Abstract
The visual processing of humans is primarily reliant upon the sensitivity of cone photoreceptors to light during daylight conditions. This underscores the importance of understanding how cone photoreceptors maintain the ability to detect light. The vertebrate retina consists of a combination of both rod and cone photoreceptors. Subsequent to light exposure, both rod and cone photoreceptors are dependent upon the recycling of vitamin A to regenerate photopigments, the proteins responsible for detecting light. Metabolic processing of vitamin A in support of rod photopigment renewal, the so-called “rod visual cycle”, is well established. However, the metabolic processing of vitamin A in support of cone photopigment renewal remains a challenge for characterization in the recently discovered “cone visual cycle”. In this review we summarize the research that has defined the rod visual cycle and our current concept of the novel cone visual cycle. Here, we highlight the research that supports the existence of a functional cone-specific visual cycle: the identification of novel enzymatic activities that contribute to retinoid recycling, the observation of vitamin A recycling in cone-dominated retinas, and the localization of some of these activities to the Müller cell. In the opinions of the authors, additional research on the possible interactions between these two visual cycles in the duplex retina is needed to understand visual detection in the human retina.
A. The rod visual cycle in the retina and RPE
A-1: The rod (rhodopsin) visual cycle involves both retina and RPE.
The term “visual cycle” was coined by George Wald in the mid-1900's to describe the ability of the eye to “re-cycle” vitamin A (vitamin A is a collective term for physiologically active retinoids) for the synthesis of visual pigments (Wald 1968) . Over 50 years later, vision research scientists have now gathered a great deal of information on the rod (rhodopsin) visual cycle (Crouch, Chader et al. 1996; Saari 2000; McBee, Palczewski et al. 2001; Rando 2001; Lamb and Pugh 2004; Travis, Golczak et al. 2006) . As originally proposed (Wald 1968), the rod visual cycle requires the involvement of both the retina and the retinal pigment epithelium (RPE) in order to properly process the retinal chromophore released from bleached rod pigment (or rhodopsin) (Dowling 1960; Zimmerman 1974; Zimmerman, Yost et al. 1974; Groenendijk, De Grip et al. 1980). Upon bleaching, all-trans retinal separates from opsin in the rod outer segment and is reduced to all-trans retinol by an NADPH-dependent all-trans specific retinol dehydrogenase (Lion, Rotmans et al. 1975; Zimmerman, Lion et al. 1975; Suzuki, Ishiguro et al. 1993; Cideciyan, Haeseleer et al. 2000; Jang, McBee et al. 2000; Rattner, Smallwood et al. 2000) . It has been proposed that the rate of retinol production is limited by the availability of NADPH, which is dependent on ATP localized to the rod outer segment but derived from mitochondria in the rod inner segment (Kolesnikov, Ala-Laurila et al. 2007). All-trans retinol is then transferred from the retina to the RPE where it is esterified by the enzyme, lecithin:retinol acyltransferase (LRAT) (Saari and Bredberg 1988; Saari and Bredberg 1989; Saari, Bredberg et al. 1993; Ruiz, Winston et al. 1999; Mondal, Ruiz et al. 2000) . RPE65, another enzyme in the RPE, catalyzes the hydrolysis of the all-trans retinyl ester (Jin, Li et al. 2005; Moiseyev, Chen et al. 2005; Redmond, Poliakov et al. 2005) and uses the energy released in the hydrolytic reaction to isomerize all-trans retinol to 11-cis retinol (Gollapalli and Rando 2003). Oxidation of 11-cis retinol to 11-cis retinal in humans is carried out by RDH5, an 11-cis specific retinol dehydrogenase and a member of the short chain acyl-CoA dehydrogenase (SCAD) family of proteins (Lion, Rotmans et al. 1975; Zimmerman, Lion et al. 1975; Suzuki, Ishiguro et al. 1993; Driessen, Janssen et al. 1995; Simon, Hellman et al. 1995; Driessen, Winkens et al. 1997; Haeseleer, Huang et al. 1998; Simon, Romert et al. 1999; Cideciyan, Haeseleer et al. 2000; Gamble, Mata et al. 2000; Jang, McBee et al. 2000); mutations in this gene result in Fundus albipunctatus phenotype (Yamamoto, Simon et al. 1999). However, the knock out of RDH5 in mice, as well as the double knock out of RDH5 and RDH11 did not produce the expected phenotype, suggesting that the oxidation of 11-cis retinol to 11-cis retinal may involve an additional yet unidentified retinol dehydrogenases (Kim, Maeda et al. 2005) (Maeda, Maeda et al. 2006). 11-cis retinal then exits the RPE and transfers back to the retina to re-combine with opsin to form rhodopsin (Perlman, Nodes et al. 1982; Pepperberg and Clack 1984; Bok 1985).
11-cis retinol can also be esterified by LRAT to form 11-cis retinyl ester (Saari, Bredberg et al. 1993; Mata and Tsin 1998) which is stored in the RPE and later released by 11-cis retinyl ester hydrolase (Blaner, Das et al. 1987; Mata, Tsin et al. 1992; Mata, Mata et al. 1996; Mata, Mata et al. 1998; Tsin, Mata et al. 2000) to supply chromophore for pigment synthesis (Mata, Villazana et al. 1998).
It is also important to point out retinal G-protein coupled receptor (RGR) has also been identified to play key roles in the rod visual cycle. RGR is expressed in the RPE and in the Müller cells (Jiang, Pandey et al. 1993; Shen, Jiang et al. 1994; Chen, Korenberg et al. 1996). It is proposed that RGR forms a complex with retinol dehydrogenase 5 (RDH5) to isomerize all-trans retinal to 11-cis retinal under light illumination (Hao, Chen et al. 2000; Chen, Lee et al. 2001; Yang and Fong 2002), thus providing an alternate pathway to obtain cis retinoids in the visual cycle. However, recent data using single and double RGR/RDH knockout mouse models suggest that RGR's role in the generation of cis retinoid for rod pigment regeneration may be not essential (Maeda, Van Hooser et al. 2003). RGR has also been found to accelerate the conversion of retinyl esters to 11-cis retinal in a light independent manner. Accordingly, it has been proposed that RGR enhances isomerohydrolase activity in the dark (Wenzel, Oberhauser et al. 2005).
A-2: The rod (rhodopsin) visual cycle requires retinoid binding proteins.
Vitamin A are fat soluble molecules which need facilitating factors (such as retinol binding proteins) to transfer in aqueous cytosolic and extracellular locations (Ho, Massey et al. 1989). It is now well established that CRBP (cellular retinol binding protein, Type I) binds all-trans retinol (Saari, Futterman et al. 1978; Napoli 2000; Noy 2000), and CRALBP (cellular retinal binding protein) binds 11-cis retinal (Futterman and Saari 1977; Futterman, Saari et al. 1977; Stubbs, Saari et al. 1979; Saari 1982; Saari, Bredberg et al. 1982) in the RPE (Bunt-Milam and Saari 1983; Bok, Ong et al. 1984).
In the retina, both of these binding proteins are expressed in Müller cells; (Bunt-Milam and Saari 1983; Eisenfeld, Bunt-Milam et al. 1985) with all-trans retinol bound to CRBP while 11-cis retinol and 11-cis retinal are bound to CRALBP(Saari 1982; Noy 2000). Vitamin A in the extracellular interphotoreceptor matrix is also associated with a retinoid binding protein known as interphotoreceptor retinoid binding protein (IRBP) (Adler and Klucznik 1982; Lai, Wiggert et al. 1982; Liou, Ma et al. 1989; Fong and Bridges 1990).
Although the transport function of these binding proteins is essential (Jones, Crouch et al. 1989; Okajima, Pepperberg et al. 1989; Pepperberg, Okajima et al. 1991; Crouch, Hazard et al. 1992) it is not entirely clear whether some binding proteins truly associate with vitamin A for this purpose, since genetic deletion of IRBP show little loss of visual recovery in mice (Liou, Matragoon et al. 1998; Palczewski, Van Hooser et al. 1999; Ripps, Peachey et al. 2000). Another important function of retinoid binding proteins (such as CRBP and CRALBP) is their ability to remove vitamin A from the site of a reaction thereby shifting the equilibrium of the reaction (by mass action) and dictating the direction of the reaction in a pathway of the visual cycle (Saari, Bredberg et al. 1994; Stecher, Gelb et al. 1999; McBee, Van Hooser et al. 2001).
B. Cone cycle in the retina: data from earlier reports in the literature
B-1: Electrophysiology data from isolated retina
In a study of recovery of cone early receptor potential (ERP), isolated frog retina was exposed to 580 and 433 nm illumination to bleach the principal and green cone pigments. Full recovery of cone ERP was observed in subsequent dark adaptation. (Goldstein 1967; Goldstein 1968; Goldstein and Wolf 1973). In comparison, rod pigment bleached by 502 nm light did not regenerate in the isolated frog retina. In similar experiments, the full recovery of 2 log units of sodium aspartate (cone) receptor potential was observed in isolated frog retina exposed to 580 nm light. However, recovery of rod pigment was not observed. (Hood and Hock 1973). These results provided the first experimental demonstration of the recovery of cone sensitivity in the isolated retina, suggesting that a cone cycle may exist in the retina in support of cone pigment regeneration.
B-2: Electrophysiology data from isolated photoreceptors
The recovery of visual sensitivity in isolated salamander rod and cone outer segments in response to added retinol and retinal after light exposure has been studied. It was shown that rods recovered light sensitivity after bleaching upon the addition of 11-cis retinal, but not 11-cis retinol. Cones however, recovered visual sensitivity from both 11-cis retinal and 11-cis retinol (Jones, Crouch et al. 1989). By exposing the cell bodies to 11-cis retinol it was found that only bleached cones (not rods) recover sensitivity after exposure to 11-cis retinol, suggesting that the 11-cis retinol may move freely along the cone photoreceptors (Jin, Jones et al. 1994). Based on these experimental results, these authors suggested that cones may access a pool of 11-cis retinoid in the retina and that cones may follow a different visual pathway for pigment regeneration.
B-3: Biochemistry data from intact retina and RPE
The predominance of 11-cis retinyl esters (as opposed to all-trans retinyl esters) has been demonstrated in the cone-dominated chicken eye (Bridges, Alvarez et al. 1987). Based on biochemical extraction and high performance liquid chromatography, it was determined that in rd (retinal degeneration), and in normal (Rhode Island Red) chicken that over half of the total retinyl palmitate recovered from the eye was located in the chicken retina, and that 11-cis retinyl palmitate accounted for nearly 100% of retinyl palmitate in the retina (Bridges, Alvarez et al. 1987).
It was later confirmed that cone-dominated chicken retina possessed a significant level of 11-cis retinyl palmitate (Rodriguez and Tsin 1989). In contrast, rod-dominated frog and cow eyes had mainly all-trans retinyl palmitate in the RPE. Based on the molar amount of retinyl ester in the retina and the rate of visual pigment regeneration, it was suggested that this pool of retinoid in the retina may be a source for cone pigment regeneration (Rodriguez and Tsin 1989).
The retinyl ester hydrolase (REH) activity in the chicken retina and RPE was then determined, using a sensitive radiometric assay. It was shown that high REH activity existed in the chicken retina to release free retinoid (from esterified retinoids) which may be used to supply visual chromophore for the metabolic renewal and regeneration of visual pigments. Based on these biochemical data, it was suggested that a cone cycle may exist in the cone-dominated chicken retina, utilizing 11-cis retinyl esters to support cone pigment regeneration (Bustamante, Ziari et al. 1995) .
B-4: Biochemistry data from cultured Müller cells from the retina
Using cultured Müller cells from chicken retina, it was demonstrated that these cells synthesized all-trans retinyl palmitate, 11-cis retinol and 11-cis retinyl palmitate from radiolabeled all-trans retinol added to the culture medium. It was also shown that most of the 11-cis retinol was released by the cell to the surrounding medium. These data indicate that the Müller cells have the ability to isomerize and esterify retinoids, two key functions to support a cone visual cycle in the retina (Das, Bhardwaj et al. 1992).
B-5: Biochemistry data from RPE65 knockout model
Results from a biochemical study of the deletion of RPE65 protein in the transgenic mouse show that RPE65 is essential for the production of 11-cis vitamin A in the visual cycle (Redmond, Yu et al. 1998). Subsequent studies on RPE65-deficient mouse models show that RPE65 supports both rod and cone functions (Seeliger, Grimm et al. 2001); (Fan, Rohrer et al. 2003) (Wenzel, von Lintig et al. 2007), suggesting an important role of this protein in both the rod and cone visual cycles in the rod-dominated mouse retina.
C. Cone cycle in the retina: data from recent reports in the literature
C-1. Demonstration of a light-driven retinoid cycle in cone-dominated retinas:
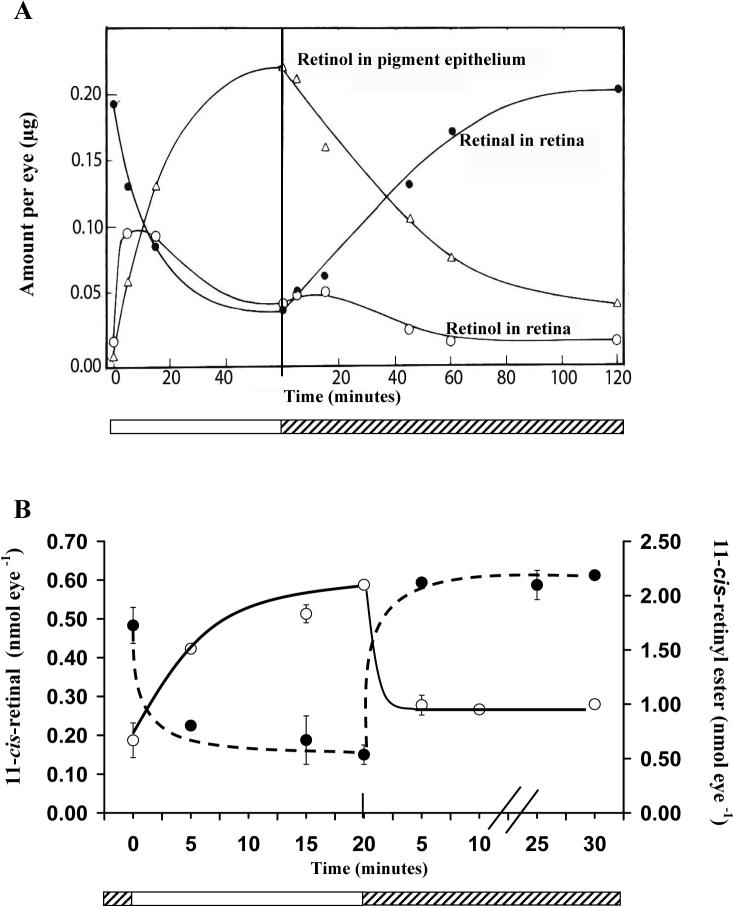
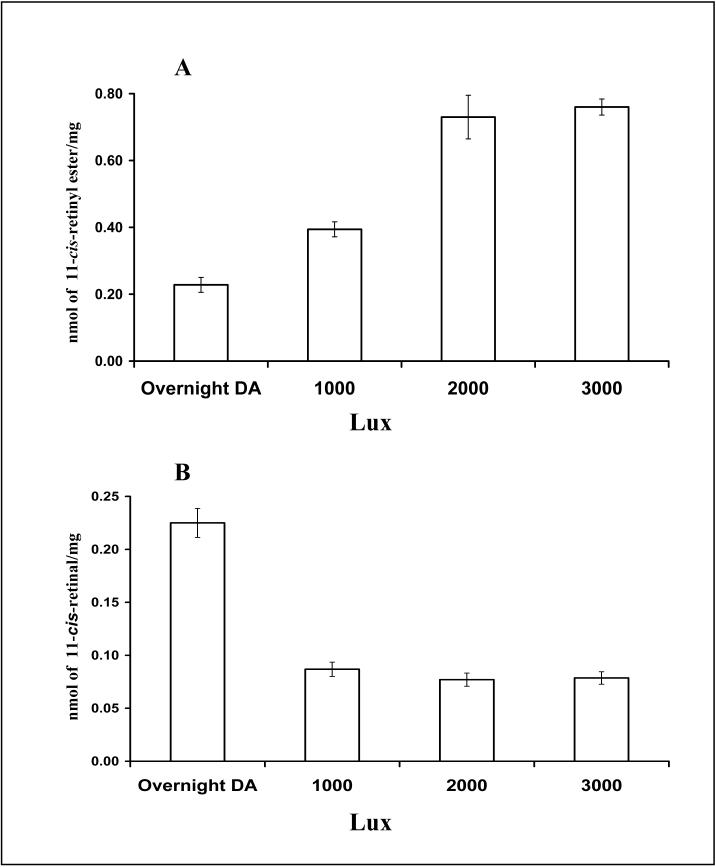
Direct evidence of a light-driven cone cycle was recently obtained in the cone-rich chicken retina (Trevino, Villazana-Espinoza et al. 2005; Villazana-Espinoza, Hatch et al. 2006b). Subsequent to dark adaptation, light exposure resulted in the accumulation of 11-cis retinyl esters in the retina and all-trans retinyl esters to the RPE. It was also shown that the rate of increase of 11-cis retinyl esters in the retina far exceeds the accumulation of all-trans retinyl ester in the RPE (1.17nmol h−1 and 0.32nmol h−1 respectively) and that the rate of depletion of 11-cis retinyl esters in the retina also exceeded that of all-trans retinyl esters in the RPE under dark conditions (−2.45 nmol h−1 and −0.027 nmol h−1 respectively). Furthermore, reciprocal changes of retinyl ester versus retinal in response to light and dark adaptation, which constitute the basis of a visual cycle, were observed in the chicken eye (see Fig. 1B). This novel retinoid cycle utilizes 11-cis retinyl esters as the primary source of visual chromophore for cone pigment regeneration (Trevino, Villazana-Espinoza et al. 2005). The kinetics of cone pigment regeneration (full recovery within 5 min.) is much faster in comparison to rod pigment regeneration (about 100 min. in albino rat, see Fig. 1A, from (Dowling 1960). Additional data have shown that increases of light intensity and period of light exposure lead to proportional increases in the amount of retinyl ester accumulation (see Fig. 2). The molar amount of retinyl ester corresponded to the molar amount of retinal released from light exposure (Villazana-Espinoza, Hatch et al. 2006b). Taken together, these results strongly support the existence of a cone visual cycle in the chicken retina.
Fig. 1.
Retinoid distribution during light and dark adaptation in the rod visual cycle in the rat (A) and the cone visual cycle in the chicken (B). A: Distribution of retinol in the retinal pigment epithelium (RPE) and in the retina of the rod-dominated eye of albino rat during light and dark adaptation. During light adaptation, retinal in the retina decreases as retinol/retinyl esters accumulate in the RPE. During dark adaptation, these processes are reversed. Rod pigments take about 100 min. to fully regenerate (i.e. recovery of retinal content in the retina) in the dark adapted rat eye and this process is supported by retinol/retinyl esters in the RPE. ○- retinol in retina , Δ -retinol/retinyl esters in the RPE, •- retinal in the retina. For details of experiments and results, see Dowling, 1960. B: Distribution of 11-cis retinyl ester ○ (solid line) and 11-cis retinal • (broken line) in the retina of the cone-dominated chicken eye during light and dark adaptation. During light adaptation, 11-cis retinal in the retina decreases while 11-cis retinyl esters accumulate in the retina. During dark adaptation, these processes are reversed. Cone pigments fully regenerate in the retina in less than 5 min. and this process is supported by 11-cis retinyl esters in the retina.  Light adaptation,
Light adaptation,  Dark adaptation For details of experiment and results see Trevino, Villazana-Espinoza et al. 2005.
Dark adaptation For details of experiment and results see Trevino, Villazana-Espinoza et al. 2005.
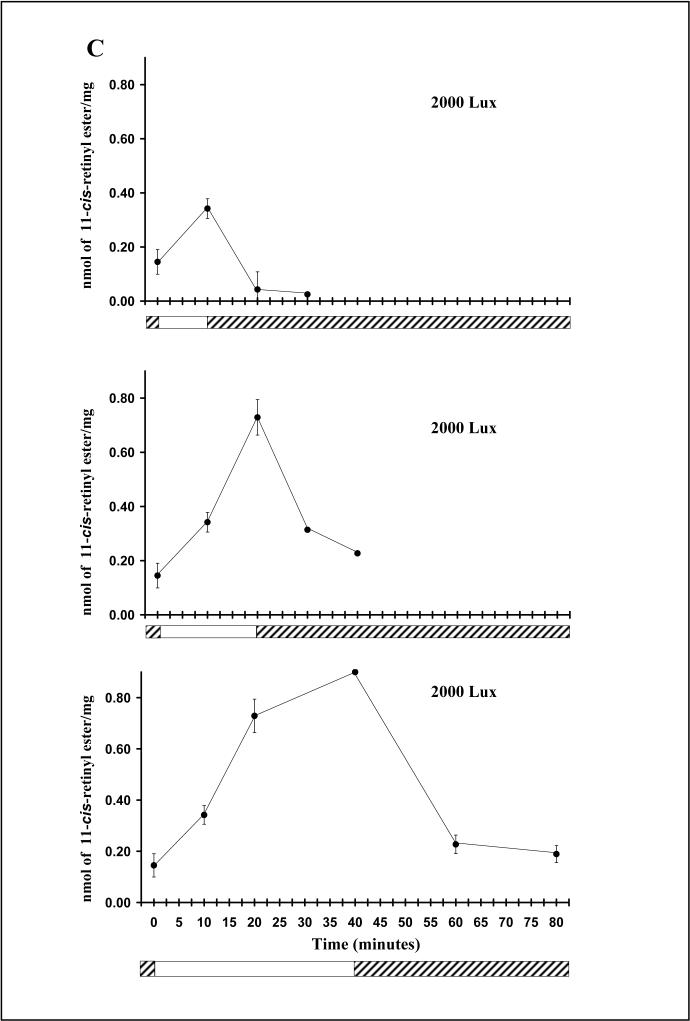
Fig. 2.
Light dependency of the chicken cone visual cycle: effect of light intensity (A, B) and duration of light exposure (C) on the accumulation of 11-cis retinyl esters in the chicken retina. A-B: Increase in retinyl ester accumulation in the chicken retina in response to increase in light intensity. Retinyl ester content in the retina increased with higher light intensity (A), indicating that this process is light-dependent. A reciprocal decrease of 11-cis retinal (B) suggests that retinyl ester in the retina is derived from retinal chromophore from bleached visual pigments. C. Increase in retinyl ester accumulation in the chicken retina in response to an increase in the duration of light exposure. At 2000 Lux, an increase in the duration of light exposure resulted in a significant increase in the amount of retinyl ester in the retina, indicating that this process is light-driven.  light adaptation,
light adaptation,  dark adaptation. For details of experiments and results, see Villazana-Espinoza, Hatch et al. 2006.
dark adaptation. For details of experiments and results, see Villazana-Espinoza, Hatch et al. 2006.
C-2. Visual Cycle Enzymes in Cone-dominated Retinas
Recent data show that membrane fractions prepared from cone-dominated retinas (chicken and ground squirrel) exhibit three visual cycle enzyme activities (Mata, Radu et al. 2002) different from those of the rod cycle. The first of these novel visual enzyme activities is associated with a retinyl ester synthase. In a series of experiments, it was shown that this retinyl ester synthase activity could not be reduced by the LRAT inhibitors trans retinol bromoacetate (tRBA) or N-ethylmaleimide (NEM) (note: LRAT is the known retinyl ester synthase in the rod cycle). These inhibition studies pointed towards a distinct retinyl ester synthase in the cone-dominated retina. Furthermore, it was also demonstrated that the ester synthase activity in the cone-dominated retina is enhanced in the presence of palmitoyl CoA, suggesting an ARAT enzyme. Figure 3A shows the effect of palmitoyl CoA and CRALBP on retinyl ester synthesis.
Figure 3.
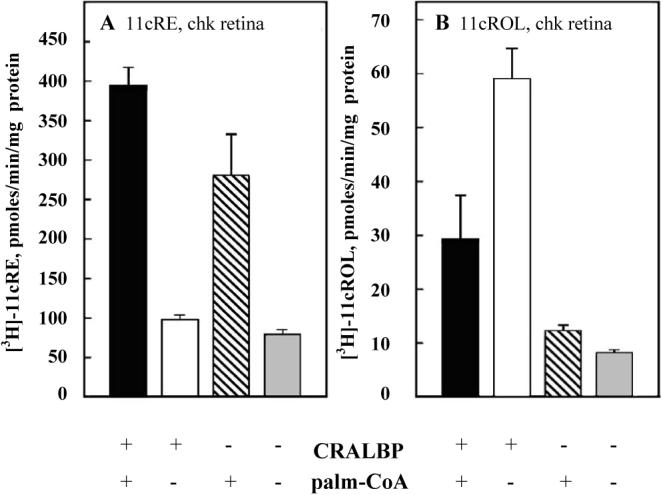
Novel properties of 11-cis retinyl ester synthase (A) and retinol isomerase (B) enzyme activities in chicken retinal membrane. A: Synthesis of 11-cis-retinyl esters by chicken retinal membranes is dependent on both CRALBP and palmitoyl CoA. B: Synthesis of 11-cis retinol from all-trans retinol by chicken retinal membranes is enhanced in the presence of CRALBP. For details of experiment and results, see Mata, Radu et al. 2002.
The second novel enzyme activity found in the cone-dominated retina is associated with that of retinol dehydrogenase. In the past it has been shown that cones regenerate opsin pigment upon the addition of 11-cis retinol (Jones, Crouch et al. 1989), suggesting that cones may posses the ability to oxidize retinol to retinal. To test the possibility of photoreceptors being able to oxidize 11-cis retinol, chicken and ground squirrel retina microsomes were assayed for 11-cis retinol dehydrogenase activity. The 11-cis retinol dehydrogenase activity in the retina was found to prefer NADP+ as a cofactor (Mata, Radu et al. 2002) rather than NAD+ (which is the cofactor for the 11-cis retinol dehydrogenase in the rod cycle, (Jang, McBee et al. 2000). Further characterization of 11-cis retinol dehydrogenase activity of the cone-dominated retina showed high specificity for pro-S-11-cis retinol and pro-S-NADPH substrates. Table 1 shows the kinetic parameters, apparent Vmax and Km, for 11-cis-retinol dehydrogenase in retinal microsomes from cone-dominated retinas.
Table 1.
Kinetic properties of novel retinol dehydrogenases (oxidase) in the conedominated ground squirrel and chicken retinas. Enzyme activities were assayed in membranes prepared from ground squirrel, chicken, bovine, and mouse retinas. In comparison to the enzyme activities of 11-cis retinol dehydrogenase in the membrane of rod-dominated bovine and mouse retinas (9 and 3 pmol/min/mg respectively), the maximum velocities of 11-cis retinol dehydrogenases in the cone-dominated ground squirrel and chicken retina were significantly higher (138 and 95 pmol/min/mg respectively). Similarly, enzyme activities of all-trans retinol dehydrogenase of cone-dominated retinas (148 and 104 pmol/min/mg, ground squirrel and chicken, respectively) far exceeded those in rod- dominated retinas (66 and 27 pmol/min/mg; bovine and mouse, respectively). Comparable Km were observed for retinol dehydrogenases in cone- and rod- dominated retinas. These results show that cone-dominated retinas have novel retinol dehydrogenases, with distinctly higher catalytic rates to convert retinol to retinal than those in rod-dominated retinas. The affinity between retinol dehydrogenase enzyme and substrate (as indicated by Km) is similar between rod- and cone-dominated retinas (for details of experiments and results, see Mata, Radu et al. 2002).
| 11-cis Retinol Dehydrogenase | All-trans Retinol Dehydrogenase | |||
|---|---|---|---|---|
| Vmax | Km | Vmax | Km | |
| Ground Squirrel | 138 ± 6.3 | 3.1 | 148 ± 8.1 | 6.1 |
| Chicken | 95 ± 4.5 | 2.9 | 104 ± 4.2 | 4.6 |
| Bovine | 9 ± 1.7 | 2.2 | 66 ± 3.4 | 2.6 |
| Mouse | 3 ± 0.7 | 1.7 | 27 ± 2.5 | 1.0 |
(Vmax is in pmol/min/mg and Km is in μM).
The third novel enzyme activity is from the all-trans retinol isomerase (or Isomerase II), which converts all-trans retinol to 11-cis retinol passively in the presence of CRALBP (see Fig. 3B) and CRBP1 (Mata, Radu et al. 2002; Mata, Ruiz et al. 2005) . This Isomerase II activity in the cone-rich retina is distinct from the Isomerase I (RPE65) in the RPE which utilizes all-trans retinyl esters as substrate (Jin, Li et al. 2005; Moiseyev, Chen et al. 2005; Redmond, Poliakov et al. 2005). Further, in the presence of CRBP1, the Isomerase present in the cone-dominated retina isomerizes all-trans retinol to 11-cis retinol without the synthesis of retinyl esters (Mata, Ruiz et al. 2005). In another study, it was shown that all-trans-retinyl ester is the direct precursor of 11-cis-retinol in chicken retinal pigment epithelium/retina membranes, suggesting that isomerohydrolase (or Isomerase I) may be involved in the synthesis of 11-cis retinol in the cone-rich chicken retina (Gollapalli and Rando 2003). However, it is possible that the combined RPE/retina membranes favored the activity of Isomerase I in the RPE. Thus it is difficult to conclude that the isomerase activity in the cone-dominated chicken eye is that of the Isomerase I. Recently, this isomerase activity was further examined by measuring the conversion of radiolabeled all-trans retinol to 11-cis retinol in the chicken eyecups and in the isolated retina. Results from these experiments show that the labeled all-trans retinol was converted to both 11-cis retinol and all-trans retinyl esters. All-trans retinyl esters had a significantly lower specific radioactivity than 11-cis retinol, thus it cannot serve as a precursor for 11-cis retinol (Villazana-Espinoza, Hatch et al. 2006a). These results further support an Isomerase II-mediated isomerization of retinol at the alcohol oxidation level.
Although the activity of an Isomerase enzyme in the chicken retina has been investigated previously (Gollapalli and Rando 2003; Mata, Ruiz et al. 2005), the location of this enzyme in the retina, and the structure of the protein remain unknown. Further work is needed to identify this Isomerase enzyme, along with biochemical characterization and localization of this protein in the cone-rich chicken retina (Villazana-Espinoza, Hatch et al. 2006a).
C-3. The role of Müller cells in the cone visual cycle
Das and his colleagues (Das, Bhardwaj et al. 1992) were the first to suggest that the Müller cell is the possible location in the retina where retinoids of a cone visual cycle are enzymatically processed and are stored. Interestingly, it was also found earlier that both CRBP and CRALBP are located in these cells (Bunt-Milam and Saari 1983; Eisenfeld, Bunt-Milam et al. 1985). In a recent study, cultured chicken Müller cells were determined to possess an 11-cis ARAT activity that specifically catalyzes the synthesis of 11-cis retinyl esters from 11-cis retinol, and this activity is enhanced in the presence of CRALBP (see Fig. 4) (Muniz, Villazana-Espinoza et al. 2006). The identification of an 11-cis ARAT activity provides an explanation for how 11-cis retinyl esters rapidly accumulate in the chicken retina upon light exposure, and supports the concept of Müller cells playing a key role in a cone visual cycle.
Figure 4.
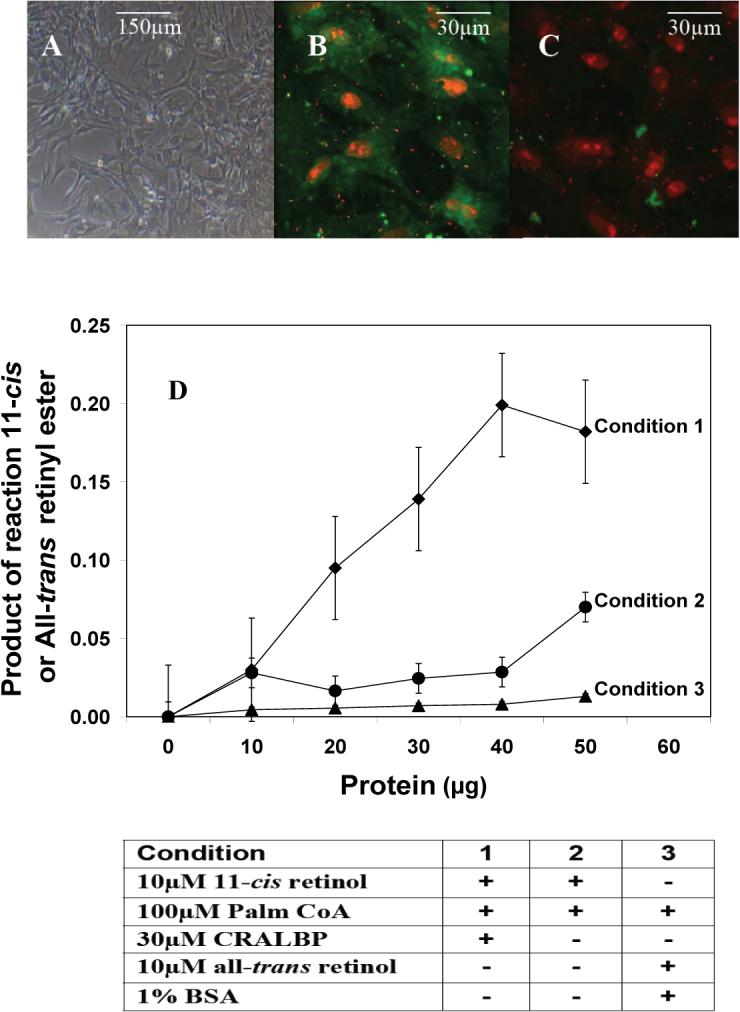
11-cis retinyl ester synthase activity in Müller cell of the chicken retina. A-C: Photomicrographs showing primary culture of chicken Müller cells. Freshly explanted Müller cells were cultured to confluence (A) and immunostained with anti-CRALBP antibody for identification of cell type (B), and negative control (C). D: Production of 11-cis retinyl ester by Müller cell membranes is specific for 11-cis retinol and requires both CRALBP and palmitoyl CoA. This 11-cis retinyl ester synthase activity in the Müller cell provides an explanation for the accumulation of 11-cis retinyl ester in the cone-dominated chicken retina. ◆ 11-cis retinyl esters under condition 1; • 11-cis retinyl esters under condition 2; ▲ all-trans retinyl esters under condition 3. For details of experiments and results, see Muniz, Villazana-Espinoza et al. 2006.
C-4. Proposed Cone Visual Cycle in cone-dominated retinas:
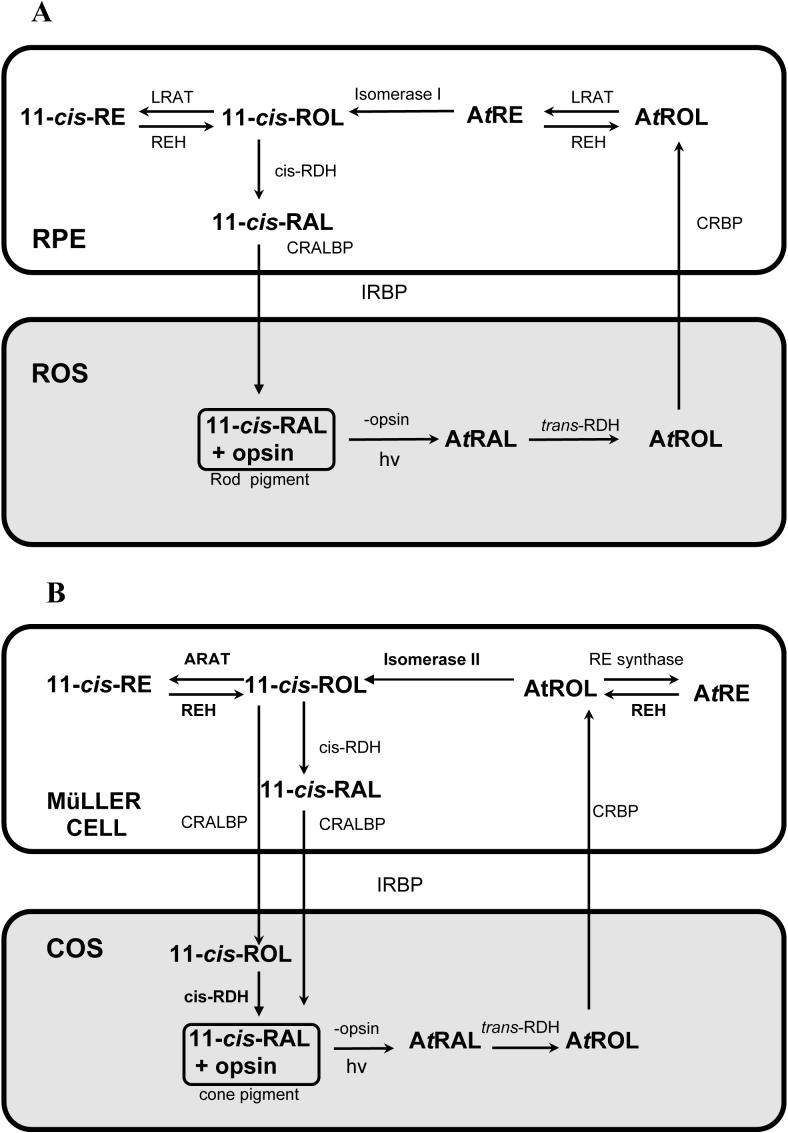
Based on experimental data summarized in previous paragraphs, it is evident that the cone-dominated chicken retina possesses a cone visual cycle to support cone pigment regeneration. Similar to the rod cycle (Fig. 5A), 11-cis retinal in the cone pigment is photo-isomerized by light absorption to all-trans retinal, then reduced to all-trans retinol by a still unidentified and uncharacterized retinol dehydrogenase (Fig. 5B). All-trans retinol is then released and transported across the interphotoreceptor matrix to Müller cells. IRBP is an abundant protein in the interphotoreceptor matrix and it is likely responsible for the transfer of retinoids between cones and Müller cells (Okajima, Pepperberg et al. 1989; Pepperberg, Okajima et al. 1991; Crouch, Hazard et al. 1992). In Müller cells, all-trans retinol is bound to CRBP (Bunt-Milam and Saari 1983; Eisenfeld, Bunt-Milam et al. 1985) and it is isomerized to 11-cis retinol by Isomerase II at the alcohol level (Mata, Radu et al. 2002; Mata, Ruiz et al. 2005). The all-trans retinol may also be esterified to all-trans retinyl ester by an unidentified and uncharacterized retinyl ester synthase. Based on results from immunochemical and enzyme activity experiments (Mata, Radu et al. 2002; Muniz, Villazana-Espinoza et al. 2006), this retinyl ester synthase is neither LRAT nor ARAT. The all-trans retinyl ester may also be isomerohydrolyzed to 11-cis retinol by Isomerase I (Gollapalli and Rando 2003). This 11-cis retinol then follows one of two paths. The first is a storage path in which 11-cis ARAT esterifies the 11-cis retinol into 11-cis retinyl ester (Mata, Radu et al. 2002; Muniz, Villazana-Espinoza et al. 2006). This 11-cis retinyl ester is then hydrolyzed by 11-cis retinyl ester hydrolase to supply 11-cis retinol for pigment regeneration (Bustamante, Ziari et al. 1995). In the second path, 11-cis retinol is oxidized by a retinol dehydrogenase to 11-cis retinal. This retinol dehydrogenase may be located in the Müller cells, and also in the cone photoreceptors (Mata, Radu et al. 2002). If the retinol dehydrogenase is located in the Müller cells, the resulting 11-cis retinal will then be bound to and protected by CRALBP. It is important to note that Müller cell CRALBP has binding affinity for both 11-cis retinol and 11-cis retinal (Maw, Kennedy et al. 1997; Noy 2000). The 11-cis retinal is then released to the interphotoreceptor matrix and transported to the photoreceptors to combine with cone opsin to form cone pigment (Fig. 5B). It is also likely that 11-cis retinol is provided to the cone photoreceptors where it is oxidized to retinal for cone pigment regeneration (Jones, Crouch et al. 1989; Mata, Radu et al. 2002).
Figure 5.
Comparison of pathways of the rod (A) and the cone (B) visual cycles. A: Pathways of the classical rhodopsin visual cycle in the retina and RPE of the eye (see sections A-1 and A-2 for details). B: Pathways of the novel cone visual cycle in the cone-dominated retina. (see Section C-4 for details). Enzymes that have been characterized with apparent kinetic constants in the cone visual pathway are indicated with bold font and they include Isomerase II (Mata, Ruiz et al. 2005), 11-cis and all-trans retinyl ester hydrolase (REH) (Bustamante, Ziari et al. 1995), 11-cis Acyl CoA Retinol Acyl Transferase (ARAT) (Mata, Radu et al. 2002; Muniz, Villazana-Espinoza et al. 2006) and retinyl dehydrogenase (RDH-oxidase) (Mata, Radu et al. 2002). Uncharacterized enzymes are indicated with normal font; these include all-trans retinyl dehydrogenase (RDH-reductase) in the cone outer segment, all-trans retinyl ester synthase, and 11-cis retinol dehydrogenase in the Müller cell. The retinoid binding proteins IRBP and CRBP/CRALBP have been located in the interphotoreceptor matrix and Müller cells, respectively (Bunt-Milam and Saari 1983; Okajima, Pepperberg et al. 1989; Crouch, Hazard et al. 1992).
D: Discussions:
The elucidation of a novel cone visual cycle in the cone-dominated retina provides the basis for further investigations on the properties of this and other cone cycles in the eye. For example, the identity of proteins and kinetic properties of many visual enzymes of this cone cycle are not known. The nature and the roles of retinol binding proteins remain to be studied. Furthermore, it will be of great interest to study how this cone cycle operates in the rod-dominated human or mouse retina as well as in duplex (fish and frog) retinas.
D-1: Structure and functions of cone visual cycle enzymes:
The activities of Isomerase I and II have been investigated (Gollapalli and Rando 2003; Mata, Ruiz et al. 2005) but the nature of the Isomerase II protein remains unknown. The activity of the all-trans retinyl ester synthase remains to be investigated since it is not a known enzyme such as LRAT (Mata, Radu et al. 2002), nor all-trans ARAT (Muniz, Villazana-Espinoza et al. 2006). The RDH in the photoreceptor and in the Müller cell have not been fully investigated at this time.
D-2: Role of retinoid binding proteins in the cone visual cycle:
The specificity and importance of CRALBP in the retinol isomerase reaction has been underscored (Mata, Ruiz et al. 2005). The contribution of retinoid-binding proteins such as CRALBP and IRBP to throughput (flux?) in specific steps of the cone visual cycle remains a topic for future research. The role of CRALBP in supporting the activity of Isomerase II in the retina may prove to be similar to the pivotal role of isomerase I in the RPE.
D-3: Property of a cone cycle in a duplex retina or in a rod-dominated retina:
It has been hypothesized that a separate cone visual cycle was first developed in duplex retinas to allow cones to avoid competition with rods for 11-cis retinal supplied by the RPE (Mata, Radu et al. 2002). Thus, it is important to examine whether a neuro-retinal cone visual cycle co-exists with the RPE rod cycle in duplex retinas, from which modern cone-rich retinas were derived, and if so, the degree to which the two visual cycles interact under bright and dim-light conditions.
Rod-dominated human retinas contain a cone-only area centralis and a cone-rich fovea/macula. It is not known if a cone cycle, similar to that described herein for cone-dominated retinas, exists in these locations to support cone pigment regeneration. Further study is clearly needed to examine properties of cone pigment regeneration in the macula region which is of great clinical significance.
E. Conclusions:
The discovery of the rod visual cycle half a century ago has led to fruitful investigations into the structure and function of many visual proteins of the rod pigment regeneration pathway. The recent discovery of a cone cycle in the cone-dominated retina offers new opportunities to study visual proteins and the pathway of cone pigment regeneration. Additional research is needed to reveal the existence of a cone cycle in the rod-dominated human and mouse retina and the interplay of this cone cycle with the rod cycle. Results from these studies will provide new knowledge on cone functions of the eye.
Acknowledgements:
We thank Drs. Joe Harrison, Bridget Thackeray, Mr. Ernest Heimsath and Mrs. Eileen K. Vidro for their critical review of the manuscript. Our research was supported by a grant from the NIH (GM08194) and the MBRS-RISE Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler AJ, Klucznik KM. Proteins and glycoproteins of the bovine interphotoreceptor matrix: composition and fractionation. Exp Eye Res. 1982;34(3):423–34. doi: 10.1016/0014-4835(82)90088-4. [DOI] [PubMed] [Google Scholar]
- Blaner WS, Das SR, et al. Hydrolysis of 11-cis- and all-trans-retinyl palmitate by homogenates of human retinal epithelial cells. J Biol Chem. 1987;262(1):53–8. [PubMed] [Google Scholar]
- Bok D. Retinal photoreceptor-pigment epithelium interactions. Friedenwald lecture. Invest Ophthalmol Vis Sci. 1985;26(12):1659–94. [PubMed] [Google Scholar]
- Bok D, Ong DE, et al. Immunocytochemical localization of cellular retinol binding protein in the rat retina. Invest Ophthalmol Vis Sci. 1984;25(8):877–83. [PubMed] [Google Scholar]
- Bridges CD, Alvarez RA, et al. Rhodopsin, vitamin A, and interstitial retinol-binding protein in the rd chicken. Invest Ophthalmol Vis Sci. 1987;28(4):613–7. [PubMed] [Google Scholar]
- Bunt-Milam AH, Saari JC. Immunocytochemical localization of two retinoid-binding proteins in vertebrate retina. J Cell Biol. 1983;97(3):703–12. doi: 10.1083/jcb.97.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante JJ, Ziari S, et al. Retinyl ester hydrolase and the visual cycle in the chicken eye. Am J Physiol. 1995;269(6 Pt 2):R1346–50. doi: 10.1152/ajpregu.1995.269.6.R1346. [DOI] [PubMed] [Google Scholar]
- Chen P, Lee TD, et al. Interaction of 11-cis-retinol dehydrogenase with the chromophore of retinal g protein-coupled receptor opsin. J Biol Chem. 2001;276(24):21098–104. doi: 10.1074/jbc.M010441200. [DOI] [PubMed] [Google Scholar]
- Chen XN, Korenberg JR, et al. Localization of the human RGR opsin gene to chromosome 10q23. Hum Genet. 1996;97(6):720–2. doi: 10.1007/BF02346179. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Haeseleer F, et al. Rod and cone visual cycle consequences of a null mutation in the 11-cis-retinol dehydrogenase gene in man. Vis Neurosci. 2000;17(5):667–78. doi: 10.1017/s0952523800175029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch RK, Chader GJ, et al. Retinoids and the visual process. Photochem Photobiol. 1996;64(4):613–21. doi: 10.1111/j.1751-1097.1996.tb03114.x. [DOI] [PubMed] [Google Scholar]
- Crouch RK, Hazard ES, et al. Interphotoreceptor retinoid-binding protein and alpha-tocopherol preserve the isomeric and oxidation state of retinol. Photochem Photobiol. 1992;56(2):251–5. doi: 10.1111/j.1751-1097.1992.tb02154.x. [DOI] [PubMed] [Google Scholar]
- Das SR, Bhardwaj N, et al. Muller cells of chicken retina synthesize 11-cisretinol. Biochem J. 1992;285(Pt 3):907–13. doi: 10.1042/bj2850907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE. Chemistry of visual adaptation in the rat. Nature (London) 1960;188:114–118. doi: 10.1038/188114a0. [DOI] [PubMed] [Google Scholar]
- Driessen CA, Janssen BP, et al. Cloning and expression of a cDNA encoding bovine retinal pigment epithelial 11-cis retinol dehydrogenase. Invest Ophthalmol Vis Sci. 1995;36(10):1988–96. [PubMed] [Google Scholar]
- Driessen CA, Winkens HJ, et al. Cloning and structural analysis of the murine GCN5L1 gene. Gene. 1997;203(1):27–31. doi: 10.1016/s0378-1119(97)00486-1. [DOI] [PubMed] [Google Scholar]
- Eisenfeld AJ, Bunt-Milam AH, et al. Localization of retinoid-binding proteins in developing rat retina. Exp Eye Res. 1985;41(3):299–304. doi: 10.1016/s0014-4835(85)80020-8. [DOI] [PubMed] [Google Scholar]
- Fan J, Rohrer B, et al. Isorhodopsin rather than rhodopsin mediates rod function in RPE65 knock-out mice. Proc Natl Acad Sci U S A. 2003;100(23):13662–7. doi: 10.1073/pnas.2234461100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong SL, Bridges CD. Interstitial retinol-binding protein: purification, characterization, molecular cloning, and sequence. Methods Enzymol. 1990;189:207–13. doi: 10.1016/0076-6879(90)89291-o. [DOI] [PubMed] [Google Scholar]
- Futterman S, Saari JC. Occurrence of 11-cis-retinal-binding protein restricted to the retina. Invest Ophthalmol Vis Sci. 1977;16(8):768–71. [PubMed] [Google Scholar]
- Futterman S, Saari JC, et al. Occurrence of a binding protein for 11-cis-retinal in retina. J Biol Chem. 1977;252(10):3267–71. [PubMed] [Google Scholar]
- Gamble MV, Mata NL, et al. Substrate specificities and 13-cis-retinoic acid inhibition of human, mouse and bovine cis-retinol dehydrogenases. Biochim Biophys Acta. 2000;1476(1):3–8. doi: 10.1016/s0167-4838(99)00232-0. [DOI] [PubMed] [Google Scholar]
- Goldstein EB. Early receptor potential of the isolated frog (Rana pipiens) retina. Vision Res. 1967;7(11):837–45. doi: 10.1016/0042-6989(67)90004-1. [DOI] [PubMed] [Google Scholar]
- Goldstein EB. Visual pigments and the early receptor potential of the isolated frog retina. Vision Res. 1968;8(8):953–63. doi: 10.1016/0042-6989(68)90070-9. [DOI] [PubMed] [Google Scholar]
- Goldstein EB, Wolf BM. Regeneration of the green-rod pigment in the isolated frog retina. Vision Res. 1973;13(3):527–34. doi: 10.1016/0042-6989(73)90022-9. [DOI] [PubMed] [Google Scholar]
- Gollapalli DR, Rando RR. All-trans-retinyl esters are the substrates for isomerization in the vertebrate visual cycle. Biochemistry. 2003;42(19):5809–18. doi: 10.1021/bi0341004. [DOI] [PubMed] [Google Scholar]
- Gollapalli DR, Rando RR. Molecular logic of 11-cis-retinoid biosynthesis in a cone-dominated species. Biochemistry. 2003;42(50):14921–9. doi: 10.1021/bi0356505. [DOI] [PubMed] [Google Scholar]
- Groenendijk GW, De Grip WJ, et al. Quantitative determination of retinals with complete retention of their geometric configuration. Biochim Biophys Acta. 1980;617(3):430–8. doi: 10.1016/0005-2760(80)90009-0. [DOI] [PubMed] [Google Scholar]
- Haeseleer F, Huang J, et al. Molecular characterization of a novel short-chain dehydrogenase/reductase that reduces all-trans-retinal. J Biol Chem. 1998;273(34):21790–9. doi: 10.1074/jbc.273.34.21790. [DOI] [PubMed] [Google Scholar]
- Hao W, Chen P, et al. Analysis of chromophore of RGR: retinal G-protein-coupled receptor from pigment epithelium. Methods Enzymol. 2000;316:413–22. doi: 10.1016/s0076-6879(00)16739-4. [DOI] [PubMed] [Google Scholar]
- Ho MT, Massey JB, et al. Mechanism of vitamin A movement between rod outer segments, interphotoreceptor retinoid-binding protein, and liposomes. J Biol Chem. 1989;264(2):928–35. [PubMed] [Google Scholar]
- Hood DC, Hock PA. Recovery of cone receptor activity in the frog's isolated retina. Vision Res. 1973;13(10):1943–51. doi: 10.1016/0042-6989(73)90065-5. [DOI] [PubMed] [Google Scholar]
- Jang GF, McBee JK, et al. Stereoisomeric specificity of the retinoid cycle in the vertebrate retina. J Biol Chem. 2000;275(36):28128–38. doi: 10.1074/jbc.M004488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Pandey S, et al. An opsin homologue in the retina and pigment epithelium. Invest Ophthalmol Vis Sci. 1993;34(13):3669–78. [PubMed] [Google Scholar]
- Jin J, Jones GJ, et al. Movement of retinal along cone and rod photoreceptors. Vis Neurosci. 1994;11(2):389–99. doi: 10.1017/s0952523800001735. [DOI] [PubMed] [Google Scholar]
- Jin M, Li S, et al. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122(3):449–59. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GJ, Crouch RK, et al. Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc Natl Acad Sci U S A. 1989;86(23):9606–10. doi: 10.1073/pnas.86.23.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TS, Maeda A, et al. Delayed dark adaptation in 11-cis-retinol dehydrogenase-deficient mice: a role of RDH11 in visual processes in vivo. J Biol Chem. 2005;280(10):8694–704. doi: 10.1074/jbc.M413172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov AV, Ala-Laurila P, et al. Visual cycle and its metabolic support in gecko photoreceptors. Vision Res. 2007;47(3):363–74. doi: 10.1016/j.visres.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Lai YL, Wiggert B, et al. Interphotoreceptor retinol-binding proteins: possible transport vehicles between compartments of the retina. Nature. 1982;298(5877):848–9. doi: 10.1038/298848a0. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN., Jr. Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23(3):307–80. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Lion F, Rotmans JP, et al. Biochemical aspects of the visual process. XXVII. Stereospecificity of ocular retinol dehydrogenases and the visual cycle. Biochim Biophys Acta. 1975;384(2):283–92. doi: 10.1016/0005-2744(75)90030-3. [DOI] [PubMed] [Google Scholar]
- Liou GI, Ma DP, et al. Human interstitial retinoid-binding protein. Gene structure and primary structure. J Biol Chem. 1989;264(14):8200–6. [PubMed] [Google Scholar]
- Liou GI, Matragoon S, et al. Visual sensitivity and interphotoreceptor retinoid binding protein in the mouse: regulation by vitamin A. Faseb J. 1998;12(1):129–38. doi: 10.1096/fasebj.12.1.129. [DOI] [PubMed] [Google Scholar]
- Maeda A, Maeda T, et al. Aberrant metabolites in mouse models of congenital blinding diseases: formation and storage of retinyl esters. Biochemistry. 2006;45(13):4210–9. doi: 10.1021/bi052382x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Van Hooser JP, et al. Evaluation of the role of the retinal G protein-coupled receptor (RGR) in the vertebrate retina in vivo. J Neurochem. 2003;85(4):944–56. doi: 10.1046/j.1471-4159.2003.01741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata JR, Mata NL, et al. Substrate specificity of retinyl ester hydrolase activity in retinal pigment epithelium. J Lipid Res. 1998;39(3):604–12. [PubMed] [Google Scholar]
- Mata NL, Mata JR, et al. Comparison of retinyl ester hydrolase activities in bovine liver and retinal pigment epithelium. J Lipid Res. 1996;37(9):1947–52. [PubMed] [Google Scholar]
- Mata NL, Radu RA, et al. Isomerization and oxidation of vitamin A in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36(1):69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata NL, Ruiz A, et al. Chicken retinas contain a retinoid isomerase activity that catalyzes the direct conversion of all-trans-retinol to 11-cis-retinol. Biochemistry. 2005;44(35):11715–21. doi: 10.1021/bi050942m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata NL, Tsin AT. Distribution of 11-cis LRAT, 11-cis RD and 11-cis REH in bovine retinal pigment epithelium membranes. Biochim Biophys Acta. 1998;1394(1):16–22. doi: 10.1016/s0005-2760(98)00078-2. [DOI] [PubMed] [Google Scholar]
- Mata NL, Tsin AT, et al. Hydrolysis of 11-cis- and all-trans-retinyl palmitate by retinal pigment epithelium microsomes. J Biol Chem. 1992;267(14):9794–9. [PubMed] [Google Scholar]
- Mata NL, Villazana ET, et al. Colocalization of 11-cis retinyl esters and retinyl ester hydrolase activity in retinal pigment epithelium plasma membrane. Invest Ophthalmol Vis Sci. 1998;39(8):1312–9. [PubMed] [Google Scholar]
- Maw MA, Kennedy B, et al. Mutation of the gene encoding cellular retinaldehyde-binding protein in autosomal recessive retinitis pigmentosa. Nat Genet. 1997;17(2):198–200. doi: 10.1038/ng1097-198. [DOI] [PubMed] [Google Scholar]
- McBee JK, Palczewski K, et al. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retin Eye Res. 2001;20(4):469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- McBee JK, Van Hooser JP, et al. Isomerization of 11-cis-retinoids to all-trans-retinoids in vitro and in vivo. J Biol Chem. 2001;276(51):48483–93. doi: 10.1074/jbc.M105840200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseyev G, Chen Y, et al. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci U S A. 2005;102(35):12413–8. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal MS, Ruiz A, et al. Lecithin retinol acyltransferase contains cysteine residues essential for catalysis. Biochemistry. 2000;39(17):5215–20. doi: 10.1021/bi9929554. [DOI] [PubMed] [Google Scholar]
- Muniz A, Villazana-Espinoza ET, et al. 11-cis-Acyl-CoA:retinol O-acyltransferase activity in the primary culture of chicken Muller cells. Biochemistry. 2006;45(40):12265–73. doi: 10.1021/bi060928p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli JL. A gene knockout corroborates the integral function of cellular retinol-binding protein in retinoid metabolism. Nutr Rev. 2000;58(8):230–6. doi: 10.1111/j.1753-4887.2000.tb01870.x. [DOI] [PubMed] [Google Scholar]
- Noy N. Retinoid-binding proteins: mediators of retinoid action. Biochem J. 2000;348(Pt 3):481–95. [PMC free article] [PubMed] [Google Scholar]
- Okajima TI, Pepperberg DR, et al. Interphotoreceptor retinoid-binding protein: role in delivery of retinol to the pigment epithelium. Exp Eye Res. 1989;49(4):629–44. doi: 10.1016/s0014-4835(89)80059-4. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Van Hooser JP, et al. Kinetics of visual pigment regeneration in excised mouse eyes and in mice with a targeted disruption of the gene encoding interphotoreceptor retinoid-binding protein or arrestin. Biochemistry. 1999;38(37):12012–9. doi: 10.1021/bi990504d. [DOI] [PubMed] [Google Scholar]
- Pepperberg DR, Clack JW. Rhodopsin photoproducts and the visual response of vertebrate rods. Vision Res. 1984;24(11):1481–6. doi: 10.1016/0042-6989(84)90310-9. [DOI] [PubMed] [Google Scholar]
- Pepperberg DR, Okajima TL, et al. Functional properties of interphotoreceptor retinoid-binding protein. Photochem Photobiol. 1991;54(6):1057–60. doi: 10.1111/j.1751-1097.1991.tb02129.x. [DOI] [PubMed] [Google Scholar]
- Perlman JI, Nodes BR, et al. Utilization of retinoids in the bullfrog retina. J Gen Physiol. 1982;80(6):885–913. doi: 10.1085/jgp.80.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando RR. The biochemistry of the visual cycle. Chem Rev. 2001;101(7):1881–96. doi: 10.1021/cr960141c. [DOI] [PubMed] [Google Scholar]
- Rattner A, Smallwood PM, et al. Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J Biol Chem. 2000;275(15):11034–43. doi: 10.1074/jbc.275.15.11034. [DOI] [PubMed] [Google Scholar]
- Redmond TM, Poliakov E, et al. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci U S A. 2005;102(38):13658–63. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond TM, Yu S, et al. Rpe65 is necessary for production of 11-cisvitamin A in the retinal visual cycle. Nat Genet. 1998;20(4):344–51. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- Ripps H, Peachey NS, et al. The rhodopsin cycle is preserved in IRBP “knockout” mice despite abnormalities in retinal structure and function. Vis Neurosci. 2000;17(1):97–105. doi: 10.1017/s095252380017110x. [DOI] [PubMed] [Google Scholar]
- Rodriguez KA, Tsin AT. Retinyl esters in the vertebrate neuroretina. Am J Physiol. 1989;256(1 Pt 2):R255–8. doi: 10.1152/ajpregu.1989.256.1.R255. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Winston A, et al. Molecular and Biochemical Characterization of Lecithin Retinol Acyltransferase. 1999. [DOI] [PubMed] [Google Scholar]
- Saari JC. Isolation of cellular retinoid-binding proteins from bovine retina with bound endogenous ligands. Methods Enzymol. 1982;81:819–26. doi: 10.1016/s0076-6879(82)81109-9. [DOI] [PubMed] [Google Scholar]
- Saari JC. Biochemistry of visual pigment regeneration: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2000;41(2):337–48. [PubMed] [Google Scholar]
- Saari JC, Bredberg DL. CoA- and non-CoA-dependent retinol esterification in retinal pigment epithelium. J Biol Chem. 1988;263(17):8084–90. [PubMed] [Google Scholar]
- Saari JC, Bredberg DL. Lecithin:retinol acyltransferase in retinal pigment epithelial microsomes. J Biol Chem. 1989;264(15):8636–40. [PubMed] [Google Scholar]
- Saari JC, Bredberg DL, et al. Retinol esterification in bovine retinal pigment epithelium: reversibility of lecithin:retinol acyltransferase. Biochem J. 1993;291(Pt 3):697–700. doi: 10.1042/bj2910697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari JC, Bredberg DL, et al. Control of substrate flow at a branch in the visual cycle. Biochemistry. 1994;33(10):3106–12. doi: 10.1021/bi00176a045. [DOI] [PubMed] [Google Scholar]
- Saari JC, Bredberg L, et al. Identification of the endogenous retinoids associated with three cellular retinoid-binding proteins from bovine retina and retinal pigment epithelium. J Biol Chem. 1982;257(22):13329–33. [PubMed] [Google Scholar]
- Saari JC, Futterman S, et al. Cellular retinol- and retinoic acid-binding proteins of bovine retina. Purification and properties. J Biol Chem. 1978;253(18):6432–6. [PubMed] [Google Scholar]
- Seeliger MW, Grimm C, et al. New views on RPE65 deficiency: the rod system is the source of vision in a mouse model of Leber congenital amaurosis. Nat Genet. 2001;29(1):70–4. doi: 10.1038/ng712. [DOI] [PubMed] [Google Scholar]
- Shen D, Jiang M, et al. A human opsin-related gene that encodes a retinaldehyde-binding protein. Biochemistry. 1994;33(44):13117–25. doi: 10.1021/bi00248a022. [DOI] [PubMed] [Google Scholar]
- Simon A, Hellman U, et al. The retinal pigment epithelial-specific 11-cis retinol dehydrogenase belongs to the family of short chain alcohol dehydrogenases. J Biol Chem. 1995;270(3):1107–12. [PubMed] [Google Scholar]
- Simon A, Romert A, et al. Intracellular localization and membrane topology of 11-cis retinol dehydrogenase in the retinal pigment epithelium suggest a compartmentalized synthesis of 11-cis retinaldehyde. J Cell Sci. 1999;112(Pt 4):549–58. doi: 10.1242/jcs.112.4.549. [DOI] [PubMed] [Google Scholar]
- Stecher H, Gelb MH, et al. Preferential release of 11-cis-retinol from retinal pigment epithelial cells in the presence of cellular retinaldehyde-binding protein. J Biol Chem. 1999;274(13):8577–85. doi: 10.1074/jbc.274.13.8577. [DOI] [PubMed] [Google Scholar]
- Stubbs GW, Saari JC, et al. 11-cis-Retinal-binding protein from bovine retina. Isolation and partial characterization. J Biol Chem. 1979;254(17):8529–33. [PubMed] [Google Scholar]
- Suzuki Y, Ishiguro S, et al. Identification and immunohistochemistry of retinol dehydrogenase from bovine retinal pigment epithelium. Biochim Biophys Acta. 1993;1163(2):201–8. doi: 10.1016/0167-4838(93)90182-q. [DOI] [PubMed] [Google Scholar]
- Travis GH, Golczak M, et al. Diseases Caused by Defects in the Visual Cycle: Retinoids as Potential Therapeutic Agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevino SG, Villazana-Espinoza ET, et al. Retinoid cycles in the cone-dominated chicken retina. J Exp Biol. 2005;208(Pt 21):4151–7. doi: 10.1242/jeb.01881. [DOI] [PubMed] [Google Scholar]
- Tsin AT, Mata NL, et al. Substrate specificities of retinyl ester hydrolases in retinal pigment epithelium. Methods Enzymol. 2000;316:384–400. doi: 10.1016/s0076-6879(00)16737-0. [DOI] [PubMed] [Google Scholar]
- Villazana-Espinoza E, Hatch A, et al. In-vitro Conversion of All-Trans Retinol to 11-cis Retinol in Chicken Eye. Invest Ophthalmol Vis Sci. 2006a ARVO E abstract #2036. [Google Scholar]
- Villazana-Espinoza ET, Hatch AL, et al. Effect of light exposure on the accumulation and depletion of retinyl ester in the chicken retina. Exp Eye Res. 2006b;83(4):871–6. doi: 10.1016/j.exer.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Wald G. Molecular basis of visual excitation. Science. 1968;162(850):230–9. doi: 10.1126/science.162.3850.230. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Oberhauser V, et al. The retinal G protein-coupled receptor (RGR) enhances isomerohydrolase activity independent of light. J Biol Chem. 2005;280(33):29874–84. doi: 10.1074/jbc.M503603200. [DOI] [PubMed] [Google Scholar]
- Wenzel A, von Lintig J, et al. RPE65 Is Essential for the Function of Cone Photoreceptors in NRL-Deficient Mice. Invest Ophthalmol Vis Sci. 2007;48(2):534–42. doi: 10.1167/iovs.06-0652. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Simon A, et al. Mutations in the gene encoding 11-cis retinol dehydrogenase cause delayed dark adaptation and fundus albipunctatus. Nat Genet. 1999;22(2):188–91. doi: 10.1038/9707. [DOI] [PubMed] [Google Scholar]
- Yang M, Fong HK. Synthesis of the all-trans-retinal chromophore of retinal G protein-coupled receptor opsin in cultured pigment epithelial cells. J Biol Chem. 2002;277(5):3318–24. doi: 10.1074/jbc.M108946200. [DOI] [PubMed] [Google Scholar]
- Zimmerman WF. The Distribution and Proportions of Vitamin A Compounds during the Visual Cycle in the Rat. Vision Research. 1974;14(9):795–802. doi: 10.1016/0042-6989(74)90143-6. [DOI] [PubMed] [Google Scholar]
- Zimmerman WF, Lion F, et al. Biochemical aspects of the visual process. XXX. Distribution of stereospecific retinol dehydrogenase activities in subcellular fractions of bovine retina and pigment epithelium. Exp Eye Res. 1975;21(4):325–332. doi: 10.1016/0014-4835(75)90043-3. [DOI] [PubMed] [Google Scholar]
- Zimmerman WF, Yost MT, et al. Dynamics and function of vitamin A compounds in rat retina after a small bleach of rhodopsin. Nature. 1974;250(461):66–7. doi: 10.1038/250066a0. [DOI] [PubMed] [Google Scholar]