Abstract
Motivation plays an important role in the control of food intake. This review will focus on recent findings using a neural systems analysis of a behavioral model for learned motivational control of eating. In this model, environmental cues that acquire motivational properties through Pavlovian conditioning can subsequently override satiety and promote eating in sated rats. Evidence will be presented that a brain network formed by the amygdala, lateral hypothalamus, and medial prefrontal cortex mediates this phenomenon of conditioned potentiation of feeding. The animal model may be informative for understanding control of eating in humans including maladaptive influences that contribute to overeating.
Keywords: amygdala, appetite, conditioning, craving, eating, environment, hypothalamus, overeating, obesity, prefrontal cortex, satiety
In Western societies and other developed countries, we are surrounded by images and messages that provide cues for food. How might these cues modulate food intake? Could pervasive cues for food contribute to overeating? Indeed, studies in both laboratory animals and humans show that in addition to signals related to energy balance, eating is also regulated by environmental or external signals that are not related to metabolic control [1], [2], [3], [4], [5]. Notably, environmental cues previously associated with food (learned cues) exert powerful control over food consumption that can override regulatory signals and stimulate eating in sated states [6], [7], [8]. The mechanisms and neural systems through which such cues influence food intake are being elucidated in animal models. Here we will review the evidence from our recent studies of a brain network that mediates regulation of food intake by motivational cues on the basis of associative learning. The forebrain network described in this research will also be discussed in the framework of research findings on food-related cues and functionally activated systems in the human brain.
Potentiation of feeding by associative learning
The behavioral model we use to study feeding is based on a paradigm originally developed by Weingarten in 1983 [6]. In this paradigm, environmental cues modulate feeding through motivational properties acquired in associative learning. The behavioral aspects of the model were described in detail in two recent reviews [9], [10]. Briefly, in a typical experiment food-restricted rats (maintained at 85% ad libitum weight) are trained in a Pavlovian conditioning procedure, in which a conditioned stimulus (CS+), such as a simple auditory tone, is paired with food delivery. In most of our experiments, an additional control stimulus, which is not followed by food delivery (CS−), is also presented during training. During such training rats learn to approach the site of food delivery during the CS+, but not during the CS−. The amount of time spent at the food cup (conditioned response, CR) during the cue that predicts food provides a well-characterized measure of associative learning. After training, sated rats are tested for food consumption when those cues are presented. The conditioned potentiation of eating is evident in such tests; sated rats consume more food in the presence of the CS+ compared to tests with CS− presentations. This learning has an associative basis and is not merely due to non-specific activation by a sensory stimulus, because only the cue paired with food (CS+) but not an unpaired cue (CS−) enhances eating (for review see [10]). Furthermore, cue-induced enhancement of eating is not simply a byproduct of the CRs that bring the rats to the food cup. Potentiation also occurs in tests when food is presented in a receptacle that is different in appearance and location from the food cup used in training [11], [12]. Thus, enhanced eating is a consequence of motivational properties acquired by an otherwise neutral cue through associative learning.
In the above-described procedures rats were trained under severe food restriction (85% of ad libitum body weight) that might have critical implications in terms of mechanisms recruited. Indeed, weight loss initiates a complex cascade of adaptive responses, including a decrease in circulating levels of leptin and insulin, and modification of gut peptide signaling, which in turn result in activation of orexigenic mechanisms in the brain [13], [14]. Most notably, leptin acts directly on neurons within the arcuate nucleus of the hypothalamus (ARH) that stimulate anabolic and orexigenic mechanisms (coexpressing neuropeptide Y and agouti-related protein), as well as on ARH neurons (coexpressing proopiomelanocortin and cocaine- and amphetamine-regulated transcript) related to catabolic and anorexic actions [15]. Thus, a change in leptin levels associated with maintenance of body weight levels at 85%, and subsequent effects on ARH neurons that could lead to synaptic plasticity [16], could critically influence an ongoing learning and memory underlying cue-food associations.
Nevertheless, severe chronic food restriction is not necessary during training to produce subsequent cue-enhanced eating in sated rats. In our recent preparation (described-below), rats trained under moderate food restriction regimen that does not produce body weight show CS-enhanced food consumption. Here, instead of chronic food restriction, rats underwent multiple acute food deprivations during training [12], [17]. Clearly future studies are needed to examine how different metabolic history—such as food deprivation (acute versus chronic) on one end and long-term access to palatable, high calorie foods on the other end of the spectrum—could provide different molecular, and behavioral background for learning about food cues, and associated motivation and consumption.
In the type of study described above potentiated feeding is induced by an explicit CS, such as a discrete auditory or visual cue. In a recent study we examined whether the environment in which food is consumed can also serve as a CS to promote eating [12]. We trained food-deprived rats to consume food pellets in a distinct environment (context). Rats in a control group were exposed to the same context but received food pellets in their home cages. During training rats were food-deprived for 20 hours prior to each training session, and allowed ad libitum access to lab chow for 24–72 hours between sessions. Then we tested sated rats for food pellet consumption in the conditioning context. Rats that were previously fed in the conditioning context when hungry consumed more food pellets in the conditioning context during tests compared to the rats in the control group that were never fed in that context. These results show that contextual CSs, similar to discrete cues, can promote food consumption (also see [18]).
The nature of CS-driven motivation to eat
Recently, we examined the motivational basis for learned potentiation of feeding. We found that CS-enhanced eating is specific to the food consumed during training [12], [17]. Sated rats showed enhanced food consumption in the conditioning context only when presented with the training pellets, but not when presented with an entirely novel food or with other familiar foods [12], [17]. This finding suggests that the basis for CS enhancement of eating is not induction of a general motivation to eat, akin to hunger, but instead appears to be due to induction of a more specific motivational state, akin to appetite or craving. Although food cravings are difficult to define, especially in animal models [19], some notable parallels can be drawn between cue-induced consumption in our model and food cravings. For example, in a recent study of people identified as restrained eaters (chronic dieters), food-related cues elicited a specific appetite/craving, rather than a general desire to eat, and such appetite was correlated with increased intake of the target food [20]. Thus, both are food selective and can be elicited by exposure to cues associated with food [19], [21]. Similar to binge eating associated with cue-elicited cravings [22], [23], animals can also consume a large amount in a very short time in the context associated with food [12], [17]. Finally, there is an apparent overlap in brain areas activated in humans by cues for preferred and/or craved food and the brain systems that mediate cue-induced feeding in the conditioned potentiation paradigm.
Amygdalar subsystems and CS-enhanced feeding
As a first step in identifying neural systems for CS-enhanced feeding, two subsystems within the amygdalar complex, the central nucleus (CEA) and the basolateral region (BLA; including basolateral, basomedial, and lateral nuclei) were examined ([24], [25], [26], for review see [10]). These amygdalar regions have been linked to a range of functions that rely on associative learning to control goal directed behavior [27], [28], [29], [30], [31], [25], [32], [33]. Most notably, both the BLA, and CEA have been implicated in various aspects of appetitive incentive motivation and reward [30], [33], [34], [35], [36]). Projections from BLA and CEA form pathways to the lateral hypothalamus [37] that could provide access to feeding systems [38]. In addition, in other settings, CEA has been shown to influence feeding (e.g., [39], [40], [41].), possibly via direct pathways to the brain stem [42], and indirect input to the paraventricular hypothalamic nucleus [43]. Against that background, selective neurotoxic lesions of the CEA or BLA were employed to test whether one or both of these regions would be critical for CS potentiation of feeding [11].
The results of studies using selective neurotoxic lesions showed that the BLA but not CEA is critical for CS-induced food consumption ([24], [25], [26], for review see [10]). During food consumption tests sated rats with either sham- or CEA-lesions showed similar robust CS-enhanced eating. In contrast, lesions of BLA abolished potentiated feeding. Notably, that impairment was specific to CS-modulated eating. There was no difference in baseline eating among the groups (as seen in a pre-test period prior to CS presentations). Furthermore, BLA-lesioned rats acquired CS-driven food cup CRs during training comparable to rats with sham and CEA lesions. Thus, rats with BLA lesions were impaired in the acquisition or expression of the learned motivational properties of the CS manifest through modulation of food consumption. Notably, this finding is in agreement with previous evidence also showing dissociable roles for BLA and CEA in some other motivational functions in appetitive learning ([44], [45], for review see [10]).
Amygdalo-hypothalamic components of the network
The fact that CS-potentiation of feeding is spared in rats with CEA lesions would appear to constrain the circuitry through which BLA plays a role in this form of learning. Our next study began to define the functional system through which the BLA gains access to feeding behavior. We focused on the BLA connections with the LHA, a region historically linked to initiation of feeding, reward and motivation [46], [38], [35].
To test whether communication between the BLA and LHA is necessary for allowing learned cues to stimulate eating, we examined rats with a preparation that disconnected the two structures. We placed unilateral, neurotoxic lesions of BLA and LHA on opposite sides of the brain (contralateral group). Because BLA outputs are largely ipsilateral, this preparation disconnected the BLA-LHA system in both hemispheres without disturbing other functional circuitries involving each of those structures. These rats were trained in CS potentiation of eating, together with rats in two control groups. One control group of rats received unilateral lesions of the BLA and LHA on the same side of the brain (ipsilateral group) to control for the amount of damage without disconnecting the BLA-LHA system. A second control group of rats received contralaterally placed sham lesions of BLA and LHA.
The disconnection of the BLA-LHA system did not affect auditory Pavlovian discrimination learning. During conditioning, rats in all groups learned to approach the food cup at the same rate and showed similar discrimination (food cup CRs) between the CS+ and CS−. However, the disconnection of BLA-LHA abolished CS-enhanced eating in food consumption tests. Rats in the control groups ate significantly more in the presence of CS+ compared to tests with the CS−, while rats with the lesion that disconnected the BLA and LHA ate the same small amount in both tests. The impairment was specific to consumption under the influence of the learned cues as all rats ate similar amounts in a pre-test period with no stimuli. Thus, these results demonstrate that the BLA and LHA form part of the brain network that allows learned motivational cues to control eating [47].
The impairment in rats with BLA-LHA disconnection was similar to the original finding with bilateral lesions of the BLA in that it did not produce deficits in CS-driven food cup CRs, but only a more selective impairment in the ability of the CS to modulate subsequent feeding based on motivational properties acquired during conditioning. In a subsequent test with those same rats, however, we found that disconnection of BLA-LHA is more selective than BLA damage and does not share a similar profile in other aspects of learning processes. As mentioned earlier, intact BLA function is needed in a number of tasks that depend on CS-acquired value to guide subsequent behavior [10]. Specifically, we examined whether the BLA-LHA system is also important in second-order conditioning—a paradigm that critically depends on the BLA. In second-order conditioning, the CS acquires the properties of a reinforcer such that the formerly neutral cue can support new learning. To test this function in the BLA-LHA disconnection preparation, those rats were again food-deprived and received pairings of a new stimulus (a second order CS) with the original auditory first-order stimulus (CS+). Interestingly, we found that the lesion disconnecting BLA and LHA did not produce any impairment in second-order conditioning. All groups acquired similar food cup CRs to the second order CS. Thus, in contrast to the impairment in CS-enhanced eating, disconnection of the BLA-LHA system did not interfere with that CS′ acquired ability to reinforce new learning [47]. This finding indicates a more selective role for the BLA-LHA system in control of food consumption that is dissociable from other associative functions of BLA. Indeed, research using the second-order conditioning paradigm has shown that BLA’s communication with the nucleus accumbens is critical in mediating that function [48].
Amygdalo-prefrontal-hypothalamic system and CS-enhanced eating
The disconnection of BLA-LHA system that selectively abolished CS-potentiated feeding implicates the LHA as a final common pathway for the influence of learned potentiation on food consumption. However, in addition to direct projections from BLA to the LHA, other indirect pathways in the forebrain could also be involved. The BLA participates in a forebrain network with many indirect pathways for gaining access to the hypothalamus, including projections from BLA to medial prefrontal cortex (mPFC) and the nucleus accumbens (ACB), along with projections to the central nucleus of the amygdala (CEA), each of which, in turn, innervates the LHA [49]. To study these routes we used functional anatomical methods to further delineate critical components of a forebrain-LHA network engaged in CS-potentiated feeding.
We employed a novel approach for functional mapping of activated circuitry in the CS-potentiation paradigm. Retrograde tract-tracing was used in conjunction with methods to identify activated neurons. The effector immediate early genes (IEGs), Arc (also known as Arg3.1, which encodes activity-regulated cytoskeleton-associated protein), and Homer 1a (H1a), were employed as markers of activated neurons [50]. Arc and H1a mRNAs are expressed in the same neurons with temporally offset appearance/disappearance, and as such can be employed to detect neuronal populations activated by two temporally distinct experiences in a single brain [50]. This approach allowed us to view the components of the BLA-LHA circuitry that are selectively activated in food consumption tests with CS presentations that stimulate eating. Thus, we assessed sated rats for food consumption in the presence of a cue that was previously paired with food (CS+), or in the presence of another cue that was never paired with food (CS−), in two consecutive tests temporally arranged for activation of Arc and Homer 1a and examined the selective induction of these IEGs in BLA, CEA, mPFC, and ACB neurons that project to LHA, as identified with the retrograde tracer (FluoroGold).
Here we found that LHA projection neurons, as defined with retrograde tracer, localized in the basomedial and adjacent basolateral nuclei within the BLA and in the mPFC were activated selectively by a cue that stimulates eating in sated rats (Figure 1, pathways indicated in red). In both regions, a significantly larger percentage of the neurons projecting to the LHA show IEG induction in response to food consumption tests with CS+ compared to tests with CS−. Thus, these findings demonstrate that both direct BLA projections and a prefrontal projection system to the LHA are activated during CS-driven eating.
Figure 1.
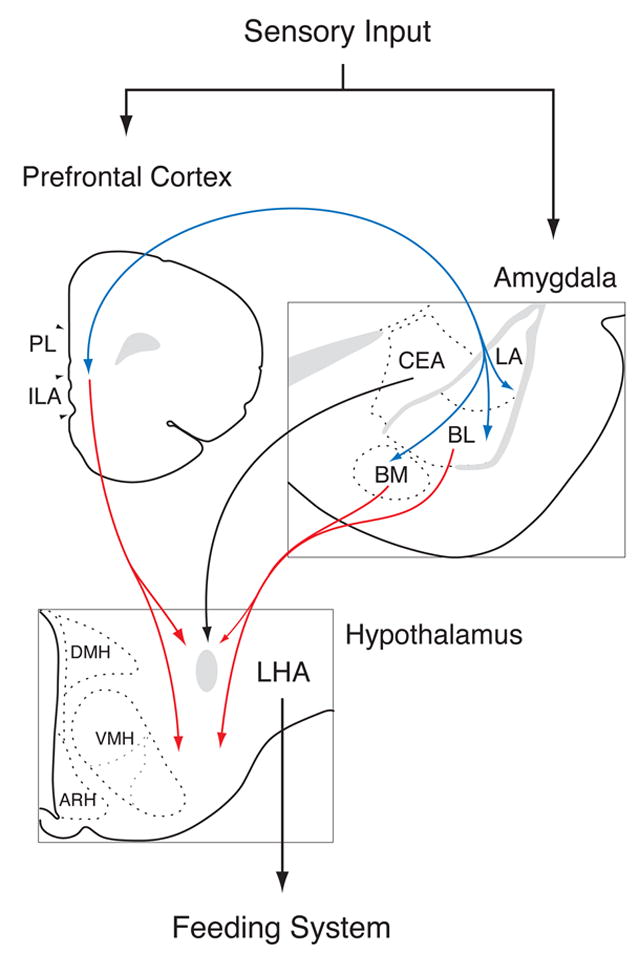
This diagram summarizes recent findings from neural systems analysis of the behavioral model, conditioned potentiation of feeding, that relies on learned cues to override metabolic signals. In that model, a cue previously paired with food when an animal was hungry can initiate eating in sated rats.
The lateral hypothalamus (LHA) is an integrative site for feeding signals, both intrinsic and extrinsic. Pathways in red show projections to the LHA with origins in the forebrain neurons that show activation by a conditioned stimulus (CS+) that promotes feeding. The origins of these pathways in the basolateral amygdala (BLA; includes BL, BM, and LA), and ventral medial prefrontal cortex (mPFC; includes PL, ILA, and mOFC) are critical functionally, as shown by lesions of each of these areas, which abolish CS-enhanced feeding. Our studies have further shown that direct projections from CEA are not critical; as indicated by lack of selective activation of that pathway by the effective CS+, and the fact that lesions of CEA spare CS-enhanced feeding. Blue line shows interconnections between the BLA and mPFC that may also play a role in regulation of feeding by learned cues.
Abbreviations: ARH, arcuate nucleus of the hypothalamus; BLA, basolateral amygdala; BL, basolateral nucleus of the amygdala; BM, basomedial nucleus of the amygdala; DMH, dorsomedial nucleus of the hypothalamus; ILA, infralimbic area; LA, lateral nucleus of the amygdala; LHA, lateral hypothalamic area; PL, prelimbic area; mOFC, medial orbitofrontal area; VMH, ventromedial nucleus of the hypothalamus. Plates modified from the atlas of Swanson [63].
Interestingly, the two other brain regions examined in that study that project to the LHA, the ACB and CEA, did not contain neurons that were selectively activated in the setting of potentiated feeding. That result suggests that anatomical routes to LHA via the ACB and CEA are not critical for learning-dependent modulation of feeding in our paradigm. This interpretation is further corroborated by results from lesion studies. In addition to the earlier described result, namely that bilateral neurotoxic CEA lesions spare potentiated feeding [24], [25], [11], we also found no impairment after disconnection of the BLA and ACB (using a preparation conceptually similar to the BLA-LHA disconnection involving lesions placed contralaterally in BLA and ACB) [10]. At the same time, it is important to note that a variety of evidence has implicated those regions of the forebrain in other aspects of motivational control in feeding [33], [51], [34], [35]. Thus, the ACB and CEA might interact with the BLA-LHA in settings dissociable from cue-driven eating. Within that context, the BLA-LHA system is needed in ACB-dependent, μ-opioid induced consumption of fat [52]. Notably the CEA, including its projection to the LHA, which is not critical for cue-‘enhanced’ consumption, may be needed to modulate feeding in aversive settings [53]. Thus, subsystems within a larger network appear to be recruited by different processes, including a more general motivation to eat, motivation for highly palatable foods, stress-regulated eating, or selective cue-driven consumption. Of course, it will be of great interest to determine whether these different subsystems interact with common or different metabolic regulators within the LHA and other components of the feeding system. Clearly, more work is needed to fully delineate the exact circuitry and mechanisms used to modulate feeding in many different settings.
Medial prefrontal cortex and CS-enhanced eating
Guided by the results of our functional anatomical work that showed selective activation of pathways from the mPFC to LHA in CS-enhanced eating, we subsequently examined whether an intact mPFC is essential for learned cues to promote food consumption [54], [17]. The lesion area in this study encompassed the prelimbic, infralimbic, and medial orbitofrontal areas to closely match the region of the mPFC that was functionally activated in potentiation tests in the earlier functional mapping experiment [55].
Rats with bilateral neurotoxic- or sham- lesions of the mPFC were trained in a conditioned potentiation paradigm in which a distinct context was paired with food consumption when the rats were hungry. The behavioral procedure was similar to one described earlier in this chapter; half of the rats were presented with experimental pellets in a distinct context (paired), while the other half of rats were placed in the context without the food (unpaired). After training, rats were sated, and then tested for food consumption in the conditioning context. Sham-lesioned rats showed conditioned potentiation of eating—rats in the paired group ate significantly more food pellets compared to the amount consumed by rats in the unpaired group during tests. In contrast, rats with mPFC lesions in paired and unpaired groups consumed similar small amounts of food pellets during the tests. Thus, consistent with our finding that mPFC neurons are engaged during potentiated feeding, we found that neurotoxic mPFC lesions produced impairment in food consumption specifically driven by conditioned motivational cues.
These results indicate that mPFC forms part of the forebrain-LHA circuitry for potentiated feeding. Within that network, the mPFC could participate via its direct projections to the LHA as suggested by our functional labeling study, as well as via additional routes. The mPFC, itself, is connected with a number of areas that, in turn, reach the LHA including ACB and mediodorsal thalamus [56], [57] [58]. Importantly, the mPFC also shares reciprocal connections with the BLA [49] that could provide an additional route for the integration of mPFC and BLA’s influence on the LHA. Interestingly projection neurons to the mPFC from a region of BLA are strongly activated by CS+ in the potentiation task (Fig. 2; our unpublished observations). Thus, a network of overlapping direct and indirect pathways between the mPFC, BLA and LHA are likely to represent a forebrain network that mediates cue-driven eating and the learning process by which cues acquire such power.
Figure 2.
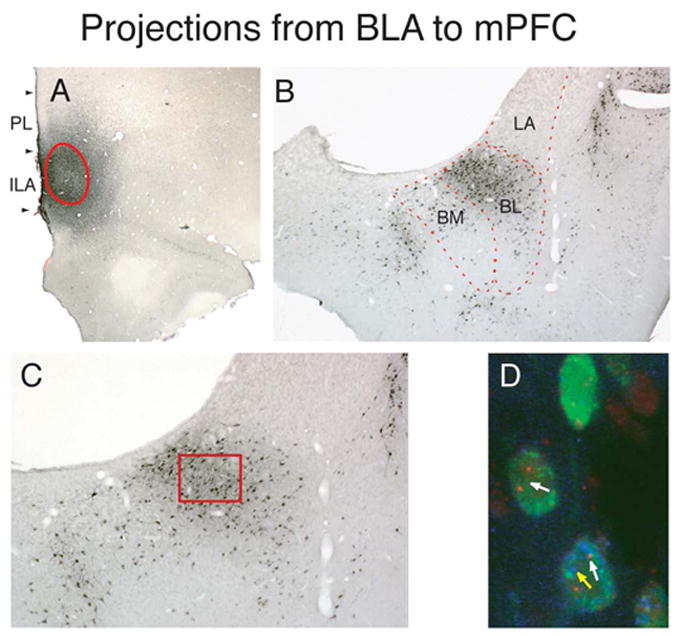
A population of neurons within the basolateral amygdala (BLA; includes BL, BM, and LA) that project to the medial prefrontal cortex (mPFC) is selectively activated by CS+. A, A photomicrograph of the mPFC in the immunohistochemically-processed tissue after retrograde tracer (FluoroGold, FG) injection. Red line encircles the area of the FG deposit within the mPFC. B, A photomicrograph of the amygdala showing retrogradely labeled neurons (black deposits) within the BLA, after FG injection in the mPFC (shown in A). C, A high power photomicrograph of the BLA. D, A high power photomicrograph showing the region of the BLA demarcated by the red box in C, after combined FG detection with double-label fluorescence in situ hybridization (FISH) for Arc and H1a. Animal injected with FG into the mPFC was tested for food consumption in the presence of CS− and CS+ in two consecutive tests temporally arranged for activation H1a and Arc, respectively. Complete analysis of labeled cells is conducted through the z axis. At the focal plane shown in the figure FG-labeled neurons (green) are also labeled with Arc intranuclear foci, INF, (red, white arrow) activated by CS+, H1a INF (blue) activated by CS− (yellow arrow), or both.
Abbreviations: BLA, basolateral amygdala; BL, basolateral nucleus of the amygdala; BM, basomedial nucleus of the amygdala; ILA, infralimbic area; PL, prelimbic area
In that context, all the studies to date, including lesions of the BLA, disconnections of the BLA-LHA systems, and mPFC lesions were made prior to training. Thus, the results of these studies do not provide insights as to whether these structures are critical only during learning, during memory maintenance and/or expression of cue-potentiated feeding. The roles of the BLA, and communication with the LHA, directly, and via mPFC, as well as the roles of the mPFC and its pathways to the LHA, might differ in various stages in the CS potentiation of feeding task.
Possible relevance of the animal model to humans
It is of special interest that the use of functional neuroimaging methods has identified forebrain regions in the human brain in a network that resembles cue-potentiated circuitry in the model described here. Most notably, the amygdala, which is critical for cue-enhanced eating, is activated in sated humans while viewing names of preferred versus neutral foods [59], and while individuals think about the sensory properties of liked or craved food [60]. Indeed activations of the amygdala and a medial region of the orbitofrontal cortex are seen in response to a number of different food-related cues [61], [59], [62], [63], [64], [60]. Viewed within its well-known role in goal-directed behavior [65], the mPFC appears particularly critical for controlling an impulse to eat under the influence of environmental cues.
Commonality in brain circuitry and features of cue-modulated eating, including induction of appetite or craving, suggest that the animal model of learned potentiation of feeding has potential relevance for understanding the control of eating in humans. Indeed, the network of neural circuitry demonstrated in animals may be directly relevant to conditions that can be studied in humans in a similar experimental setting. Notably, a role for cues in food consumption has been described in terms quite similar to potentiated feeding in laboratory animals. Using Pavlovian conditioning procedures, conditioned potentiation of food consumption has been reported in preschool children [8]. Similar to the finding in laboratory animals, conditioned cues had the ability to promote eating in children who had consumed food snacks prior to the potentiation tests. Thus ability of cues and the feeding environment to contribute to food consumption as studied in animals may be relevant to conditions that motivate eating (and perhaps overeating) in people.
Concluding remarks
Evidence reviewed here from studies that have combined behavioral and functional anatomical methods highlight the BLA, mPFC, and LHA as a network critical for the regulation of feeding by learned, motivational cues in an animal model. This emerging knowledge is informative for understanding a basis for eating in humans that can be driven by environmental factors apart from metabolic control. In the current context of concern about weight control one might ask whether the operation of the neural systems and behavioral mechanisms in this model contribute maladaptively to obesity? Whether potentiated feeding, referring to greater food consumption in the presence of learned cues than would otherwise occur, represents a form of overeating that could contribute to body weight gain in the long term is an important, as yet unanswered question. As a possible scenario, however, consider the fact that an increasingly larger proportion of the total dietary intake in the USA is consumed in distinct environments such as fast food chains [66]. Notably, fast food and other chain restaurants are designed to have a relatively uniform, recognizable appearance and provide relatively limited menu choices of distinctive items. Such settings, based on studies of potentiated feeding in laboratory animals, would seem to be ideal for associating specific food-context with hunger, which through learning, could subsequently promote appetite for and consumption of such foods in a similar setting, even in otherwise relatively satiated states. Further work is clearly needed to determine whether the animal model is applicable to human eating in such terms, and whether conditioned enhancement of food consumption is a contributing factor to overeating in a manner that could lead to body weight gain.
Acknowledgments
Supported by National Institute of Mental Health Grants MH67252 (G.D.P.) and MH60179 (M.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rodin J. Current status of the internal-external hypothesis for obesity. American Psychologist. 1981;36:361–372. doi: 10.1037//0003-066x.36.4.361. [DOI] [PubMed] [Google Scholar]
- 2.Booth DA. Mood- and nutrient-conditioned appetites. Cultural and physiological bases for eating disorders. Ann NY Acad Sci. 1989;575:122–135. doi: 10.1111/j.1749-6632.1989.tb53237.x. [DOI] [PubMed] [Google Scholar]
- 3.de Castro JM. Socio-cultural determinations of meal size and frequency. Br J Nutr. 1997;77:S39–S55. doi: 10.1079/bjn19970103. [DOI] [PubMed] [Google Scholar]
- 4.Woods SC. Signals that influence food intake and body weight. Physiol Behav. 2005;86:709–716. doi: 10.1016/j.physbeh.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 5.Popkin BM, Duffey K, Gordon-Larsen P. Environmental influences on food choice, physical activity and energy balance. Physiol Behav. 2005;86:603–613. doi: 10.1016/j.physbeh.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 6.Weingarten HP. Conditioned cues elicit feeding in sated rats: A role for learning in meal initiation. Science. 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- 7.Cornell CE, Rodin J, Weingarten HP. Stimulus-induced eating when satiated. Physiol Behav. 1989;45:695–704. doi: 10.1016/0031-9384(89)90281-3. [DOI] [PubMed] [Google Scholar]
- 8.Birch LL, McPhee L, Sullivan S, Johnson S. Conditioned meal initiation in young children. Appetite. 1989;13:105–113. doi: 10.1016/0195-6663(89)90108-6. [DOI] [PubMed] [Google Scholar]
- 9.Petrovich GD, Gallagher M. Amygdala sybsystems and control of feeding behavior by learned cues. Ann NY Acad Sci. 2003;985:251–262. doi: 10.1111/j.1749-6632.2003.tb07086.x. [DOI] [PubMed] [Google Scholar]
- 10.Holland PC, Petrovich GD. A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiol Behav. 2005;86:747–761. doi: 10.1016/j.physbeh.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiology & Behavior. 2002;76:117–129. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- 12.Petrovich GD, Ross CA, Gallagher M, Holland PC. Learned contextual cue potentiates eating in rats. Physiol Behav. 2006 doi: 10.1016/j.physbeh.2006.09.031. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz MW, Woods SC, Porte DJ, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 14.Moran TH. Gut peptide signaling in the controls of food intake. Obesity. 2006;14(Suppl 5):250S–253S. doi: 10.1038/oby.2006.318. [DOI] [PubMed] [Google Scholar]
- 15.Flier JS. Obesity wars: Molecular progress confronts an expending epidemic. Cell. 2004;116:37–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 16.Pinto S, Roseberry AG, Liu H, Diano S, Cai SMX, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 17.Petrovich GD, Ross CA, Holland PC, Gallagher M. Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats. 2007 doi: 10.1523/JNEUROSCI.5001-06.2007. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Merrer J, Stephens DN. Food-induced behavioral sensitization, its cross-sensitization to cocaine and morphine, pharmacological blockade, and effect on food intake. J Neurosci. 2006;26:7163–7171. doi: 10.1523/JNEUROSCI.5345-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weingarten HP, Elston D. The phenomenology of food craving. Appetite. 1990;15:231–246. doi: 10.1016/0195-6663(90)90023-2. [DOI] [PubMed] [Google Scholar]
- 20.Feodoroff I, Polivy J, Herman CP. The specificity of restrained versus unrestrained eaters’ responses to food cues: general desire to eat, or craving for the cued food? Appetite. 2003;41:7–13. doi: 10.1016/s0195-6663(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 21.Tiggemann M, Kemps E. The phenomenology of food cravings: The role of mental imagery. Appetite. 2005;45:305–313. doi: 10.1016/j.appet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Jansen A. A learning model of binge eating: cue reactivity and cue exposure. Behav Res Ther. 1998;36:257–272. doi: 10.1016/s0005-7967(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 23.Sobik L, Hutchison K, Craighead L. Cue-elicited craving for food: a fresh approach to the study of binge eating. Appetite. 2005;44:253–261. doi: 10.1016/j.appet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher M, Holland PC. Understanding the function of the central nucleus: Is simple conditioning enough? In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss; New York: 1992. [Google Scholar]
- 25.Gallagher M. The amygdala and associative learning. In: Aggleton JP, editor. The Amygdala: A functional analysis. Oxford University Press; New York: 2000. pp. 311–329. [Google Scholar]
- 26.Holland PC, Hatfield T, Gallagher M. Rats with basolateral amygdala lesions show normal increases in conditioned stimulus processing but reduced conditioned potentiation of eating. Behav Neurosci. 2001;115:945–950. [PubMed] [Google Scholar]
- 27.Kapp BS, Pascoe JP, Bixler MA. The amygdala: A neuroanatomical systems approach to its contribution to aversive conditioning. In: Squire L, Butters N, editors. The Neuropsychology of Memory. The Guilford Press; New York: 1984. pp. 473–428. [Google Scholar]
- 28.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 29.Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 30.Holland P, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- 31.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 32.Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 33.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 34.Phillips AG, Ahn S, Howland JG. Amygdalar control of the mesocorticolimbic dopamine system: parallel pathways to motivated behavior. Neuroscience & Biobehavioral Reviews. 2003;27:543–554. doi: 10.1016/j.neubiorev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 36.Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. TINS. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Research Reviews. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- 38.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: Hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 39.Giraudo SQ, Kotz CM, Billington CJ, Levine AS. Association between the amygdala and nucleus of the solitary tract in μ-opioid induced feeding in the rat. Brain Research. 1998;802:184–188. doi: 10.1016/s0006-8993(98)00602-7. [DOI] [PubMed] [Google Scholar]
- 40.Roozendaal B, Oldenburger WP, Strubbe JH, Koolhaas JM, Bohus B. The central amygdala is involved in the conditioned but not in the meal-induced cephalic insulin response in the rat. Neurosci Lett. 1990;116:210–215. doi: 10.1016/0304-3940(90)90412-3. [DOI] [PubMed] [Google Scholar]
- 41.Giraudo SQ, Billington CJ, Levine AS. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the central nucleus of the amygdala and in the paraventricular nucleus in the rat. Brain Res. 1998;782:18–23. doi: 10.1016/s0006-8993(97)01140-2. [DOI] [PubMed] [Google Scholar]
- 42.Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- 43.Prewitt CM, Herman JP. Anatomical interactions between the central amygdaloid nucleus and the hypothalamic paraventricular nucleus of the rat: a dual tract-tracing analysis. J Chem Neuroanat. 1988;15:173–185. doi: 10.1016/s0891-0618(98)00045-3. [DOI] [PubMed] [Google Scholar]
- 44.Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. Eur J Neurosci. 2000;12:405–413. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- 46.Wise RA. Lateral hypothalamic electrical stimulation: does it make animals “hungry”? Brain Research. 1974;67:187–209. doi: 10.1016/0006-8993(74)90272-8. [DOI] [PubMed] [Google Scholar]
- 47.Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. Journal of Neuroscience. 2002;22:8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Setlow B, Holland PC, Gallagher M. Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive Pavlovian second-order conditioned responses. Behav Neurosci. 2002;116:267–275. doi: 10.1037//0735-7044.116.2.267. [DOI] [PubMed] [Google Scholar]
- 49.Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 50.Vazdarjanova A, McNaughton BL, Barnes CA, Worley PF, Guzowski JF. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. Journal of Neuroscience. 2002;22:10067–10071. doi: 10.1523/JNEUROSCI.22-23-10067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berridge KC, Robinson TE. Parsing reward. TINS. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 52.Will MJ, Kelley AE. Intra-acumbens μ-opioid-induced fat intake depends on activation of basolateral amygdala-lateral hypothalamic pathway. Soc Neurosci. 2005;31:532.521. Abst. [Google Scholar]
- 53.Petrovich GD, Ross CA, Holland PC, Gallagher M. Central but not basolateral amygdala is critical for control of feeding by aversive, conditioned cues. Soc Neurosci. 2006;32 doi: 10.1523/JNEUROSCI.3656-09.2009. Abst. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrovich GD, Holland PC, Gallagher M. Medial prefrontal cortex is necessary for an appetitive conditioned stimulus to promote eating in sated rats. Soc Neurosci Abstr. 2005;31 doi: 10.1523/JNEUROSCI.5001-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci. 2005;25:8295–8302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical Organization of the Efferent Projections of the Medial Prefrontal Cortex in the Rat: An Anterograde Tract-Tracing Study With Phaseolus vulgaris Leucoagglutinin. Journal of Comparative Neurology. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 57.Hurley KM, Herbert H, Moga MM, Saper CB. Efferent Projections of the Infralimbic Cortex of the Rat. Journal of Comparative Neurology. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- 58.Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to hypothalamus in the rat. Journal of Comparative Neurology. 2001;432:307–328. doi: 10.1002/cne.1105. [DOI] [PubMed] [Google Scholar]
- 59.Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J Neurosci. 2003;23:9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. NeuroImage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 61.LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- 62.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 63.Killgore WDS, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. NeuroImage. 2003;19:1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- 64.Hinton EC, Parkinson JA, Holland AJ, Arana FS, Roberts AC, Owen AM. Neural contributions to the motivational control of appetite in humans. European Journal of Neuroscience. 2004;20:1411–1418. doi: 10.1111/j.1460-9568.2004.03589.x. [DOI] [PubMed] [Google Scholar]
- 65.O’Doherty J. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 66.Nielsen SJ, Siega-Riz AM, Popkin BM. Trends in food locations and sources among adolescents and young adults. Preventive Medicine. 2002;35:107–113. doi: 10.1006/pmed.2002.1037. [DOI] [PubMed] [Google Scholar]


