Abstract
The results of controlled non-human animal and human laboratory studies are mixed regarding whether women and men respond differently to stimulant drugs. In order to assess potential gender differences in the effects of d-amphetamine, we conducted a retrospective-analysis of six studies conducted in our laboratory that used identical procedures and measures. Thirteen women and fourteen men learned to discriminate 15 mg oral d-amphetamine. After acquiring the discrimination (i.e., =80% correct responding on 4 consecutive sessions), the effects of a range of doses of d-amphetamine (0, 2.5, 5, 10, and 15 mg) alone and in combination with other drugs, were assessed. Only data from sessions in which d-amphetamine was administered alone were included in this analysis. d-Amphetamine functioned as a discriminative-stimulus and dose-dependently increased drug-appropriate responding. Women and men did not differ in their ability to discriminate d-amphetamine. Women and men differed on participant-ratings of high (women < men), nausea (women > men) and sluggish (women < men), women also experienced greater increases in diastolic pressure than men. Because the results of this study may have been confounded by the training procedures, future research should use other behavioral arrangements (e.g. drug self-administration) to determine if women and men respond differently to the effects of d-amphetamine.
INTRODUCTION
Results from epidemiological studies suggest that women may be more vulnerable to stimulant (e.g., cocaine or methamphetamine) dependence than men (e.g. Brecht et al., 2004; Westermeyer and Boedicker, 2000). For example, in one sample of 350 methamphetamine abusers (56% male), women advanced to regular use, defined as using 3 or more days per week, more quickly than men (1.6 years and 2.6 years, respectively) and entered treatment after fewer years of drug use (8.8 years and 9.7 years, respectively) (Brecht et al., 2004). In another sample of 642 patients (57% male) admitted to a treatment program, women reported using cocaine for fewer years prior to entry than men (2.8 vs. 4 years, respectively). In addition, women in that sample were diagnosed with cocaine abuse or dependence at higher rates than men (13% vs. 7%, respectively) (Westermeyer and Boedicker, 2000). Finally, data collected from 1,047 prescription stimulant abusers (60% male) between 1995 and 1998 revealed that women were 2.6 times more likely to develop prescription stimulant dependence than men (Wu and Schlenger, 2003). The aforementioned studies suggest that women may be more susceptible to stimulant abuse and dependence than men. The biological, behavioral or sociocultural variables that mediate these differences are not known. Perhaps women are more likely to seek treatment than men. Alternatively, the behavioral effects of stimulants may be more robust in women than men.
Results from pre-clinic al laboratory studies are mixed regarding differences between females and males in terms of behavioral responses to stimulants. Several studies have demonstrated that female rats acquire drug self-administration more rapidly and escalate drug intake more quickly than male rats, regardless of estrous cycle phase (e.g. Festa et al., 2004; Lynch and Carroll, 1999; Roth and Carroll, 2004). In one study, for example, female rats acquired cocaine self-administration (i.e. ≥ 100 infusions in a six hour period over five consecutive sessions) significantly faster than male rats (7.6 vs. 16.7 days, respectively) (Lynch and Carroll, 1999). A larger percentage of female rats also met self-administration acquisition criteria than male rats (70% vs. 30%, respectively) (Lynch and Carroll, 1999). The results of other studies, by contrast, suggest that there are few, if any, differences between male and female rats (Anderson and van Haaren, 1999; Caine et al., 2004; Craft and Stratmann, 1996; Stratmann and Craft, 1997; Haney et al., 1995). Two studies, for instance, failed to find sex differences in the discriminative-stimulus effects of cocaine in rats (Anderson and van Haaren, 1999; Craft and Stratmann, 1996).
Consistent with the pre-clinical laboratory data, results from human laboratory studies are also mixed. Results from several studies suggest that men may be more sensitive to the behavioral and physiological effects of stimulants such as d-amphetamine and cocaine (e.g. Lukas et al., 1996; Sofuoglu et al., 1999, 2000; White et al., 2002). In one study, for example, men achieved significantly higher plasma cocaine levels and reported a greater number of euphoric events compared to women following administration of 0.9 mg/kg intranasal cocaine (Lukas et al., 1996). The results from other human laboratory studies, by contrast, suggest that women may have a more robust response to stimulants than men (e.g. Evans et al., 1999; Kosten et al., 1996; McCance-Katz et al., 2005; Singha et al., 2000). For example, following administration of 80 mg/kg oral cocaine, women reported higher ratings of “bad drug effect” and “nervous” compared to men (Singha et al., 2000).
Given the mixed results described above, the present retrospective analysis was conducted to examine possible gender differences in responses to d-amphetamine. Data from six studies that used identical drug-discrimination procedures were combined. Each of the studies was designed as a pretreatment study in which d-amphetamine was given in combination with another drug. d-Amphetamine alone was common to all studies and data collected from sessions in which drug combinations were administered were not included in this retrospective analysis. The discriminative-stimulus effects of d-amphetamine (0, 2.5, 5, 10 and 15 mg) were assessed in 13 women and 14 men with a history of non-therapeutic stimulant use. A drug (15 mg d-amphetamine) versus not drug (placebo) discrimination procedure was utilized in each study. To the best of our knowledge, this is the first analysis of gender differences in the discriminative-stimulus effects of d-amphetamine in humans. In order to more fully assess potential gender differences in response to d-amphetamine, data from self-report questionnaires, a performance task, and cardiovascular measures were also analyzed.
METHODS
Six studies were included in this retrospective analysis (Lile et al. 2005a, 2005b; Rush et al., 2003, 2004; Stoops et al., unpublished data, 2006a). Each study was designed as a pretreatment study in which d-amphetamine was given in combination with risperidone (Rush et al., 2003), alprazolam (Rush et al., 2004), aripiprazole (Lile et al., 2005a; Stoops et al., 2006a), oxazepam (Lile et al., 2005b), or fluphenazine (Stoops et al., unpublished data). In all studies, medications were administered acutely and a minimum of 24 hours separated all drug administrations. Data collected from sessions in which drug combinations were administered are not included in this analysis. All studies employed identical experimental procedures and were conducted in the same laboratory. The Institutional Review Board at the University of Kentucky approved all protocols and informed consent documents.
Participants
Thirteen adult women and 14 adult men that were recruited via newspaper ads, flyers, and word-of-mouth participated and were included in this analysis. If a participant had enrolled in more than one of the studies, only data from the first study in which they participated was used. Participants were paid $40/session to participate in addition to performance-based payment as outlined below. Participants provided written informed consent, and completed questionnaires assessing drug use, medical and psychiatric histories, prior to participating. All participants were in good physical and psychiatric health as determined by the medical and psychiatric questionnaires, and clinical laboratory chemistries. Participants were without contraindications to d-amphetamine. Drug urine screens conducted during screening were negative for benzodiazepines, barbiturates, coca ine, and opioids (Abuscreen ONTRAK, Roche Diagnostic Systems, Nutley, NJ). In the female participants, urine pregnancy tests before study participation and prior to each session had to be negative.
General Procedures
Participants enrolled as outpatients at the Laboratory of Human Behavioral Pharmacology at the University of Kentucky, Monday through Friday, for up to 37 experimental sessions. Sessions typically began between 8:00 am and 10:00 am. The time of day that sessions were conducted was held constant for each volunteer. Participants were informed that during their participation they would receive a stimulant like d-amphetamine (Dexedrine®, Glaxosmithkline, Research Triangle Park, NC) or a placebo. For each study, participants also received another drug alone or in combination with d-amphetamine during some sessions. As noted above, data from these sessions were not included in the analyses. Participants were told that the purpose of the study was to determine if they could detect the presence of a drug and how the drug affects mood and behavior. Other than receiving this general information, participants were blind to the type of drug administered and were given no instructions regarding what they were “supposed” to do or what outcomes might be expected.
Prior to initiating drug testing, participants completed two “practice” sessions. These practice sessions were used to familiarize participants with the drug-discrimination task, participant-rated drug-effect questionnaires, performance measure, and daily laboratory routine. No medications were administered on these days.
Throughout the study, participants were requested to refrain from using all psychoactive drugs, and from using caffeine and solid food for 4 hours prior to a scheduled experimental session, and alcohol for 12 hours prior to a scheduled experimental session. Individual participants arrived at the laboratory at approximately the same time each day and provided a urine sample before drug administration, which was screened for the presence of amphetamine, barbiturates, benzodiazepines, cocaine, opioids, and THC. These urine samples were occasionally positive for amphetamine, which was likely attributable to the experimental administration of d-amphetamine. If a urine sample was positive for anything other than THC, d-amphetamine, or other experimentally administered drugs, the experimental session was cancelled and rescheduled. Participants also provided an expired air specimen, which was assayed for the presence of alcohol using a hand-held Breathalyzer (Intoximeters, Inc., St. Louis, MO). All expired air specimens had to be negative in order for a session to begin.
On experimental session days, participants completed the participant-rated drug-effect questionnaires and performance task approximately 30 minutes prior to drug administration and 1, 2, 3, 4 and 5 hours after drug administration. Drug administration took place approximately one hour after a participant arrived at the laboratory. A minimum of 24 hours separated all drug administrations. When not completing the drug-discrimination task, participant-rated questionnaires, and performance tasks, participants were allowed to engage in recreational activities (e.g., watch television, play cards or read) or socialize with each other. Participants were instructed not to discuss their drug effects with each other during the experimental session or outside the laboratory.
Drug Discrimination Procedures
Each experiment consisted of three phases, which were completed in fixed order: 1) sampling phase, 2) test-of-acquisition phase, and 3) test phase. Because these procedures have been described previously, they are only briefly described here (for details, see Rush et al., 2003).
Sampling phase
All participants completed two sampling sessions to acquaint them with the drug effects. During each sampling session, participants ingested capsules that contained a total of 15-mg d-amphetamine. d-Amphetamine was identified by letter code (e.g., DRUG A), but the participants were not explicitly informed of the capsules’ contents. Instructions were given to participants prior to each medication administration (for complete instructions, see Rush et al., 2003).
Test-of-acquisition phase
Following the sampling phase, a test-of-acquisition phase was conducted to determine if participants could discriminate 15-mg d-amphetamine. On test-of-acquisition days, participants ingested capsules under double-blind conditions, but were not told whether the capsules contained 15-mg d-amphetamine (e.g., DRUG A) or placebo (e.g., NOT DRUG A). Participants were not explicitly instructed that they would be attempting to acquire a drug versus placebo discrimination. After capsule administration, participants completed the drug-discrimination task, participant-rated drug-effect questionnaires and performance measures periodically for five hours. After completing these tasks at the five-hour observation, participants opened a sealed envelope that informed the participant and the research assistant of the identity of the drug administered (i.e., DRUG A or NOT DRUG A). The criterion for having acquired the discrimination was =80% correct responding on four consecutive sessions on the drug-discrimination task described below. Participants unable to meet this criterion within 12 sessions were dismissed from the study. The order of drug administration was determined randomly except that each participant received each training condition, 15-mg d-amphetamine and placebo, at least twice.
Test phase
Following the test-of-acquisition phase, participants entered a test phase to determine whether other doses of d-amphetamine shared discriminative-stimulus effects with the training dose. During the test phase, participants also received d-amphetamine in combination with another medication. Data collected during these sessions were omitted from the analyses. As noted above, participants were instructed that there would be days on which they would not be given any feedback concerning the accuracy of their drug-discrimination performance, and that on these days they would be credited with the largest amount of money earned on either response option (i.e., the DRUG A option and NOT DRUG A option). Thus, these days were identical to acquisition days except that participants did not receive any feedback concerning their drug-discrimination performance. Participants were not told the purpose of these “test” days, nor did they know when they were scheduled until after they opened the sealed envelope.
Doses of d-amphetamine administered in the test phase were 0, 2.5, 5, 10, and 15 mg. The order of drug administration during this phase of the experiment was random except that an active drug dose was never administered on more than three consecutive sessions. Test of acquisition sessions were also included during the test phase to ensure accurate discrimination was maintained.
Drug-Discrimination Measure
A point-distribution drug-discrimination task was completed 1, 2, 3, 4, and 5 hours after oral drug administration on an Apple Macintosh computer (Apple Computer, Inc., Cupertino, CA). In this procedure, the participant distributed 100 points between two options (i.e., DRUG A or NOT DRUG A). Points accumulated on the correct option were exchangeable for money at a rate of $0.08/point. Thus, participants were able to earn a maximum of $40.00/session on this task. The dependent measure in this task was percent d-amphetamine-appropriate responding.
Participant-Rated, Performance and Cardiovascular Measures
Participant-rated drug-effect questionnaires and the performance measure were administered on an Apple Macintosh computer. The questionnaires and performance task were completed approximately 30 minutes before drug administration, and 1, 2, 3, 4, and 5 hours after drug administration.
Addiction Research Center Inventory
The short form of the ARCI consisted of 49 true/false questions and contained five major subscales: the morphine-benzedrine group (MBG; a measure of euphoria), the pentobarbital, chlorpromazine, alcohol group (PCAG; a measure of sedation), the lysergic acid diethylamide (LSD; a measure of dysphoria) and the benzedrine group and amphetamine scales (BG and A, respectively; stimulant-sensitive scales) (Jasinski, 1977; Martin et al., 1971).
Adjective-Rating Scale
The Adjective-Rating Scale consists of 32 items and contained two subscales: Sedative and Stimulant (Oliveto et al., 1992). Participants rated each item using the computer mouse to point to and select among one of five response options: Not at All, A Little Bit, Moderately, Quite a Bit, and Extremely (scored numerically from 0 to 4, respectively).
Drug-Effect Questionnaire
The Drug-Effect Questionnaire consisted of 20 items (for the individual items, see Rush et al., 2003). Participants rated each item with a 5-point scale identical to the one described above.
Stimulant Sensitive Adjectives
The Stimulant-Sensitive Adjective-Rating Scale consisted of 21 items (Di Marino et al., 1998). Participants rated each adjective using a 5-point scale identical to the one described above. Responses to individual items are summed to create a composite score, with a maximum total score of 84.
Digit-Symbol-Substitution Test (DSST)
A computerized version of the DSST, which has been described previously, was used in this experiment (McLeod et al., 1982). Briefly, participants used a numeric keypad to enter a geometric pattern associated with one of nine digits displayed on a video screen. Participants had 90 seconds to enter as many geometric patterns as possible. The dependent measure was the percent of geometric patterns the participant entered correctly (i.e., percent correct).
Heart Rate and Blood Pressure
Heart rate and blood pressure were recorded using an automated blood pressure monitor (DINAMAP, Johnson and Johnson, Alexandria, TX). Heart rate and blood pressure were recorded immediately before participants completed the behavioral tasks.
Drug Administration
All drug conditions were administered in a double-blind fashion. d-Amphetamine doses were administered in three capsules that were prepared by over-encapsulating commercially available generic drug in size 0 capsules. Each d-amphetamine capsule contained either 2.5 or 5 mg. Cornstarch or lactose was used to fill the remainder of all the capsules. Placebo capsules contained only cornstarch or lactose. Administering the appropriate number of active and placebo capsules varied d-amphetamine dose. Capsules were taken orally with approximately 150 ml of water.
Drug administration procedures were designed to ensure that participants swallowed the capsules and did not open them in their mouths and taste the contents. To accomplish this, the research assistant: a) watched the participant to ensure that he/she swallowed the capsules and did not remove them from his/her mouth, b) conducted a brief oral examination to ensure that the participant was not hiding the capsules under his/her tongue, and c) spoke with the participant to determine if he/she had anything in his/her mouth.
Data Analysis
Demographic data for the women and men were compared using unpaired t-tests. Statistical analyses of group data were conducted to examine drug effects on the drug-discrimination task, self-reported drug-effect questionnaires, performance, and cardiovascular measures. Data were analyzed statistically as raw scores (Stat View 5.0.1, SAS Institute Inc., Cary, NC). For all statistical analyses, effects were considered significant for p ≤ 0.05. Drug-discrimination data were analyzed statistically as the total percent of points allocated to the drug option across the five-hour session (i.e., percent drug-appropriate responding). For the 15 mg d-amphetamine alone and placebo conditions, data were averaged across the four sessions of the acquisition phase in which the participant met the discrimination criterion as well as all exposures to these conditions in the test phase. Participant-rated drug-effect, performance, and cardiovascular data were analyzed statistically as peak effect (i.e. the maximum effect of d-amphetamine between hours one and five). Data were analyzed by a two-factor mixed model ANOVA with d-amphetamine Dose (0, 2.5, 5, 10, and 15 mg) as a within participant factor and Gender (male or female) as a between participant factor. If the interaction of Dose and Gender obtained statistical significance in the ANOVA, Fisher’s Protected Least Significant Difference (PLSD) post-hoc tests were conducted to determine whether men and women differed significantly at each dose.
RESULTS
Demographics
The men were significantly heavier (Menmean = 88 kg [range: 71-131 kg], Womenmean = 63 kg [range: 52-70 kg]; p < 0.001) and older (Menmean = 23 years [range: 20-28 years], Womenmean = 21 years [range: 18-24 years]) than the women (Table 1). The women and men did not differ significantly in years of education, self-reported alcohol consumption, daily caffeine or cigarette use, lifetime use of amphetamines, cocaine, marijuana, or opioids, nor did they differ on scores on the Michigan Alcohol Screening Test (MAST) or Drug Abuse Screening Test (DAST) (Table 1).
Table 1.
Demographic Characteristics: Means, standard deviations and t-values from unpaired t-tests. Bold values indicate a significant effect (p ≤ .05)
| DEMOGRAPHICS: | Women Mean (SD): | Men Mean (SD): | t-value (df = 25): |
|---|---|---|---|
| Age (years) | 21.2 (1.6) | 23.3 (2.0) | 2.9 |
| Weight (kg) | 63.5 (5.4) | 88.1 (17.5) | 4.9 |
| Education (years) | 15.1 (1.3) | 15.2 (1.9) | 0.2 |
| MAST (score) | 2.6 (2.1) | 2.4 (2.0) | 0.2 |
| DAST (score) | 1.5 (1.6) | 1.6 (1.3) | 0.2 |
| Substance Use Licit: | |||
| Caffeine Use (mg/day) | 108.1 (112.4) | 158.4 (169.6) | 0.9 |
| Cigarettes (per day) | 4.2 (6.8) | 2.2 (4.3) | 0.9 |
| Alcohol (drinks/ week) | 7.3 (8.3) | 8.1 (5.4) | 0.3 |
|
Substance Use Illicit:
(# times used in lifetime) |
|||
| Amphetamine | 0.2 (0.6) | 0.4 (1.6) | 0.4 |
| Cocaine | 2.5 (8.3) | 0.3 (0.8) | 1.0 |
| Marijuana | 203.1 (330.6) | 76.8 (144.8) | 1.3 |
| Opiates | 0.4 (0.5) | 0.4 (0.6) | 0.1 |
Drug-Discrimination Data
Women and men did not differ significantly in terms of the number of trials needed to acquire the d-amphetamine discrimination (mean of 6.7 and 5.6 trials, respectively). d-Amphetamine dose-dependently increased drug-appropriate responding (F4, 25 = 25.1, p < 0.001) (Figure 1). The dose-related effects of d-amphetamine did not vary significantly as a function of Gender.
Figure 1. Percent drug-appropriate responding.
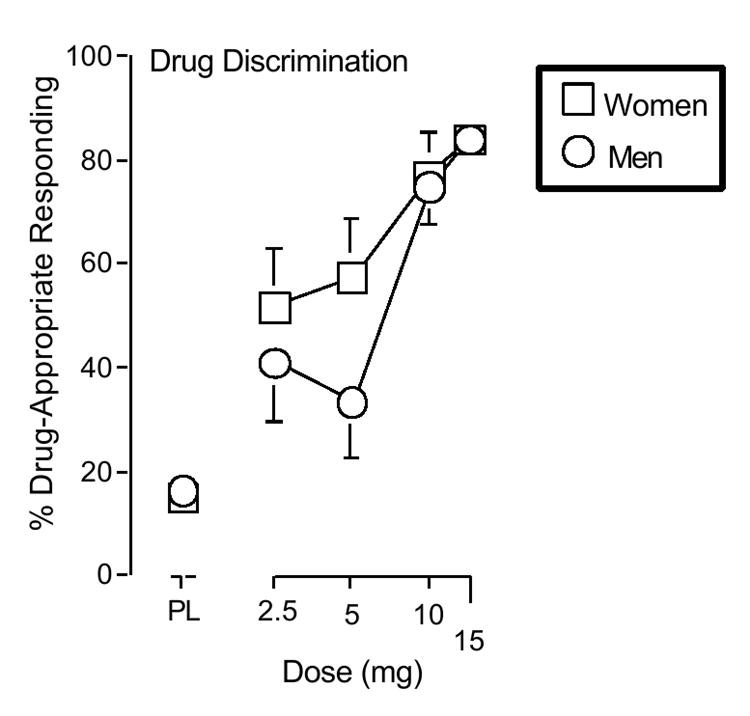
X-axes: d-Amphetamine dose. Data points above PL represent placebo. Data points show means of 13 women (squares) and 14 men (circles). Unidirectional brackets are included for clarity and indicate 1 S.E.M.
Participant-Rated Drug-Effect Questionnaires
Addiction Research Center Inventory (ARCI)
d-Amphetamine dose-dependently increased scores on the A (F4, 25 = 10.7, p < 0.001), BG(F4, 25 = 5.8, p < 0.001), LSD (F4, 25 = 5.5, p < 0.001), and MBG (F4, 25 = 7.0, p < 0.001) scales of the ARCI. The dose-related effects of d-amphetamine did not vary significantly as a function of Gender (data not shown).
Adjective-Rating Scale
d-Amphetamine dose-dependently increased scores on the Stimulant Scale (F4, 25 = 8.2, p < 0.001) and decreased scores on the Sedative Scale (F4, 25 = 3.8, p < 0.01) of the Adjective-Rating Scale. Scores on the Stimulant and Sedative scales of the Adjective-Rating Scale did not vary significantly as a function of Gender (data not shown).
Drug Effect Questionnaire
The interaction of Dose and Gender attained statistical significance on three measures of the drug effect questionnaire: high (F4, 100 = 2.9, p ≤ 0.05), nausea (F4, 100 = 2.4, p ≤ 0.05) and sluggish (F4, 100 = 2.5, p ≤ 0.05). The significant interaction of Dose and Gender on ratings of high was attributable to the men reporting greater increases than the women following the 10 and 15 mg dose conditions (Figure 2). The significant interaction of Dose and Gender on ratings of nausea was attributable to the women reporting greater increases than the men following the 2.5 and 5 mg dose conditions (Figure 2). The significant interaction of Dose and Gender on ratings of Sluggish was due to the men reporting greater increases than the women following the 2.5 and 15 mg dose conditions, while the women reported greater increases than men following the 5 mg dose condition (data not shown).
Figure 2.
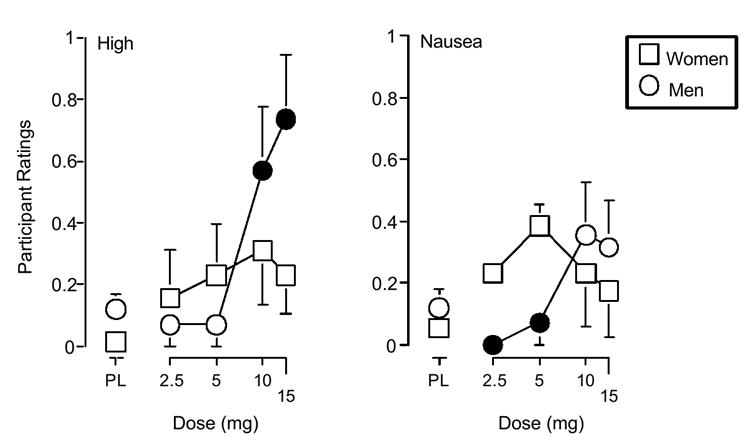
Effects of d-amphetamine dose and sex on ratings of high and nausea from the drug effect questionnaire. Filled symbols indicate those values that are significantly different from the corresponding value (p ≤ 0.05, Fisher’s [PLSD] post hoc test). All other details are as in figure 1.
d-Amphetamine dose-dependently increased scores on 16 other items on the Drug Effect Questionnaire: active/ alert/ energetic (F4, 25 = 16.7, p < 0.001), any effect (F4, 25 = 15.2, p < 0.001), bad effects (F4, 25 = 3.1, p < 0.05), euphoric (F4, 25 = 3.5, p < 0.05), good effects (F4, 25 = 16.5, p < 0.001), irregular heart beat (F4, 25 = 9.0, p < 0.001), like drug (F4, 25 = 13.5, p < 0.001), nervous (F4, 25 = 5.6, p < 0.001), performance improved (F4, 25 = 6.0, p < 0.001), willing to pay for (F4, 25 = 3.9, p < 0.05), restless (F4, 25 = 5.0, p < 0.05), rush (F4, 25 = 10.1, p < 0.001), shaky/jittery (F4, 25 = 5.9, p < 0.001), stimulated (F4, 25 = 11.9, p < 0.001), willing to take again (F4, 25 = 12.2, p < 0.001), and talkative/friendly (F4, 25 = 15.2, p < 0.001). Scores on these items did not vary significantly as a function of Gender. Figure 3 shows the effects of d-amphetamine on measures of any effect, good effects, like drug, stimulated, and take again from the Drug-Effect Questionnaire.
Figure 3.
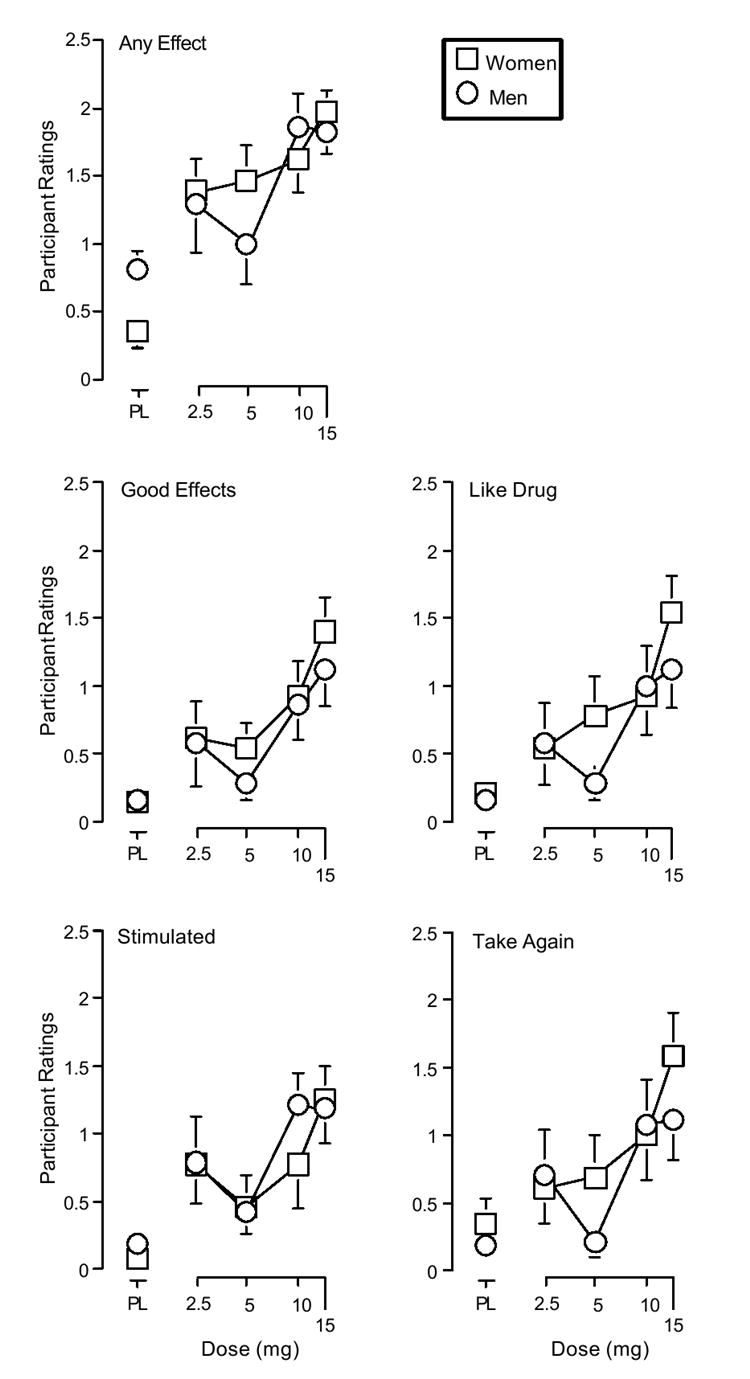
Effects of d-amphetamine and placebo on ratings of any effect, good effects, like drug, stimulated, and take again from the drug effect questionnaire. All other details are as in figure 1.
Stimulant-Sensitive Adjective-Rating Scale
d-Amphetamine dose-dependently increased scores on the Stimulant-Sensitive Adjective-Rating Scale (F4, 25 = 12.1, p < 0.001). Scores on this scale did not vary significantly as a function of Gender (data not shown).
Digit-Symbol Substitution Test
All doses of d-amphetamine improved performance on the DSST relative to placebo as measured by percent trials correct (F4, 25 = 10.36, p < 0.001). The effects of d-amphetamine did not vary significantly as a function of Gender (data not shown).
Heart Rate and Blood Pressure
The interaction of Dose and Gender attained statistical significance for diastolic pressure (F4 , 100 = 2.7, p ≤ 0.001). This interaction was attributable to the women having greater increases than the men under the 10 and 15 mg dose conditions (data not shown). d-Amphetamine dose-dependently increased systolic pressure and heart rate, but these effects did not vary significantly as a function of Gender (data not shown).
DISCUSSION
The results of this study suggest that women and men are not differentially sensitive to the discriminative-stimulus effects of d-amphetamine. There was not a significant difference between women and men in terms of the number of trials needed to meet the discrimination criterion during the test-of-acquisition phase. In addition, the d-amphetamine dose-response function was nearly identical in women and men. To the best of our knowledge, this is the first study to compare the discriminative-stimulus effects of d-amphetamine in humans as a function of gender.
Consistent with our results, previous studies conducted in rats trained to discriminate cocaine did not reveal sex differences (Anderson and van Haaren, 1999; Craft and Stratmann, 1996). The present data also correspond well with results from another retrospective analysis of data collected from drug discrimination studies conducted in our laboratory in which 13 (6 women, 7 men) participants were trained to discriminate 0.375 mg triazolam (Vansickel et al., 2006). Despite epidemiological data suggesting that women are more likely to abuse and become dependent on benzodiazepines, no gender differences were found. The drug-discrimination procedure used in this analysis provides participants with similar recent behavioral and pharmacological histories, which may be important determinants of subsequent drug effects (e.g. Singha et al., 1999). As described above, women and men were required to meet a predetermined discrimination criterion before advancing to the test phase. As a result, potential gender differences might have been obscured. In fact, drug discrimination may be an effective paradigm for minimizing individual differences.
Men had greater increases on ratings of high than women, which is consistent with results from previous human laboratory studies (e.g. Sofuoglu et al., 1999; 2000). Women, by contrast, reported greater feelings of nausea than men following low doses of d-amphetamine (i.e. 2.5 and 5mg). One potential explanation for this finding may be that women and men were at different areas of the d-amphetamine dose-response curve perhaps because weight-adjusted dosing was not used in the current study. However, the present findings are consistent with results of previous human laboratory studies in which women reported greater increases in ratings of bad drug effect (e.g. Evans et al., 1999; Singha et al., 2000). Overall, the present findings, along with those from previous studies, suggest men may be more sensitive to the positive effects of stimulants while women are more susceptible to the negative effects (Lukas et al., 1996; Singha et al., 2000).
The results of the current experiment are consistent with results from previous human laboratory studies that revealed only a few differences between women and men on a number of items from subject-rated drug-effect questionnaires following administration of stimulants (e.g. Evans et al., 1999; Kosten et al., 1996; Lukas et al., 1996; Sofuoglu et al., 1999, 2000; White et al., 2002). In the present experiment, differences between women and men were noted on only three of 28 subject-rated items.
d-Amphetamine dose-dependently increased blood pressure and heart rate. No gender differences were observed on systolic pressure or heart rate. There was, however, a significant interaction of Dose and Gender on diastolic pressure. Women had greater increases in diastolic pressure than men following the administration of higher d-amphetamine doses (i.e. 10 and 15 mg). The reason for this difference is unknown. The most parsimonious explanation is that women received functionally higher doses of d-amphetamine. As noted above, weight-adjusted dosing was not used and the women weighed significantly less than the men. However, despite receiving functionally higher doses, most of the behavioral and physiological effects of d-amphetamine were strikingly similar in women and men. Thus, under the current experimental conditions, the women may have actually been less sensitive to the behavioral and cardiovascular effects (i.e., heart rate and systolic pressure) of d-amphetamine than men. Future research on the effects of stimulants in women and men should use weight-adjusted dosing.
Changes in menstrual cycle and hormone levels were not evaluated in the current study, which is a limitation. There is evidence to support the role of these variables in altering the behavioral effects of stimulants in females (e.g. Carroll et al., 2004; Lynch et al., 2002; Lynch, 2006; Terner and de Wit, 2006). Women have been shown to have an enhanced response (i.e. increased ratings of stimulant-like effects) to d-amphetamine (15 mg) during the follicular phase compared to the luteal phase of their menstrual cycle and this effect is positively correlated with salivary estradiol levels (White et al., 2002). Further, administration of estradiol (0.25 mg) has been shown to enhance the discriminative-stimulus effects of a low dose of d-amphetamine in women (i.e. 3.125 mg/70 kg), as well as some of the participant-rated effects (Lile et al., unpublished data). Conversely, progesterone (200 mg) pretreatment has been shown to attenuate some of the effects of 0.4 mg/kg smoked cocaine (Sofuoglu et al., 2002). Future studies should examine the role of menstrual cycle and circulating hormone levels in altering sensitivity to the discriminative-stimulus effects of stimulants.
In conclusion, women and men do not appear to differ in response to the discriminative-stimulus effects of d-amphetamine, although, a few differences were found on participant-rated drug effect questionnaires as well as diastolic pressure. As reviewed above, female rats acquire cocaine self-administration significantly faster than male rats, and a larger percentage of female rats meet acquisition criterion than male rats (Lynch and Carroll, 1999). Futures studies should examine the reinforcing effects of d-amphetamine in women and men. A modified progressive-ratio procedure, for example, has recently been shown to be sensitive to individual differences in humans (Stoops et al., 2006b). In that study, high sensation-seekers were found to reach higher break points than low sensation-seekers on a modified progressive-ratio procedure when responding for d-amphetamine capsules (Stoops et al., 2006b). Whether women and men might differentially self-administer d-amphetamine under this behavioral arrangement is unknown.
Acknowledgments
The authors with to thank Frances P. Wagner, R.N., for her expert nursing assistance and Michelle Gray, Derek Roe, John Blackburn, Jamie Haga, Allison Weber, Abigail Osborne, Matt Weaver, Brad Cooper and Joe Kingery for their expert technical assistance. The National Institute on Drug Abuse Grants DA (RO1) 10325, 017711, and 13567 (C.R.R.) as well as DA (T32) 007304 (Thomas Garrity) supported this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson KG, van Haaren F. Cocaine discrimination and time-course effects in male and female Wistar rats. Eur J Pharmacol. 1999;382:69–74. doi: 10.1016/s0014-2999(99)00597-x. [DOI] [PubMed] [Google Scholar]
- Brecht M, O’Brien A, von Mayrhauser C, Anglin MD. Methamphetamine use behaviors and gender differences. Addict Behav. 2004;29:89–106. doi: 10.1016/s0306-4603(03)00082-0. [DOI] [PubMed] [Google Scholar]
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormaone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology. 2004;29:929–942. doi: 10.1038/sj.npp.1300387. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends pharmacol sci. 2004;25(5):273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Craft RM, Stratmann JA. Discriminative stimulus effects of cocaine in female versus male rats. Drug Alcohol Depend. 1996;42:27–37. doi: 10.1016/0376-8716(96)01259-8. [DOI] [PubMed] [Google Scholar]
- Di Marino ME, Haberny KA, Felch LJ, Walsh SL, Preston KL, Bigelow GE. Development of a subjective rating scale sensitive to acute cocaine administration. National Institute on Drug Abuse Research Monograph Series. Problems of Drug Dependence; Proceedings of the 59th Annual Scientific Meeting; 1998; 1997. p. 139. [Google Scholar]
- Evans SM, Haney M, Fischman M, Foltin R. Limited sex differences in response to “binge” smoked cocaine use in humans. Neuropsychopharmacology. 1999;21:445–454. doi: 10.1016/S0893-133X(98)00120-1. [DOI] [PubMed] [Google Scholar]
- Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin S, Foltz R, Jenab S, Quinones-Jinab V. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology. 2004;46:672–687. doi: 10.1016/j.neuropharm.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Jasinski D. Assessment of the abuse potentiality of morphine-like drugs (methods used in man) In: Morton WR, editor. Drug Addiction I. New York: Springer-Verlag; 1977. pp. 197–258. [Google Scholar]
- Kosten TR, Kosten TA, McDougle CJ, Hameedi FA, McCance EF, Rosen MI, Oliveto AH, Price LH. Gender differences in response to intranasal cocaine administration to humans. Biol Psychiatry. 1996;39(2):147–148. doi: 10.1016/0006-3223(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Vansickel AR, Glaser PEA, Hays LR, Rush CR. Aripiprazole attenuates the discriminative-stimulus and participant-rated effects of d-amphetamine in humans. Neuropsychopharmacology. 2005a;30:2103–2114. doi: 10.1038/sj.npp.1300803. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Wagner FP, Glaser PEA, Rush CR. Oxazepam does not modulate the behavioral effects of d-amphetamine in humans. Pharmacol Biochem Behav. 2005b;82(2):270–279. doi: 10.1016/j.pbb.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kendall S, Babalonis S, Martin C, Kelly T. Estradiol enhances the discriminative-stimulus effects and some of the participant-rated effects of d-amphetamine in healthy pre-menopausal women. Neuropsychopharmacology. 2007 doi: 10.1016/j.pbb.2007.04.022. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, Kragie L, Mendelson JH. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology. 1996;125:346–354. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;44:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol. 2006;14(1):34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Hart CL, Boyarsky B, Kosten T, Jatlow P. Gender effects following repeated administration of cocaine and alcohol in humans. Subst Use Misuse. 2005;40(4):511–528. doi: 10.1081/ja-200030693. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling JE. An automated version of the digit symbol substitution test (DSST) Behav Res Meth Inst. 1982;14:463–466. [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, Fenwick JW. Caffeine drug-discrimination in humans: acquisition, specificity and correlation with self-reports. J Pharmacol Exp Ther. 1992;261:885–894. [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long-or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR, Glaser PEA. Alprazolam attenuates the discriminative-stimulus effects of d-amphetamine in humans. J Clin Psychopharmacol. 2004;24:410–420. doi: 10.1097/01.jcp.0000130553.55630.ad. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR, Glaser PEA, Hays LS. Risperidone attenuates the discriminative-stimulus effects of d-amphetamine in humans. J Pharmacol Exp Ther. 2003;306:195–204. doi: 10.1124/jpet.102.048439. [DOI] [PubMed] [Google Scholar]
- Singha AK, McCance-Katz EF, Heck S, Kosten TR. Individual differences in humans responding under a cocaine discrimination procedure: discriminators versus nondiscriminators. Exp Clin Psychopharmacol. 1999;7(4):391–398. doi: 10.1037//1064-1297.7.4.391. [DOI] [PubMed] [Google Scholar]
- Singha AK, McCance-Katz EF, Petrakis I, Kosten TR, Oliveto AH. Sex differences in self-reported and physiological response to oral cocaine and placebo in humans. Am J Drug Alcohol Abuse. 2000;26(4):643–657. doi: 10.1081/ada-100101900. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7(3):274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Brown S, Dudish-Poulsen S, Hatsukami DK. Individual differences in the subjective response to smoked cocaine in humans. Am J Drug Alcohol Abuse. 2000;26(4):591–602. doi: 10.1081/ada-100101897. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72:431–435. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PEA, Rush CR. A low dose of aripiprazole attenuates some of the abuse-related effects of d-amphetamine. Drug Alcohol Depend. 2006a;84(2):206–209. doi: 10.1016/j.drugalcdep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Robbins CG, Martin CA, Rush CR, Kelly TH. The reinforcing, subject-rated, performance, and cardiovascular effects of d-amphetamine: influence of sensation-seeking status. Addictive Behavior. 2006 doi: 10.1016/j.addbeh.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann JA, Craft RM. Intracranial self-stimulation in female and male rats: no sex differences using a rate-independent procedure. Drug Alcohol Depend. 1997;46:31–40. doi: 10.1016/s0376-8716(97)00040-9. [DOI] [PubMed] [Google Scholar]
- Terner J, de Wit H. Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend. 2006;84:1–13. doi: 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Rush CR, Hays LR. Discriminative-stimulus effects of triazolam in women and men. Am J Drug Alcohol Abuse. 2006;32(3):329–349. doi: 10.1080/00952990500479266. [DOI] [PubMed] [Google Scholar]
- Westermeyer J, Boedicker AE. Course, severity, and treatment of substance abuse among women versus men. American Journal of Drug and Alcohol Abuse. 2000;26(4):523–535. doi: 10.1081/ada-100101893. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJH, de Wit H. Differential subjective effects of d-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Wu L, Schlenger WE. Psychostimulant dependence in a community sample. Subst Use Misuse. 2003;38(2):221–248. doi: 10.1081/JA-120017246. [DOI] [PMC free article] [PubMed] [Google Scholar]


