility of isolated human uterine smooth muscle. J Physiol. 2003;522P:P64. [Google Scholar]
- 88.Noble K, Zhang J, Wray S. Lipid rafts, the sarcoplasmic reticulum and uterine calcium signaling: an integrated approach. J Physiol. 2006;572:29–35. doi: 10.1113/jphysiol.2005.098475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ku CY, Word RA, Sanborn BM. Differential expression of protein kinase A, AKAP79, and PP2B in pregnant human myometrial membranes prior to and during labor. J Soc Gynecol Invest. 2005;12:421–427. doi: 10.1016/j.jsgi.2005.04.002. [DOI] [PubMed] [Google Scholar]


