Abstract
N-methyl-D-aspartate (NMDA) receptors are critical for neuronal development and synaptic plasticity. The molecular mechanisms underlying the synaptic localization and functional regulation of NMDA receptors have been the subject of extensive studies. In particular, phosphorylation has emerged as a fundamental mechanism that regulates NMDA receptor trafficking and can alter the channel properties of NMDA receptors. Here we summarize recent advances in the characterization of NMDA receptor phosphorylation, emphasizing subunit-specific phosphorylation, which differentially controls the trafficking and surface expression of NMDA receptors.
Keywords: NMDA receptors, Phosphorylation, Kinase, Glutamate
1. Introduction
Ionotropic glutamate receptors mediate most excitatory neuronal transmission in the brain and play essential roles in the regulation of synaptic activity. Dysfunction of these receptors contributes to many neurological and psychiatric disorders, including Alzheimer's disease, Parkinson's disease, and schizophrenia (Cull-Candy et al., 2001; Waxman and Lynch, 2005). Depending on their specific response to different pharmacological agents, ionotropic glutamate receptors can be subdivided into N-methyl-D-aspartate (NMDA), a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate receptors (Dingledine et al., 1999; Hollmann and Heinemann, 1994). Among the ionotropic glutamate receptors, NMDA receptor channels have several unique features, including voltage-sensitive block by extracellular Mg2+, high permeability to Ca2+, and unusually slow activation/deactivation kinetics (Cull-Candy et al., 2001). The Mg2+ block acts as a molecular coincidence switch, with Mg2+ being removed from the pore of the channel when postsynaptic cells are depolarized. The relief of the Mg2+ block, together with agonist binding, leads to Ca2+ influx through the NMDA receptor channel that in turn regulates synaptic strength through Ca2+-activated signaling cascades.
Three families of genes (NR1, NR2 and NR3) have been identified that encode the NMDA receptor subunits (Cull-Candy et al., 2001). Functional NMDA receptors are tetramers composed of two essential NR1 subunits assembling with two NR2 subunits or in some cases, an NR2 and an NR3 subunit (Cull-Candy and Leszkiewicz, 2004). Crystallographic analysis, in combination with biochemical and electrophysiological studies, indicates that the NR1-NR2 heterodimer is the functional unit in tetrameric NMDA receptors (Furukawa et al., 2005). A unique feature of NMDA receptors is that receptor activation requires the binding of the co-agonist glycine in addition to glutamate (Erreger et al., 2004). Therefore, functional NMDA receptors require both an NR1 subunit, which contains the glycine binding site, and an NR2 subunit, which binds to glutamate. In addition to the formation of diheteromeric receptors (e.g. NR1/NR2B), there is compelling evidence for the existence of triheteromeric NMDA receptors (e.g. NR1/NR2A/NR2B) (Cull-Candy and Leszkiewicz, 2004). Many studies have demonstrated that NR2 and NR3 subunits confer distinct electrophysiological properties to the NMDA receptors (Cull-Candy and Leszkiewicz, 2004). Therefore, variability in NMDA receptor subunit composition is an important source of diversity for functional regulation of NMDA receptors.
The specific subunit composition of NMDA receptors varies at distinct synapses in different developmental stages. The NR1 subunit is a single gene product and, as an essential subunit, is found ubiquitously throughout the brain. In contrast, NR2 subunits (NR2A-D) are encoded by four distinct genes and are differentially expressed throughout the brain and during development. Among NR2 subunits, the expression patterns of NR2A and NR2B are relatively broad and both are developmentally regulated, with a concurrent decrease in NR2B and increase in NR2A expression as neurons mature. NR2C is restricted primarily to the cerebellum and is expressed later in development. In contrast, NR2D is predominantly expressed early in development and is localized mainly in thalamic and hypothalamic nuclei and in the brainstem (Monyer et al., 1994). The NR3A subunit is widely distributed early in development (Ciabarra et al., 1995; Sucher et al., 1995), whereas NR3B is restricted primarily to motor neurons (Chatterton et al., 2002). Endogenous NMDA receptors typically contain NR1 and NR2 subunits, with NR3 subunits only incorporated in a subpopulation of NMDA receptors playing a modulatory role (Cull-Candy and Leszkiewicz, 2004).
NMDA receptor subunits contain a long extracellular N-terminal domain, three true transmembrane segments, a re-entrant pore loop, and an intracellular C-terminal domain of variable length. The C-terminal domain is the most divergent region of the protein when comparing NMDA receptor subunits, consistent with it playing a critical role in the diversity conferred on NMDA receptors by different subunit compositions. Whereas the N-terminal domain and extracellular loop form the ligand-binding pocket (Furukawa and Gouaux, 2003), the C-terminal tail regulates receptor interactions with a variety of cytosolic proteins. These protein-protein interactions dictate the precise intracellular trafficking and localization of NMDA receptors. In addition, different NMDA receptor subunits can couple receptors to distinct intracellular signaling complexes. For example, NR2B specifically interacts with the protein SynGAP, which is a Ras GTPase activating protein demonstrated to selectively inhibit NMDA-stimulated ERK signaling (Kim et al., 2005). Also, NR2A and NR2B bind to active calcium/calmodulin-dependent protein kinase II (CaMKII) with different affinities (Strack and Colbran, 1998), which results in different forms of synaptic plasticity (Barria and Malinow, 2005). Finally, the C-termini of NMDA receptor subunits are substrates for post-translational modifications such as phosphorylation. Phosphorylation regulates many cellular processes including protein activity, localization and mobility. In addition, phosphorylation is an important regulator of many protein-protein interactions. Direct phosphorylation of ionotropic glutamate receptors is a key mechanism regulating channel function and receptor localization at synapses (Lee, 2006).
2. Functional regulation of NMDA receptors by serine/threonine phosphorylation
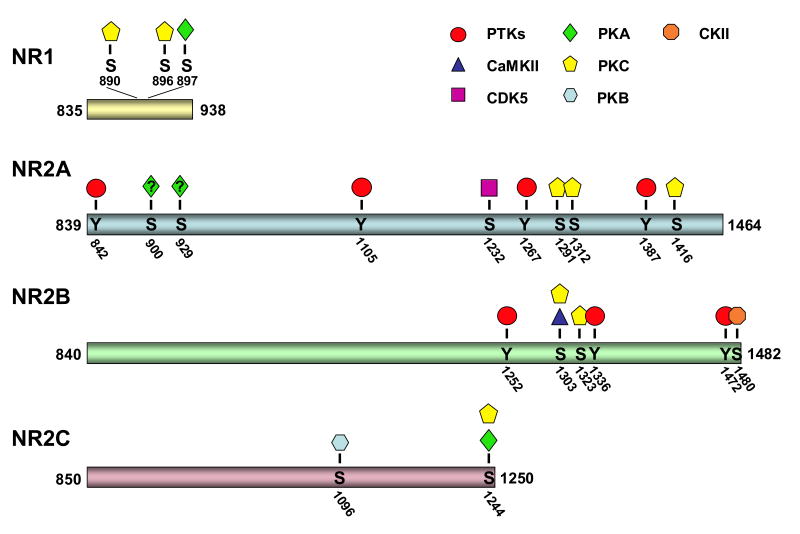
Many serine/threonine phosphorylation sites have been identified in NMDA receptor subunits, which are substrates for cAMP-dependent protein kinase A (PKA), protein kinase C (PKC), protein kinase B (PKB), CaMKII, cyclin-dependent kinase-5 (Cdk5), and casein kinase II (CKII) (Figure 1). These kinases can regulate intracellular trafficking or channel properties of NMDA receptors, resulting in changes in synaptic strength underlying many forms of synaptic plasticity (Lee, 2006). Although in some cases the regulation of NMDA receptors by phosphorylation is due to the phosphorylation of other neuronal substrates (Lan et al., 2001; Sigel et al., 1994; Zheng et al., 1999), in this review we will focus on the recent progress in characterizing the direct phosphorylation of NMDA receptors.
Figure 1.
Phosphorylation sites on the cytosolic tails of NMDA receptor subunits. The cytosolic tails of NMDA receptor subunits NR1, NR2A, NR2B and NR2C contain about 100, 630, 640 and 400 amino acids, respectively. Each tail is phosphorylated on serine/threonine and/or tyrosine residues by a variety of kinases. Among identified phosphorylation sites: NR2A is uniquely phosphorylated by Cdk5; NR2B is uniquely phosphorylated by CKII; and NR2C is uniquely phosphorylated by PKB.
There is ample evidence that NMDA receptor function is regulated by a variety of protein kinases (Mammen et al., 1999; Roche et al., 1994). The majority of studies have focused on the second messenger activated serine/threonine kinases. For example, activation of PKC potentiates NMDA receptor-mediated currents (Chen and Huang, 1992). It has been suggested that the effect of PKC on NMDA receptor regulation is the result of an increase in the opening rate of NMDA receptor channels and upregulation of receptor surface expression (Lan et al., 2001; Lu et al., 1999). However, there is also evidence that PKC activation can suppress NMDA receptor-mediated currents (Markram and Segal, 1992). These findings are supported by findings showing that PKC activation induces a rapid dispersal of NMDA receptors from synaptic sites (Fong et al., 2002). As with PKC, PKA also regulates NMDA receptor function. Activation of PKA increases the amplitude of NMDA receptor-mediated excitatory postsynaptic currents (Raman et al., 1996). Consistently, synaptic targeting of NMDA receptors is increased by PKA activation (Crump et al., 2001). In addition, recent studies have shown that PKA inhibitors reduce the relative fraction of Ca2+ influx through NMDA receptors, suggesting that PKA regulates calcium permeability of NMDA receptors (Skeberdis et al., 2006).
Although NR1 is an essential NMDA receptor subunit and as such is expressed throughout the brain, there is considerable diversity imparted on NR1 due to alternative splicing of exons 5, 21, and 22, which generate eight NR1 protein variants. Exon 5, which encodes the extracellular N-terminal domain, modulates the pharmacological properties of NMDA receptors (Rumbaugh et al., 2000; Traynelis et al., 1998; Traynelis et al., 1995). Exon 21 (also known as the C1 cassette) and exon 22 encode the intracellular C-terminal domain and mRNA splicing at these exons regulates protein-protein interactions, receptor trafficking, and NR1 phosphorylation (Ehlers et al., 1998; Ehlers et al., 1996; Lin et al., 1998; Mu et al., 2003; Okabe et al., 1999; Scott et al., 2001; Standley et al., 2000; Tingley et al., 1993). NR1 is phosphorylated by PKC on two residues (S890 and S896) within exon 21 (Tingley et al., 1997). Phosphorylation of S890 disrupts the clustering of the NR1 subunit (Tingley et al., 1997). S896 is also phosphorylated by PKC; however, phosphorylation of this residue alone has no effect on NR1 clustering, instead phosphorylation of S896 together with PKA phosphorylation of S897 are required to increase NMDA receptor surface expression (Scott et al., 2001). Interestingly, S896 and S897 of NR1 are highly phosphorylated in ER and Golgi, suggesting that phosphorylation at these two sites is an important regulator of intracellular trafficking of the NR1 subunit through the biosynthetic pathway (Scott et al., 2003). Recent studies indicate that NR1 is phosphorylated by two different PKC isoforms in cerebellar granule cells (Sanchez-Perez and Felipo, 2005), with S890 being preferentially phosphorylated by PKCg and S896 by PKCa. Therefore, S890 and S896 phosphorylation are differentially regulated and this may play a unique role in receptor regulation depending on the spatio-temporal distribution and activation of particular isoforms of PKC.
Although NR2 subunits are also necessary components of NMDA receptor complexes, unlike NR1, each NR2 subunit confers distinct channel properties that differentially affect synaptic NMDA receptor function. For example, NMDA receptors containing the NR2A subunit display fast kinetics with ∼100 ms deactivation time constant (Cull-Candy and Leszkiewicz, 2004). Expression of NR2A gradually increases to a steady-state level as neurons mature (Cull-Candy et al., 2001). Thus, as the newly synthesized NR2A is incorporated into synapses during development, there is a decrease in decay time of NMDA receptor-mediated currents. Most studies suggest that NR2A is confined to synaptic sites (Li et al., 2002; Stocca and Vicini, 1998; Tovar and Westbrook, 1999), and the C-terminal domain of NR2A is essential for the synaptic localization of NR2A-containing NMDA receptors (Steigerwald et al., 2000). However, recent studies in cultured hippocampal neurons report that NR2A can also be targeted to extra-synaptic regions (Thomas et al., 2006).
PKC can potentiate NR2A-containing receptor currents via the phosphorylation of NR2A on S1291 and S1312 (Grant et al., 2001; Jones and Leonard, 2005). Another PKC target in NR2A, S1416, is phosphorylated by PKC in vitro (Gardoni et al., 2001). Phosphorylation of S1416 decreases the binding affinity of aCaMKII for NR2A, providing a molecular mechanism for a direct cross talk between aCaMKII and PKC signaling pathways. In addition to PKC and CaMKII, Cdk5 also phosphorylates NR2A (Li et al., 2001), which enhances NMDA receptor activity, and inhibition of this phosphorylation protects CA1 pyramidal neurons from ischemic insults (Wang et al., 2003). By measuring NMDA receptor currents from NR1/NR2A expressing HEK-293 cells, S900 and S929 were identified as putative phosphorylation sites based on alanine-scanning mutagenesis analysis, but the relevant kinase remains to be identified (Krupp et al., 2002). De-phosphorylation of S900 and S929 by protein phosphatase IIb (calcineurin) modulates desensitization of NR1/NR2A-containing NMDA receptors (Krupp et al., 2002).
NR2B is found in most brain regions early in neuronal development (Cull-Candy et al., 2001). Although the expression of NR2B declines somewhat as animals reach maturity, it remains substantial in cortex and hippocampus even in adult. Compared with NR2A, NR2B-containing NMDA receptors exhibit slow kinetics with ∼250 ms deactivation time constant (Cull-Candy and Leszkiewicz, 2004). The decreased NR2B/NR2A ratio is thought to account for the developmental switch in decay time of NMDA receptor-mediated currents (Cull-Candy et al., 2001). NR2B is located at both synaptic and extrasynaptic compartments early in development, and as neurons mature NR2B become enriched at extrasynaptic sites (Li et al., 2002; Tovar and Westbrook, 1999). It has been demonstrated that NR2B-containing NMDA receptors undergo more robust endocytosis than NR2A-containing receptors and preferentially traffic through recycling endosomes (Lavezzari et al., 2004; Roche et al., 2001; Scott et al., 2004). In addition, NR2B-containing NMDA receptors have higher surface mobility than NR2A-containing receptors (Groc et al., 2006).
As with NR2A, NR2B-containing receptors are regulated by PKC. Sequence alignment of NR2A and NR2B shows that S1303 and S1323 of NR2B are analogous to the PKC substrates, S1291 and S1312, on NR2A. Phosphorylation of synthetic peptides indicates that these two sites on NR2B are PKC substrates in vitro (Liao et al., 2001). Studies in oocytes show that phosphorylation of S1303 and S1323 is required for PKC potentiation of NR1/NR2B receptor currents (Liao et al., 2001). Intriguingly, earlier studies demonstrated that S1303 of NR2B is also phosphorylated by CaMKII in vitro and in hippocampal neurons (Omkumar et al., 1996). Phosphorylation of S1303 by CaMKII inhibits receptor-kinase interactions and promotes slow dissociation of preformed CaMKII-NR2B complexes (Strack et al., 2000). However, recent studies in striatum are at odds with these findings, showing that reduced phosphorylation of S1303 is correlated with the dissociation of CaMKII-NR2B complex during cocaine treatment (Liu et al., 2006). Nevertheless, it appears that CaMKII phosphorylation of S1303 regulates NMDA receptors in a different way from PKC phosphorylation of the same site. Therefore, although there is no doubt that NR2B S1303 can be phosphorylated, the physiologically relevant kinase could be PKC or CaMKII or both.
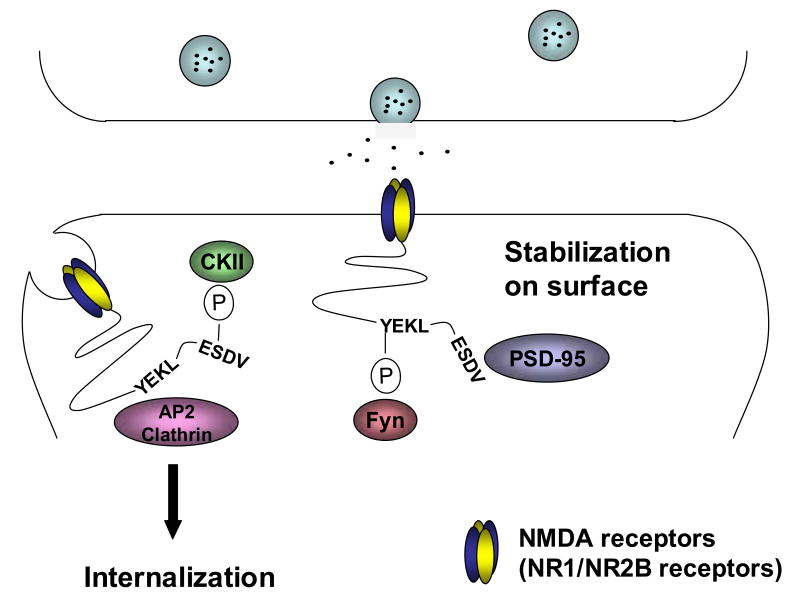
NR2B is also phosphorylated by CKII on S1480 within the PDZ domain binding site at the extreme C-terminus, and phosphorylation of S1480 disrupts the interaction between NR2B and PSD-95 (Chung et al., 2004) (Figure 2). Phosphorylation within the PDZ ligand on receptors/channels that bind to PDZ proteins is a common regulatory mechanism, which disrupts protein-protein interactions. For example, PKA phosphorylation of the potassium channel Kir 2.3 within the PDZ binding domain disrupts binding to the PSD-95 family of proteins (Cohen et al., 1996). Although phosphorylation of the PDZ binding domain of a variety of channels/receptors can disrupt binding to PDZ proteins, the relevant kinases differ. CKII phosphorylation on S1480 of NR2B is the first example of CKII regulating PSD-95 binding to a receptor, and S1480 phosphorylation ultimately regulates NMDA receptor surface expression (Figure 2).
Figure 2.
Differential regulation of NR2B-containing NMDA receptor trafficking by phosphorylation. Phosphorylation of NR2B on S1480 within the PDZ ligand by CKII disrupts the interaction between surface NMDA receptors and the PSD-95 family of proteins, causing internalization of surface-expressed NMDA receptors. However, phosphorylation of NR2B on Y1472 by Fyn kinase disrupts the interaction between NMDA receptors and the AP2-clathrin endocytic complex, leading to stabilization of the receptor on the cell surface. YEKL is a consensus AP-2 adaptor binding site, whereas ESDV is a consensus PDZ ligand.
NR2C-containing NMDA receptors possess unique channel properties, including low conductance openings exhibiting specific kinetics and low sensitivity to magnesium. The majority of NR2C is expressed in cerebellar granule cells, consistent with a unique role in cerebellum. In addition to its dominant expression in cerebellar granule cells, several recent studies have suggested that NR2C may also play an important role in other areas of the brain. For instance, three recent studies indicate that NR2C-containing NMDA receptors are expressed on the processes of oligodendrocytes, which are responsible for myelination (Karadottir et al., 2005; Micu et al., 2006; Salter and Fern, 2005). These oligodendrocyte NMDA receptors are activated during ischemia to mediate calcium accumulation in myelin; however, the physiological roles of these receptors during ischemia are not known. In addition, one group has recently reported that NR2C-containing receptors are expressed in spiny stellate cells in layer 4 rodent somatosensory cortex (Binshtok et al., 2006). These NR2C-containing receptors are expressed at intracortical synapses whereas thalamocortical synapses contain NR2A-containing NMDA receptors. Therefore, distinct trafficking mechanisms likely exist to selectively target the different NMDA receptor subtypes to the different postsynaptic locations in these neurons.
Until recently, there were no studies on NR2C phosphorylation. However, new studies demonstrate that NR2C, like other NMDA receptor subunits, is specifically phosphorylated by a variety of kinases. NR2C is phosphorylated at S1244 by PKA and PKC (Chen et al., 2006). Although this residue is located adjacent to the PDZ binding motif, phosphorylation of S1244 does not influence the PDZ interaction nor does it affect surface-expression of NR2C. However, a phosphomimetic mutation at S1244 accelerates the kinetics of NMDA-evoked currents, suggesting that phosphorylation of NR2C on S1244 maybe important in regulating the channel properties of NMDA receptor in the cerebellum. NR2C is also phosphorylated by protein kinase B (PKB) at S1096 (Chen et al., 2005). Although this serine is conserved in other NR2 subunits, the PKB recognition motif is not, suggesting that NR2C may be uniquely phosphorylated by PKB. The analogous serine in NR2B is the well-characterized CaMKII phosphorylation site (S1303) (Figure 1). Phosphorylation of S1096 on NR2C regulates receptor binding to 14-3-3e (Chen et al., 2005). The 14-3-3 family of proteins have been shown to mediate ER export of a variety of proteins (O'Kelly et al., 2002; Yuan et al., 2003). Interestingly, mutation of S1096 to alanine reduces the surface expression of NR2C-containing NMDA receptors in HEK-293 cells, suggesting that PKB phosphorylation of NR2C modulates receptor trafficking by regulating the interaction between NR2C and 14-3-3e.
3. Tyrosine phosphorylation of NMDA receptors
In addition to serine/threonine phosphorylation, NMDA receptor function is also regulated by protein tyrosine kinases (PTKs). For example, NMDA receptor currents are potentiated by increasing PTK activity and reduced by decreasing PTK activity (Wang and Salter, 1994; Wang et al., 1996). PTKs, especially Src and Fyn, are important modulators of NMDA receptors. Early studies characterizing the molecular components of the PSD revealed that several proteins were highly phosphorylated on tyrosine residues (Ellis et al., 1988). Subsequent analyses revealed that NR2B is the predominant tyrosine-phosphorylated protein in the PSD (Moon et al., 1994). NR2A and NR2D are also tyrosine phosphorylated; however, NR1 does not appear to be phosphorylated on tyrosine residues (Dunah et al., 1998; Lau and Huganir, 1995). Three tyrosine phosphorylation sites on NR2A (Y1292, Y1325 and Y1387) have been identified as targets for Src-mediated phosphorylation (Yang and Leonard, 2001; Zheng et al., 1998). Tyrosine phosphorylation of NR2A potentiates NMDA receptor currents (Kohr and Seeburg, 1996; Zheng et al., 1998). In addition, Y842 of NR2A is also phosphorylated, and de-phosphorylation of this residue may regulate the interaction of NMDA receptor with the AP-2 adaptor, a protein complex that is involved in clathrin-coated endocytic vesicle formation (Vissel et al., 2001). NR2B also contains three tyrosine phosphorylation sites (Y1252, Y1336 and Y1472), which are phosphorylated by Fyn, with Y1472 as the major phosphorylation site (Nakazawa et al., 2001; Takasu et al., 2002). Y1472 is within a tyrosine-based internalization motif (YEKL), which binds directly to the medium chain of the AP-2 adaptor (Lavezzari et al., 2003; Roche et al., 2001). Phosphorylation of NR2B Y1472 disrupts its binding to AP-2, thereby resulting in inhibition of NR2B-mediated endocytosis (Figure 2). Interestingly, tyrosine phosphorylation of NR2B is controlled by STEP (striatal enriched tyrosine phosphatase), a family of brain-specific protein tyrosine phosphatases found at the PSD of glutamatergic synapses (Salter and Kalia, 2004). Recent studies suggested that STEP activation promotes de-phosphorylation of NR2B on Y1472, leading to increased endocytosis of NMDA receptors (Snyder et al., 2005). Y1472 is only a few amino acids away from the CKII phosphorylation site on NR2B, which also regulates surface expression of NMDA receptors (Lavezzari et al., 2003; Prybylowski et al., 2005). These phosphorylation sites appear to have opposing roles, as phosphorylation of Y1472 stabilizes NMDA receptors on the plasma membrane, whereas phosphorylation of S1480 decreases surface expression (Figure 2). Therefore, surface expression and trafficking of NR2B-containing NMDA receptors are specifically regulated by both serine/threonine and tyrosine phosphorylation.
4. Conclusion
Protein phosphorylation is an important mechanism modulating the function of NMDA receptors. Although there has been considerable progress in studying NMDA receptor regulation by phosphorylation, many aspects of NMDA receptor phosphorylation remain to be explored. For example, phosphorylation of NR3A and NR3B has not been reported. However, based on the studies in other NMDA receptor subunits and given the structural similarity of these subunits with other NMDA family members, it is expected that NR3A and NR3B are also phosphoproteins. Although much is known about the phosphorylation of NMDA receptors, regulation of de-phosphorylation by protein phosphatases remains relatively unexplored. De-phosphorylation events also modulate the dynamic activity of synapses and provide a bi-directional control of synaptic activity. Thus, characterizing the opposing actions of protein kinases and protein phosphatases will allow us to gain a more complete understanding of the phosphorylation regulation that underlies neurotransmission and synaptic plasticity in the brain. In addition, many phosphorylation sites in NR1 and NR2 subunits have only been studied in vitro or in heterologous cells. Therefore, identifying and characterizing the phosphorylation sites in neurons will provide important insights into the subunit-specific signals that contribute to the regulation of NMDA receptor function. The major challenge in NMDA receptor phosphorylation studies is to reveal the functional significance of each phosphorylation event and understand how multiple phosphorylation events are coordinated in response to stimuli that control synaptic activity.
Acknowledgments
We thank John T.R. Isaac for valuable comments on the manuscript. This work was supported by the NINDS Intramural Research Program, National Institutes of Health and a NINDS Career Transition Award (to B. C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Fleidervish IA, Sprengel R, Gutnick MJ. NMDA receptors in layer 4 spiny stellate cells of the mouse barrel cortex contain the NR2C subunit. The Journal of neuroscience. 2006;26:708–715. doi: 10.1523/JNEUROSCI.4409-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415:793–798. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- Chen BS, Braud S, Badger JD, 2nd, Isaac JT, Roche KW. Regulation of NR1/NR2C N-methyl-D-aspartate (NMDA) receptors by phosphorylation. The Journal of biological chemistry. 2006;281:16583–16590. doi: 10.1074/jbc.M513029200. [DOI] [PubMed] [Google Scholar]
- Chen BS, Isaac JT, Roche KW. PKB Phosphorylation of the NMDA Receptor Subunit NR2C and Binding to 14-3-3ε. Society for Neuroscience 35th Annual Meeting Abstract 844.9 2005 [Google Scholar]
- Chen L, Huang LY. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Huang YH, Lau LF, Huganir RL. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. The Journal of neuroscience. 2004;24:10248–10259. doi: 10.1523/JNEUROSCI.0546-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciabarra AM, Sullivan JM, Gahn LG, Pecht G, Heinemann S, Sevarino KA. Cloning and characterization of chi-1: a developmentally regulated member of a novel class of the ionotropic glutamate receptor family. The Journal of neuroscience. 1995;15:6498–6508. doi: 10.1523/JNEUROSCI.15-10-06498.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NA, Brenman JE, Snyder SH, Bredt DS. Binding of the inward rectifier K+ channel Kir 2.3 to PSD-95 is regulated by protein kinase A phosphorylation. Neuron. 1996;17:759–767. doi: 10.1016/s0896-6273(00)80207-x. [DOI] [PubMed] [Google Scholar]
- Crump FT, Dillman KS, Craig AM. cAMP-dependent protein kinase mediates activity-regulated synaptic targeting of NMDA receptors. The Journal of neuroscience. 2001;21:5079–5088. doi: 10.1523/JNEUROSCI.21-14-05079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Current opinion in neurobiology. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Science's STKE 2004. 2004 doi: 10.1126/stke.2552004re16. re16. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacological reviews. 1999;51:7–61. [PubMed] [Google Scholar]
- Dunah AW, Yasuda RP, Wolfe BB. Developmental regulation of tyrosine phosphorylation of the NR2D NMDA glutamate receptor subunit in rat central nervous system. Journal of neurochemistry. 1998;71:1926–1934. doi: 10.1046/j.1471-4159.1998.71051926.x. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Fung ET, O'Brien RJ, Huganir RL. Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. The Journal of neuroscience. 1998;18:720–730. doi: 10.1523/JNEUROSCI.18-02-00720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD, Zhang S, Bernhadt JP, Huganir RL. Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell. 1996;84:745–755. doi: 10.1016/s0092-8674(00)81052-1. [DOI] [PubMed] [Google Scholar]
- Ellis PD, Bissoon N, Gurd JW. Synaptic protein tyrosine kinase: partial characterization and identification of endogenous substrates. Journal of neurochemistry. 1988;51:611–620. doi: 10.1111/j.1471-4159.1988.tb01082.x. [DOI] [PubMed] [Google Scholar]
- Erreger K, Chen PE, Wyllie DJ, Traynelis SF. Glutamate receptor gating. Critical reviews in neurobiology. 2004;16:187–224. doi: 10.1615/critrevneurobiol.v16.i3.10. [DOI] [PubMed] [Google Scholar]
- Fong DK, Rao A, Crump FT, Craig AM. Rapid synaptic remodeling by protein kinase C: reciprocal translocation of NMDA receptors and calcium/calmodulin-dependent kinase II. The Journal of neuroscience. 2002;22:2153–2164. doi: 10.1523/JNEUROSCI.22-06-02153.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H, Gouaux E. Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. The EMBO journal. 2003;22:2873–2885. doi: 10.1093/emboj/cdg303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438:185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Bellone C, Cattabeni F, Di Luca M. Protein kinase C activation modulates alpha-calmodulin kinase II binding to NR2A subunit of N-methyl-D-aspartate receptor complex. The Journal of biological chemistry. 2001;276:7609–7613. doi: 10.1074/jbc.M009922200. [DOI] [PubMed] [Google Scholar]
- Grant ER, Guttmann RP, Seifert KM, Lynch DR. A region of the rat N-methyl-D-aspartate receptor 2A subunit that is sufficient for potentiation by phorbol esters. Neuroscience letters. 2001;310:9–12. doi: 10.1016/s0304-3940(01)02085-7. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18769–18774. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annual review of neuroscience. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Jones ML, Leonard JP. PKC site mutations reveal differential modulation by insulin of NMDA receptors containing NR2A or NR2B subunits. Journal of neurochemistry. 2005;92:1431–1438. doi: 10.1111/j.1471-4159.2004.02985.x. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Kohr G, Seeburg PH. Subtype-specific regulation of recombinant NMDA receptor-channels by protein tyrosine kinases of the src family. The Journal of physiology. 1996;492( Pt 2):445–452. doi: 10.1113/jphysiol.1996.sp021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. Calcineurin acts via the C-terminus of NR2A to modulate desensitization of NMDA receptors. Neuropharmacology. 2002;42:593–602. doi: 10.1016/s0028-3908(02)00031-x. [DOI] [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MV, Zukin RS. Protein kinase C modulates NMDA receptor trafficking and gating. Nature neuroscience. 2001;4:382–390. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- Lau LF, Huganir RL. Differential tyrosine phosphorylation of N-methyl-D-aspartate receptor subunits. The Journal of biological chemistry. 1995;270:20036–20041. doi: 10.1074/jbc.270.34.20036. [DOI] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. The Journal of neuroscience. 2004;24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Lee R, Roche KW. Differential binding of the AP-2 adaptor complex and PSD-95 to the C-terminus of the NMDA receptor subunit NR2B regulates surface expression. Neuropharmacology. 2003;45:729–737. doi: 10.1016/s0028-3908(03)00308-3. [DOI] [PubMed] [Google Scholar]
- Lee HK. Synaptic plasticity and phosphorylation. Pharmacology & therapeutics. 2006;112:810–832. doi: 10.1016/j.pharmthera.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Chen N, Luo T, Otsu Y, Murphy TH, Raymond LA. Differential regulation of synaptic and extra-synaptic NMDA receptors. Nature neuroscience. 2002;5:833–834. doi: 10.1038/nn912. [DOI] [PubMed] [Google Scholar]
- Li BS, Sun MK, Zhang L, Takahashi S, Ma W, Vinade L, Kulkarni AB, Brady RO, Pant HC. Regulation of NMDA receptors by cyclin-dependent kinase-5. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12742–12747. doi: 10.1073/pnas.211428098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao GY, Wagner DA, Hsu MH, Leonard JP. Evidence for direct protein kinase-C mediated modulation of N-methyl-D-aspartate receptor current. Molecular pharmacology. 2001;59:960–964. doi: 10.1124/mol.59.5.960. [DOI] [PubMed] [Google Scholar]
- Lin JW, Wyszynski M, Madhavan R, Sealock R, Kim JU, Sheng M. Yotiao, a novel protein of neuromuscular junction and brain that interacts with specific splice variants of NMDA receptor subunit NR1. The Journal of neuroscience. 1998;18:2017–2027. doi: 10.1523/JNEUROSCI.18-06-02017.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Chu XP, Mao LM, Wang M, Lan HX, Li MH, Zhang GC, Parelkar NK, Fibuch EE, Haines M, Neve KA, Liu F, Xiong ZG, Wang JQ. Modulation of D2R-NR2B interactions in response to cocaine. Neuron. 2006;52:897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Lu WY, Xiong ZG, Lei S, Orser BA, Dudek E, Browning MD, MacDonald JF. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nature neuroscience. 1999;2:331–338. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- Mammen AL, Kamboj S, Huganir RL. Protein phosphorylation of ligand-gated ion channels. Methods in enzymology. 1999;294:353–370. doi: 10.1016/s0076-6879(99)94022-3. [DOI] [PubMed] [Google Scholar]
- Markram H, Segal M. Activation of protein kinase C suppresses responses to NMDA in rat CA1 hippocampal neurones. The Journal of physiology. 1992;457:491–501. doi: 10.1113/jphysiol.1992.sp019389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, Zamponi GW, Wang W, Stys PK. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–992. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Moon IS, Apperson ML, Kennedy MB. The major tyrosine-phosphorylated protein in the postsynaptic density fraction is N-methyl-D-aspartate receptor subunit 2B. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:3954–3958. doi: 10.1073/pnas.91.9.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Otsuka T, Horton AC, Scott DB, Ehlers MD. Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron. 2003;40:581–594. doi: 10.1016/s0896-6273(03)00676-7. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. The Journal of biological chemistry. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- O'Kelly I, Butler MH, Zilberberg N, Goldstein SA. Forward transport. 14-3-3 binding overcomes retention in endoplasmic reticulum by dibasic signals. Cell. 2002;111:577–588. doi: 10.1016/s0092-8674(02)01040-1. [DOI] [PubMed] [Google Scholar]
- Okabe S, Miwa A, Okado H. Alternative splicing of the C-terminal domain regulates cell surface expression of the NMDA receptor NR1 subunit. The Journal of neuroscience. 1999;19:7781–7792. doi: 10.1523/JNEUROSCI.19-18-07781.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omkumar RV, Kiely MJ, Rosenstein AJ, Min KT, Kennedy MB. Identification of a phosphorylation site for calcium/calmodulindependent protein kinase II in the NR2B subunit of the N-methyl-D-aspartate receptor. The Journal of biological chemistry. 1996;271:31670–31678. doi: 10.1074/jbc.271.49.31670. [DOI] [PubMed] [Google Scholar]
- Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Tong G, Jahr CE. Beta-adrenergic regulation of synaptic NMDA receptors by cAMP-dependent protein kinase. Neuron. 1996;16:415–421. doi: 10.1016/s0896-6273(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nature neuroscience. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Roche KW, Tingley WG, Huganir RL. Glutamate receptor phosphorylation and synaptic plasticity. Current opinion in neurobiology. 1994;4:383–388. doi: 10.1016/0959-4388(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Prybylowski K, Wang JF, Vicini S. Exon 5 and spermine regulate deactivation of NMDA receptor subtypes. J Neurophysiol. 2000;83:1300–1306. doi: 10.1152/jn.2000.83.3.1300. [DOI] [PubMed] [Google Scholar]
- Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nature reviews. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- Sanchez-Perez AM, Felipo V. Serines 890 and 896 of the NMDA receptor subunit NR1 are differentially phosphorylated by protein kinase C isoforms. Neurochemistry international. 2005;47:84–91. doi: 10.1016/j.neuint.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Ehlers MD. Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology. 2003;45:755–767. doi: 10.1016/s0028-3908(03)00250-8. [DOI] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. The Journal of neuroscience. 2001;21:3063–3072. doi: 10.1523/JNEUROSCI.21-09-03063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DB, Michailidis I, Mu Y, Logothetis D, Ehlers MD. Endocytosis and degradative sorting of NMDA receptors by conserved membrane-proximal signals. The Journal of neuroscience. 2004;24:7096–7109. doi: 10.1523/JNEUROSCI.0780-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E, Baur R, Malherbe P. Protein kinase C transiently activated heteromeric N-methyl-D-aspartate receptor channels independent of the phosphorylatable C-terminal splice domain and of consensus phosphorylation sites. The Journal of biological chemistry. 1994;269:8204–8208. [PubMed] [Google Scholar]
- Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, Zukin RS. Protein kinase A regulates calcium permeability of NMDA receptors. Nature neuroscience. 2006;9:501–510. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nature neuroscience. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Standley S, Roche KW, McCallum J, Sans N, Wenthold RJ. PDZ domain suppression of an ER retention signal in NMDA receptor NR1 splice variants. Neuron. 2000;28:887–898. doi: 10.1016/s0896-6273(00)00161-6. [DOI] [PubMed] [Google Scholar]
- Steigerwald F, Schulz TW, Schenker LT, Kennedy MB, Seeburg PH, Kohr G. C-Terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. The Journal of neuroscience. 2000;20:4573–4581. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. The Journal of physiology. 1998;507( Pt 1):13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S, Colbran RJ. Autophosphorylation-dependent targeting of calcium/ calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl- D-aspartate receptor. The Journal of biological chemistry. 1998;273:20689–20692. doi: 10.1074/jbc.273.33.20689. [DOI] [PubMed] [Google Scholar]
- Strack S, McNeill RB, Colbran RJ. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. The Journal of biological chemistry. 2000;275:23798–23806. doi: 10.1074/jbc.M001471200. [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Akbarian S, Chi CL, Leclerc CL, Awobuluyi M, Deitcher DL, Wu MK, Yuan JP, Jones EG, Lipton SA. Developmental and regional expression pattern of a novel NMDA receptor-like subunit (NMDAR-L) in the rodent brain. The Journal of neuroscience. 1995;15:6509–6520. doi: 10.1523/JNEUROSCI.15-10-06509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasu MA, Dalva MB, Zigmond RE, Greenberg ME. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science. 2002;295:491–495. doi: 10.1126/science.1065983. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. Journal of neurophysiology. 2006;95:1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. The Journal of biological chemistry. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- Tingley WG, Roche KW, Thompson AK, Huganir RL. Regulation of NMDA receptor phosphorylation by alternative splicing of the C-terminal domain. Nature. 1993;364:70–73. doi: 10.1038/364070a0. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. The Journal of neuroscience. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Burgess MF, Zheng F, Lyuboslavsky P, Powers JL. Control of voltage-independent zinc inhibition of NMDA receptors by the NR1 subunit. The Journal of neuroscience. 1998;18:6163–6175. doi: 10.1523/JNEUROSCI.18-16-06163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Hartley M, Heinemann SF. Science. Vol. 268. New York NY: 1995. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines; pp. 873–876. [DOI] [PubMed] [Google Scholar]
- Vissel B, Krupp JJ, Heinemann SF, Westbrook GL. A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nature neuroscience. 2001;4:587–596. doi: 10.1038/88404. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu S, Fu Y, Wang JH, Lu Y. Cdk5 activation induces hippocampal CA1 cell death by directly phosphorylating NMDA receptors. Nature neuroscience. 2003;6:1039–1047. doi: 10.1038/nn1119. [DOI] [PubMed] [Google Scholar]
- Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- Wang YT, Yu XM, Salter MW. Ca(2+)-independent reduction of N-methyl-D-aspartate channel activity by protein tyrosine phosphatase. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:1721–1725. doi: 10.1073/pnas.93.4.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. The Neuroscientist. 2005;11:37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- Yang M, Leonard JP. Identification of mouse NMDA receptor subunit NR2A C-terminal tyrosine sites phosphorylated by coexpression with v-Src. Journal of neurochemistry. 2001;77:580–588. doi: 10.1046/j.1471-4159.2001.00255.x. [DOI] [PubMed] [Google Scholar]
- Yuan H, Michelsen K, Schwappach B. 14-3-3 dimers probe the assembly status of multimeric membrane proteins. Current biology. 2003;13:638–646. doi: 10.1016/s0960-9822(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Zheng F, Gingrich MB, Traynelis SF, Conn PJ. Tyrosine kinase potentiates NMDA receptor currents by reducing tonic zinc inhibition. Nature neuroscience. 1998;1:185–191. doi: 10.1038/634. [DOI] [PubMed] [Google Scholar]
- Zheng X, Zhang L, Wang AP, Bennett MV, Zukin RS. Protein kinase C potentiation of N-methyl-D-aspartate receptor activity is not mediated by phosphorylation of N-methyl-D-aspartate receptor subunits. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:15262–15267. doi: 10.1073/pnas.96.26.15262. [DOI] [PMC free article] [PubMed] [Google Scholar]