Abstract
Background
Mechanisms purported to contribute to the pathophysiology of heart failure (HF) with normal ejection fraction (HFnlEF) include diastolic dysfunction, vascular and ventricular (LV) systolic stiffening, and volume expansion. We characterized LV volume, effective arterial (Ea), LV end-systolic (Ees) and LV diastolic elastance and relaxation non-invasively in consecutive HFnlEF patients and appropriate controls in the community.
Methods and Results
Olmsted County, Minnesota residents without CV disease (CON; n=617); with hypertension but no HF (HTN; n=719); or with HFnlEF (n=244) were prospectively enrolled. End-diastolic volume index (EDVI) was determined by echo-Doppler. Ees was determined using blood pressure, stroke volume, EF, timing intervals and estimated normalized ventricular elastance at end-diastole. Tissue Doppler e′ velocity was used to estimate the time constant of relaxation (τ). EDV and Doppler-derived end diastolic pressure (EDP) were used to derive the diastolic curve fitting (α) and stiffness (β) constants (EDP = αEDVβ). Comparisons were adjusted for age, sex and body size. HFnlEF patients had more severe renal dysfunction, yet smaller end-diastolic volume index and cardiac output and increased EDP compared to both hypertensive and healthy controls. Atrial elastance and ventricular end-systolic elastance were similarly increased in hypertensive controls and HFnlEF compared to healthy controls. In contrast, HFnlEF patients had more impaired relaxation and increased diastolic stiffness compared with either control group.
Conclusions
Based on these cross-sectional observations, we speculate that progression of diastolic dysfunction plays a key role in the development of HF symptoms in persons with hypertensive heart disease
Keywords: Heart failure, Diastole, Hypertension, Mechanics
Heart failure (HF) with normal ejection fraction (EF; HFnlEF) is a major public health problem of increasing prevalence.1 In contrast to the improvements in survival observed in patients with HF and reduced EF, mortality for patients with HFnlEF has remained stable, emphasizing the lack of proven therapies.1 An important barrier to advances in therapy is relative uncertainty regarding the fundamental pathophysiologic mechanisms. Left ventricular (LV) diastolic dysfunction (impaired relaxation and increased passive diastolic stiffness), increased systolic ventricular-vascular stiffening, and cardiac volume overload have been implicated in previous seminal studies.2–9 While well designed, these important studies were small, with both control and HFnlEF cohorts subject to potential limitations in regards to selection and referral bias, and in some instances, with populations pre-selected for features of cardiac remodeling or dysfunction. The relative incidence of each putative mechanism remains to be defined in a large, prospectively enrolled, control and heart failure populations recruited from the same community and studied in a comprehensive and uniform manner.
In this study of Olmsted County, Minnesota, residents, we employed previously validated non-invasive methods to assess LV volume,10 end-systolic LV11 and effective arterial stiffness (elastance),12 LV relaxation13,14 and diastolic elastance15 in order to compare cardiac structure and ventricular-vascular function in consecutive patients with HFnlEF to those observed in randomly selected persons without cardiovascular disease, or with hypertension, but no HF. We hypothesized that more advanced diastolic dysfunction and systolic ventricular-vascular stiffening distinguish HFnlEF from disease-free and hypertensive controls without HF in this community.
METHODS
Study setting
The unique aspects of Olmsted County, Minnesota, favoring population-based research have been previously described.16 The study was approved by the Mayo Institutional Review Board. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Identification of patients and study procedures
Subject groups were: 1. Non-obese controls without cardiovascular disease (CON); 2. Subjects with hypertension but without HF (HTN); and 3. Patients with HFnlEF. To recruit the first two groups, a random sample of the population ≥ 45 years old was prospectively identified and evaluated as previously described.16 Data from this study has previously been published, but these subsets and many of the indices presented here have not. Medical records were reviewed by trained nurse abstractors using established criteria for hypertension and HF. Clinical diagnoses of coronary artery disease, diabetes mellitus, valvular heart disease, cardiomyopathy, atrial fibrillation and transient ischemic attack or stroke were recorded. Each participant had measurement of cuff blood pressure, height and weight, with calculation of body mass index (BMI) and body surface area (BSA). Echocardiographic assessment of EF was performed by M-mode, quantitative and semiquantitative two-dimensional (2D) methods. Subjects with EF <50% were excluded. Of 2042 participants, 617 had none of the above validated or suspected cardiovascular diagnoses, a systolic pressure <140 mmHg at the time of echocardiography and a BMI <30 kg/m2, thus constituting the CON group. Subjects with hypertension but no HF (n=719) constituted the HTN group. The HFnlEF group was prospectively identified in an Olmsted County HF surveillance study by real-time interrogation of electronic medical records using natural language processing techniques.17 Briefly, all in- and out-patient electronic notes were searched (most within 24 hours of presentation) using a wide range of terms indicative of HF, enabling rapid identification of all potential cases of HF with a diagnostic sensitivity of 100%.17 The final diagnosis of HF was validated by trained nurse abstractors using the Framingham criteria. Of 811 HF patients identified between 9/10/2003 and 8/24/2005, 570 (70%) consented for participation and 516 (91%) underwent echocardiography within a median (25th, 75th percentile) of one (1,5) day of diagnosis. Of these, 276 patients had EF ≥50%. Hemodynamically significant valve disease was detected on Doppler echocardiography in 32 (11.6%) patients who were excluded. The remaining 244 patients made up the HFnlEF group. Reflecting the ethnic composition of the community, subjects were almost exclusively Caucasian.
Plasma brain natriuretic peptide (BNP) was determined by immunoradiometric assay (nonextracted) using antibody to human BNP (Shionogi Co. Ltd., Tokyo, Japan). Glomerular filtration rate (GFR) was estimated using the simplified Modification of Diet in Renal Disease Study equation. All echocardiograms were performed by registered diagnostic cardiac sonographers using standardized instruments and techniques16 and were reviewed by a cardiologist (CSL, MMR).
Assessment of cardiac volume
LV volume was determined in each subject by three methods. The Teichholz method18 used short-axis LV dimension measured from 2D or M-mode images. This was available in 532 (86%) CON, 551 (77%) HTN and 222 (91%) HFnlEF subjects. In 73 subjects, LV short-axis dimension was measured from both 2D and M-mode images and correlated well (r=0.73, p<0.001) with no systematic error (using Bland-Altman analysis, mean difference ± SD = 0.79 ± 4.2 mm) and no relationship between mean difference and the average of the two methods (r=0.02, p=0.85). LV volume calculated by the area-length formula10 used both long- and short-axis LV dimensions. This was available in 496 (80%) CON, 492 (68%) HTN and 188 (77%) HFnlEF subjects. LV volume was also calculated independent of geometric assumptions by dividing stroke volume (SV; using left ventricular outflow tract dimension and pulsed wave Doppler velocity profile) by EF. This was available in 611 (99%) CON, 697 (97%) HTN and 223 (91%) HFnlEF subjects. Left atrial volume (LAV) was calculated by the ellipse formula.19 LV mass and relative wall thickness (RWT) were calculated by standard methods.10 Measurements were indexed (I) to BSA where appropriate. LV hypertrophy (LVH) was defined as LV mass index >95 g/m2 (females) or >115 g/m2 (males) and LV geometry classified as normal, concentric remodeling, concentric LVH or eccentric LVH.10
Determination of vascular function
Effective arterial elastance (Ea) was estimated as end-systolic pressure (ESP)/SV.12 ESP was estimated as systolic pressure*0.9, as previously validated.11,12 Total arterial compliance (Ca) was estimated by SV/pulse pressure ratio20 and systemic vascular resistance index (SVRI) by [(mean arterial pressure/cardiac index)*80].
Determination of LV end-systolic elastance
The modified single-beat method was used to estimate end-systolic elastance (Ees) from arm-cuff pressures, SV, pre-ejection and total systolic periods determined on continuous wave Doppler of aortic flow, EF, and an estimated normalized ventricular elastance at arterial end-diastole, as previously validated11,21 and employed in recent studies.4,22,23
Determination of early LV relaxation velocity and filling pressures
The medial mitral annular early diastolic velocity (e′) was determined by spectral tissue Doppler imaging using standard methods. The e′ velocity is relatively preload-independent and inversely related to the time constant of isovolumic relaxation τ, which was derived by the formula [τ = (14.70 – 100e′)/0.15].13,14 Early transmitral flow velocity (E) was measured by pulse wave Doppler. End-diastolic pressure (EDP) was estimated as: [EDP = 11.96 + 0.596*E/e’] as previously determined from Doppler and invasive EDP measurements at our institution.13
Determination of LV diastolic stiffness
The recently developed and validated single-beat approach proposed by Klotz et al was used to characterize the end-diastolic pressure-volume relationship (EDPVR, where EDP = αEDVβ; α = curve fitting constant and β = diastolic stiffness constant).15 Based on the premise that volume-normalized EDPVRs share a common shape, this method allows the estimation of α and β, and hence the entire EDPVR, from a single pressure-volume point. Measured EDP and EDV were used to derive α and β in each subject. A modified method was used when EDP >28 mmHg to address the recognized mathematical limitations of the original equations (see Appendix). In order to account for covariance in α and β,24 both of which are indicative of the shape and position of the EDPVR, derived α and β in each subject were used to predict the EDV at a common EDP of 20 mmHg (EDV20). Comparison of EDV20 indexed to BSA (EDVI20) was then used as a comparison of overall diastolic stiffness between groups.
Statistical methods
Categorical variables were compared using the Pearson Chi-square test. Continuous variables were log transformed as necessary and compared between groups using one-way ANOVA with Bonferroni correction for multiple unadjusted comparisons. Regression analysis was used to adjust for age and sex and BSA or the presence of other diseases in group comparisons, where the dependent variable was the normally distributed continuous (linear least-squares regression) or categorical (logistic regression) outcome variable of interest, and factors entered into the model were age, sex, BSA, and group (dummy variable). Any interaction between these variables was also evaluated and accounted for as appropriate. All analyses were two-sided and significance was judged at p<0.05.
RESULTS
Subject characteristics
HFnlEF patients were older, more obese, had higher prevalence of coronary artery disease and diabetes and had lower GFR than HTN or CON (Table 1).
Table 1. Subject characteristics.
| CON (n=617) | HTN (n=719) | HfnlEF (n=244) | |
|---|---|---|---|
| Age (range), years | 57 (45–96) | 66 (46–91)* | 76 (22–99) *† |
| Males, % | 45 | 44 | 45 |
| Height, cm | 169±10 | 167±10* | 165±13* |
| Weight, kg | 73±13 | 84±19* | 86±25* |
| Body surface area, m2 | 1.85±0.21 | 1.96±0.26* | 1.97±0.31* |
| Body mass index, kg/m2 | 25.4±2.7 | 29.8±5.9* | 32.2±20.7*† |
| Hypertension, % | 0 | 100* | 96* |
| Coronary artery disease, % | 0 | 16* | 53*† |
| Diabetes mellitus, % | 0 | 11* | 37*† |
| Glomerular filtration rate, ml/min/1.73m2 | 74.4±14.1 | 74.7±37.0 | 64.3±28.1*† |
| BNP (Shionogi), pg/ml | 20.0±40.3 | 30.5±45.2* | 260.7±330.2*† |
| Log BNP (Shionogi, pg/ml) | 1.06±0.41 | 1.23±0.46* | 2.15±0.55*† |
| Ejection fraction, % | 63±5 | 65±6 | 62±6*† |
| Heart rate, bpm | 65±10 | 67±12 | 71±15*† |
| Systolic blood pressure, mmHg | 118±12 | 143±21* | 132±23*† |
| Diastolic blood pressure, mmHg | 70±8 | 76±11* | 67±14† |
| Pulse pressure, mmHg | 48±11 | 67±18* | 65±20* |
Data are mean ± SD unless otherwise stated. Unadjusted analysis.
p<0.05 vs CON;
p<0.05 vs HTN
BSA, body surface area; BNP, B-type natriuretic peptide
LV structure
Adjusting for age and sex, EDVI in HFnlEF was similar (Area-length) or smaller (Teichholz and Doppler) compared to CON, and smaller (by all 3 methods) compared to HTN (Table 2). Adjusting for age and sex, SVI in HFnlEF was smaller compared to CON or HTN, while cardiac index in HFnlEF was similar to that in CON but reduced compared to HTN. Adjusting for age and sex, LV mass index, RWT and LV mass to volume ratio were increased in HFnlEF and HTN compared to CON, but these parameters were similar in HFnlEF and HTN. The %LVH was greater in HTN and HFnlEF than in CON but similar in HFnlEF and HTN. LV geometry patterns varied considerably in both control populations and in HFnlEF. While HFnlEF patients had more concentric LVH and less normal geometry compared to CON, these patterns were not significantly different compared to HTN after adjusting for age.
Table 2. Measures of cardiovascular structure and function.
| CON (n=617) | HTN (n=719) | HfnlEF (n=244) | ||
|---|---|---|---|---|
| LV structure | ||||
|
| ||||
| Teichholz | 110.6±23.6 | 113.3±26.1 | 110.2±32.6 | |
| EDV, ml | Area-length | 123.2±30.3 | 125.9±32.9 | 119.4±39.3† |
| Doppler | 134.4±31.4 | 141.1±35.5 | 132.8±37.7† | |
| Teichholz | 60.6±10.9 | 59.7±12.2 | 56.4±14.4*† | |
| EDVI, ml/m2 | Area-length | 66.6±12.3 | 64.9±13.9 | 60.9±16.1† |
| Doppler | 72.5±12.9 | 72.2±15.5 | 68.1±16.6*† | |
| Stroke volume index, ml/m2 | 45.8±7.5 | 46.3±9.5 | 42.3±10.0*† | |
| Cardiac index, l/min/m2 | 2.94±0.57 | 3.04±0.70 | 2.95±0.79† | |
| LV mass, g | 164.2±38.8 | 195.0±53.2* | 200.4±67.1* | |
| LV mass index, g/m2 | 88.8±16.3 | 100.2±22.7* | 102.1±29.0* | |
| LV mass/EDV, mg/ml | 1.50±0.28 | 1.75±0.39* | 1.85±0.47* | |
| Relative wall thickness | 0.38±0.06 | 0.42±0.07* | 0.45±0.10* | |
| % LV hypertrophy | 18% | 40%* | 42%* | |
| % Normal geometry | 66 | 39* | 31* | |
| % Concentric remodeling | 16 | 21 | 27 | |
| % Concentric hypertrophy | 5 | 21* | 26* | |
| % Eccentric hypertrophy | 13 | 19 | 16 | |
|
| ||||
| Vascular function | ||||
| Effective arterial elastance (Ea), mmHg/ml | 1.30±0.30 | 1.50±0.41* | 1.53±0.43* | |
| Systemic vascular resistance index, dyne.s.cm−5.m2 | 2424±521 | 2703±657* | 2588±873* | |
| Arterial compliance, ml/mmHg | 1.86±0.58 | 1.45±0.55* | 1.41±0.93* | |
|
| ||||
| LV systolic function | ||||
| End-systolic elastance (Ees), mmHg/ml | 1.99±0.59 | 2.30±0.80* | 2.39±0.87* | |
| Ees*LV mass | 319.7±96.4 | 439.6±163.7* | 461.8±209.7* | |
| Ees*EDV | 215.5±60.7 | 256.3±86.3* | 254.0±105.3* | |
| Ea/Ees | 0.68±0.13 | 0.68±0.17 | 0.69±0.22 | |
|
| ||||
| LV diastolic function | ||||
| E, m/s | 0.660±0.131 | 0.671±0.169* | 0.979±0.347*† | |
| A, m/s | 0.561±0.161 | 0.722±0.203* | 0.848±0.267*† | |
| E/A ratio | 1.25±0.38 | 0.99±0.37* | 1.21±0.69*† | |
| Deceleration time, ms | 222±33 | 239±43 | 208±54*† | |
| e′, m/s | 0.094±0.035 | 0.077±0.039* | 0.060±0.021*† | |
| τ, ms | 35.2±23.4 | 46.8±26.0* | 58.1±14.3*† | |
| E/e’ ratio | 7.55±2.29 | 9.43±3.32* | 18.43±9.65*† | |
| LV end-diastolic pressure (EDP), mmHg | 16.5±1.4 | 17.6±2.0* | 22.9±5.7*† | |
| Diastolic stiffness constant (β) | 5.96±0.06 | 6.05±0.41* | 7.09±3.55*† | |
| EDVI20, ml/m2 | 61.7±11.4 | 59.7±11.9* | 55.7±14.5*† | |
| EDP/EDV, mmHg/ml | 0.16±0.04 | 0.16±0.05 | 0.23±0.11*† | |
Data are mean ± SD; Comparisons adjusted for age and sex, as well as body surface area (BSA) where appropriate;
p<0.05 vs CON;
p<0.05 vs HTN; LV, left ventricular; EDV, end-diastolic volume; I, indexed to BSA
Vascular function
Adjusting for age, sex and BSA where appropriate, Ea, SVRI and pulse pressure were increased while Ca was decreased in HFnlEF and HTN compared to CON, but all these parameters were similar in HFnlEF and HTN (Table 2). Unadjusted comparisons gave similar results.
LV Systolic stiffness
Adjusting for age, sex and BSA, Ees was increased in HFnlEF and HTN compared to CON but was similar in HFnlEF and HTN (Table 2). Similar results were observed in unadjusted comparisons and after normalizing Ees for LV mass (Ees*LV mass) and EDV (Ees*EDV) (and adjusting for age and sex), suggesting that the differences in Ees could not be solely attributed to differences in chamber size. Systolic vascular-ventricular coupling ratio (Ea/Ees) was preserved across groups. Predicted ESPVR equations derived from group-averaged data are given in Figure 1.
Figure 1.
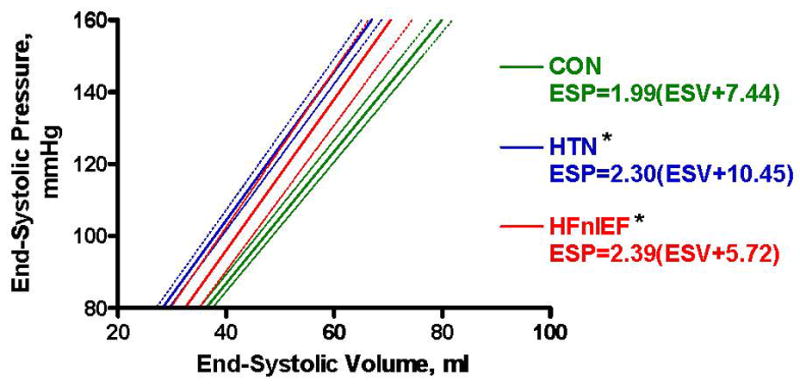
Schematic of group-averaged end-systolic pressure-volume relationship (ESPVR), where ESP = Ees(ESV-V0) (Ees = end-systolic elastance; V0 = volume intercept). Solid lines represent the mean ESPVR and dotted lines the 95% confidence intervals for each group. For comparison of Ees (slope) between groups, *p<0.05 vs CON.
Estimated LV filling pressures
EDP was higher in HFnlEF compared to both CON and HTN (Figure 2), with corroborating evidence of elevated filling pressures provided by plasma BNP and left atrial volume index measurements.
Figure 2.
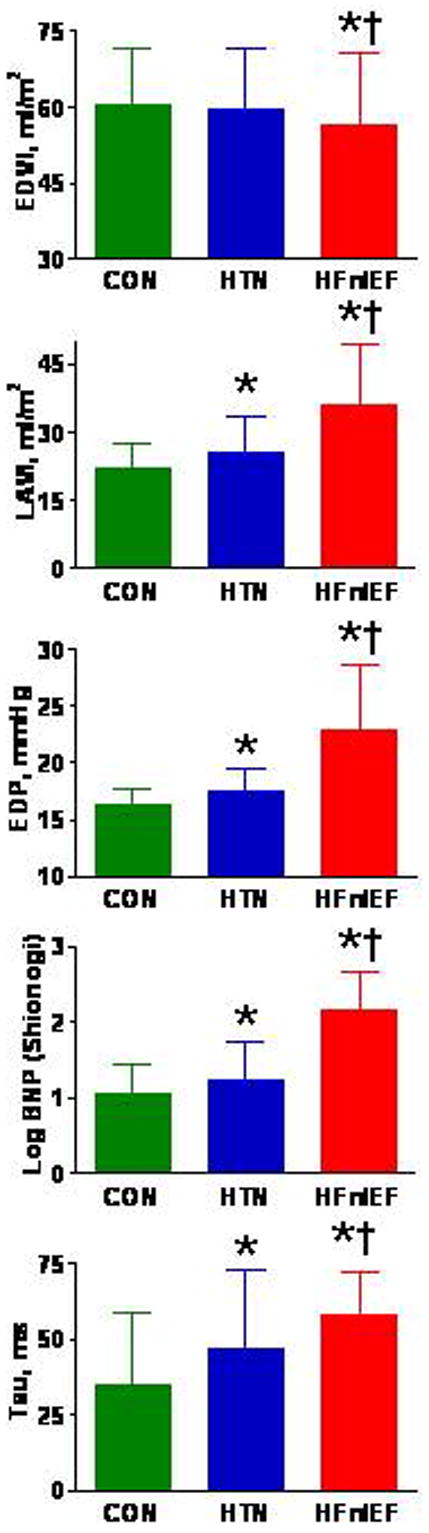
Bar graphs of indexed end-diastolic volume (EDVI), indexed left atrial volume (LAVI), end-diastolic pressure (EDP), plasma brain natriuretic peptide (BNP) and derived tau by subject group. Data are mean ± SD; *p<0.05 vs CON; † p<0.05 vs HTN.
LV diastolic function
In both unadjusted and adjusted (adjusting for age, sex and BSA) comparisons, HFnlEF patients had more impaired relaxation (lower e′, longer τ) and higher β compared to CON and HTN (Table 2). Adjusting for age and sex and controlling for covariance in α and β, overall diastolic LV stiffness was higher (lower EDVI20) in HFnlEF than in CON or HTN (Table 2). Predicted EDPVR curves derived from group-averaged data are illustrated in Figure 3.
Figure 3.
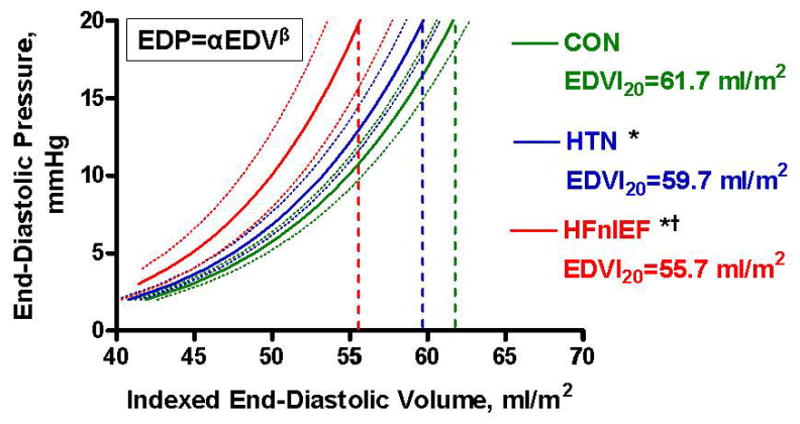
Schematic of group-averaged end-diastolic pressure-volume relationship (EDPVR), where EDP = αEDVβ (α = curve fitting constant; β = diastolic stiffness constant). Solid lines represent the mean EDPVR and dotted lines the 95% confidence intervals for each group. For comparison of indexed EDV at a common EDP of 20 mmHg (EDVI20) between groups, *p<0.05 vs CON; † p<0.05 vs HTN.
Further analyses
In view of the large age range of subjects (Table 1) and recognizing that unaccounted confounders may be present at the extremes of ages, a sub-analysis of subjects aged 60–95 years was performed and gave similar results (Table 3). Further recognizing potential confounding effects of diabetes and renal function, we adjusted for these in addition to adjusting for age, sex and body size (Table 4). Overall results were similar.
Table 3. Subgroup analysis in subjects aged 60 to 95 years.
| CON (n=211) | HTN (n=519) | HfnlEF (n=214) | ||
|---|---|---|---|---|
| Teichholz | 59.4±12.1 | 60.0±12.7 | 56.7±14.2* † | |
| EDVI, ml/m2 | Area-length | 63.7±12.8 | 64.7±14.0 | 60.8±15.6† |
| Doppler | 72.0±13.4 | 73.4±16.0 | 68.1±16.6* † | |
| Effective arterial elastance, mmHg/ml | 1.35±0.32 | 1.53±0.43* | 1.54±0.43* | |
| End-systolic elastance, mmHg/ml | 2.12±0.64 | 2.37±0.83* | 2.42±0.88 | |
| EDVI20, ml/m2 | 60.5±12.8 | 60.0±12.3 | 55.7±14.3*† | |
| τ, ms | 41.3±27.7 | 49.1±28.0 | 59.5±13.1* † | |
Data are mean ± SD; Comparisons adjusted for age, sex and body surface area (BSA) where appropriate;
p<0.05 vs CON;
p<0.05 vs HTN; EDVI, end-diastolic volume indexed to BSA
Table 4. Analysis adjusting for renal function, diabetes, age, sex and body size.
| CON (n=617) | HTN (n=719) | HfnlEF (n=244) | |
|---|---|---|---|
| Effective arterial elastance, mmHg/ml | 1.30±0.30 | 1.50±0.41* | 1.53±0.43* |
| End-systolic elastance, mmHg/ml | 1.99±0.59 | 2.30±0.80* | 2.39±0.87* |
| EDVI20, ml/m2 | 61.7±11.4 | 59.7±11.9 | 55.7±14.5*† |
| τ, ms | 35.2±23.4 | 46.8±26.0* | 58.1±14.3*† |
Data are mean ± SD; Comparisons adjusted for glomerular filtration rate, diabetes, age, sex and body surface area (BSA);
p<0.05 vs CON;
p<0.05 vs HTN; EDVI, end-diastolic volume indexed to BSA
DISCUSSION
This is the largest population-based study to date comparing vascular and ventricular structure and function in a HFnlEF cohort to that observed in healthy and hypertensive control populations without HF. The current study serves to confirm, clarify and extend smaller, seminal studies describing a variety of structural and functional perturbations in more select cohorts with HFnlEF. Several findings are noteworthy. The HFnlEF cohort had worse renal function, yet smaller LV volume and cardiac output as compared to hypertensive controls. While LV mass was, on average, increased in HFnlEF as compared to healthy controls, HFnlEF patients did not have more severe LVH than hypertensive controls. Compared to healthy controls, the HFnlEF cohort had increases in both the resistive and pulsatile components of vascular load with proportional increases in LV systolic stiffness. However, these abnormalities were similar to those observed in hypertensive controls without HF. In contrast, diastolic dysfunction (both impairment in relaxation and increases in diastolic stiffness) was more severe in HFnlEF patients as compared to healthy or hypertensive controls.
The current findings are consistent with previous studies which utilized invasive assessment of LV function in HFnlEF. Liu et al. used conductance catheters with preload reduction (multiple-beat method) in 10 patients with LVH and normal EF (7 with HFnlEF) and found impaired relaxation with increased diastolic stiffness in this group compared to 8 younger, healthy controls.25 All subjects were referred for cardiac catheterization at a tertiary center.25 In a landmark invasive study using a single-beat method, Zile et al. also found more impaired relaxation and higher diastolic stiffness in HFnlEF (n=47). These HFnlEF patients were predominantly male with echocardiographic evidence of LVH recruited at a Veterans Administration Hospital as part of a clinical trial and were compared to 10 healthy age-matched controls.2 In both these studies, the control group had no cardiovascular disease, raising concern as to whether the observed differences were specifically attributable to HFnlEF, or to hypertensive heart disease. Borbely et al. measured chamber and myocyte stiffness in 12 HFnlEF patients and 8 controls, and found increased estimated LV diastolic stiffness in HFnlEF by invasive measurements.26 However, nearly half the HFnlEF and 75% of control patients had previously undergone cardiac transplantation, thus confounding effects of occult rejection or immunosuppression may have influenced the findings.
Other studies employed non-invasive methods to characterize diastolic function.27 Ahmed et al identified 26 patients with LVH and HFnlEF undergoing echocardiography at their tertiary center and showed that these patients had more severe diastolic dysfunction than 39 non-hypertensive controls, 14 hypertensive controls and 23 controls with LVH but no HF.6 The inclusion of hypertensive controls was a strength of this study which focused on HFnlEF patients with LVH.
In the current study, consecutive cases of HFnlEF identified in both the inpatient and outpatient settings, and not pre-selected for any geometric characteristics, were compared to large, randomly-selected and prospectively enrolled control populations from the same community, with all subjects studied in a similar manner and using analyses adjusted for potential effects of age, sex and body size. The current results are consistent with the aforementioned studies in that relaxation and passive diastolic stiffness were impaired in HFnlEF compared to disease-free controls. Further, the current data confirm that compared to hypertensive controls, HFnlEF patients have more severe diastolic dysfunction. While the predominant cardiovascular abnormalities and contributing comorbidities in HFnlEF patients may vary according to a number of demographic parameters, it is noteworthy that the presence of diastolic dysfunction is a consistent finding in HFnlEF patients identified in this community and in the diverse settings included in previous studies.2,5–9,25,26
In contrast, Kawaguchi et al., using either invasive (conductance catheters and multiple-beat model) or non-invasive (single-beat model) measurements, found that relaxation was not significantly different in HFnlEF (n=10) compared to young controls (n=9) and age- and blood pressure-matched controls (n=25), except during stress (isometric handgrip).4 Additionally, although higher EDPs were observed in HFnlEF, this was due to a parallel upward shift of the diastolic pressure-volume curve, rather than to a steeper curve (i.e. β stiffness coefficients were similar), suggesting that exaggerated external forces, rather than increased passive diastolic stiffness was present in HFnlEF. However, the large variability in β observed in the HFnlEF group (range ≈ 0.01 to 0.05 mmHg/ml) may have prevented demonstration of differences in β in the small numbers of subjects enrolled. Importantly, this study showed that HFnlEF patients had increased Ea and Ees, suggesting that vascular and LV systolic stiffening may contribute to the pathophysiology of HFnlEF by exaggerating systolic load and diastolic dysfunction during exercise. These patients were studied over a 14-year period at a referral center, and while predominantly female, the mean age was lower than that observed in most population-based studies. Although we also found that Ea and Ees were increased in HFnlEF compared to healthy controls, these indices were not further increased in HFnlEF compared to hypertensive controls in the current study as well as others.5,6,9 Nonetheless, these data do not exclude a role for increased vascular and LV systolic stiffening in the pathophysiology of HFnlEF, particularly during exercise or other stressors where such changes exaggerate hypertensive responses and induce further, load dependent diastolic dysfunction.
The potential for a subgroup of HFnlEF patients to have LV dilatation and a “high output” form of HF has been reported.3 Maurer et al. used 3-dimensional and Doppler echocardiography to characterize LV volumes and pressures non-invasively at a tertiary referral center in the New York metropolitan area. Among 35 patients with hypertension and HFnlEF, a subgroup (n=29) of younger, more obese subjects had increased LV volumes associated with increased EDP but no change in Ees or Ea compared to healthy controls. These investigators concluded that many (most in their series) HFnlEF patients may have volume overload, without intrinsic diastolic dysfunction as a mechanism for increased filling pressures. In contrast, our data show that on average, compared to healthy or hypertensive controls, HFnlEF patients have normal or decreased LV volumes respectively. Since ventricular volumes vary with body size, sex and possibly age in persons without cardiovascular disease, we were careful to adjust for these parameters in all volume comparisons. We accounted not only for the short-axis but also for the long-axis LV dimension when calculating volumes. A further Doppler-based method was used to estimate volumes independent of geometric assumptions. All 3 methods gave the consistent picture that ventricular enlargement was not present in the majority of HFnlEF patients despite more impaired renal function in these patients. In fact, stroke volume and cardiac index were lower in HFnlEF than in hypertensive controls. As emphasized previously, however, the current analysis is restricted to group comparisons; as LV volume is a continuous variable with a fairly normal distribution in the HFnlEF population, some patients with HFnlEF will have increased LV volume even though the distribution curve as a whole was not shifted towards larger volumes. Indeed, our findings underscore the variable LV geometric patterns present in HFnlEF.
More recently, Melenovsky et al.9 used non-invasive methods to study 37 HFnlEF patients, 40 hypertensive and 56 non-hypertensive age-, gender- and race-matched controls recruited from an urban setting in Baltimore, Maryland. This population was largely African American, and HFnlEF patients were younger (by a decade) than observed here, more obese, and more predominately female. As in our study, LV volume did not vary significantly among groups, estimated filling pressures were highest in HFnlEF, and both Ees and Ea were similarly increased in hypertensive controls and HFnlEF compared to disease-free controls. However, both the HFnlEF and hypertensive groups had much more dramatic LVH than we observed, and while estimated LV diastolic pressures were higher in HFnlEF, many parameters displayed substantial overlap, with little disparity between these two groups. Although LV diastolic stiffness was not estimated, the prior study found left atrial enlargement and impaired atrial function in HFnlEF, leading the authors to speculate that impaired atrial function may also play a key role in the transition to HFnlEF among patients with cardiovascular disease. This hypothesis is consistent with clinical studies documenting that new onset atrial fibrillation is a common precipitant of episodes of acutely decompensated HF, regardless of EF.28,29 We too found increased left atrial volume in HFnlEF compared to either control group. Melenovsky et al. further found that total epicardial cardiac volume was highest in HFnlEF patients and speculated that external forces may contribute to elevation of filling pressures.
The variable LV geometry patterns observed in HFnlEF patients in our study is noteworthy and consistent with several2,4,28,30 prior studies, underscoring that despite traditional teaching, concentric LVH or concentric remodeling is not invariably present in HFnlEF. Indeed, there may be important geographic and race-specific differences, with marked concentric LVH being more common in some populations, such as African Americans, as seen in studies where these groups are more prominently represented.9 Finally, the similar RWT and LV mass to volume ratio observed in HTN and HFnlEF suggest that factors other than chamber geometry additionally mediate increased diastolic stiffness in HFnlEF. Changes in the cardiomyocytes themselves26 and/or the extracellular matrix31,32 may mediate diastolic stiffening and represent potential therapeutic targets in the treatment and/or prevention of HFnlEF.
Limitations
Our data are purely observational and cannot prove causality. The more impaired diastolic dysfunction in HFnlEF could be a marker for, rather than a mediator of, progression to HF. Although invasive measurements were not performed, each of the methods employed to characterize pressure-volume relationships was validated against gold-standard invasive techniques.
Future directions
While total vascular load and indirect measures of vascular stiffness were obtained here, further study is needed to evaluate more direct and perhaps regional measures of vascular stiffening, and other assessments of arterial impedance and its impact such as characteristic impedance, wave reflections, and pulse wave velocity. Hemodynamic data obtained during exercise and other stresses may be key in differentiating HFnlEF from hypertensive controls. The study population was mainly white and potential differences in other racial groups should be examined. Finally, the functional significance of different geometric patterns in HFnlEF deserves further study.
Conclusion
In this large, population-based study, HFnlEF patients had reduced LV volumes and cardiac output compared to hypertensive controls despite more renal impairment. While HFnlEF patients displayed vascular and LV systolic stiffening as compared to normal controls, HFnlEF was distinguished from hypertensive heart disease by the presence of more severe diastolic dysfunction, and increased left atrial size. Thus, these data support efforts to ameliorate diastolic dysfunction in order to prevent or treat HFnlEF. While we speculate that progression of diastolic dysfunction plays a key role in the development of HF symptoms in persons with hypertensive heart disease and a normal EF, further studies characterizing potential differential responses to exercise and other stressors may reveal additional pathophysiological mechanisms and therapeutic targets.
Acknowledgments
Funding sources
Public Health Service (NIH HL63281-4, NIH HL72435-2, and NIH HL55502-7).
Appendix
A recognized limitation of the original predictions used in the single-beat EDPVR method15 was the break down of the equations as measured EDP approached 30mmHg. This limitation was due to the arbitrary choice of V30 (estimated EDV at 30mmHg) as a starting point in the original derivation equations for a and β, which therefore became unstable as measured EDP approached 30mmHg (>28mmHg). This mathematical instability was overcome by simply using an estimate of EDV at a pressure of 15mmHg (V15) instead of V30 for cases where measured EDP >28mmHg. V15 was derived from the EDV normalized curve in the same fashion as V3015(Burkoff D, MD, PhD, electronic personal communication, 2006). Similar to the original derivations, α and β were then calculated by solving the simultaneous equations:
| [Equation 1] |
| [Equation 2] |
Where Pm = measured pressure (measured EDP) and Vm = measured volume (measured EDV) Dividing [1] by [2] and solving for β:
Substituting into [1]:
EDPVR curves derived using V15 and V30 were well-correlated at multiple parts of the curves.
Footnotes
Conflict of interest disclosures
None.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–9. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 3.Maurer MS, King DL, El-Khoury Rumbarger L, Packer M, Burkhoff D. Left heart failure with a normal ejection fraction: identification of different pathophysiologic mechanisms. J Card Fail. 2005;11:177–87. doi: 10.1016/j.cardfail.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–20. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 5.Baicu CF, Zile MR, Aurigemma GP, Gaasch WH. Left ventricular systolic performance, function, and contractility in patients with diastolic heart failure. Circulation. 2005;111:2306–12. doi: 10.1161/01.CIR.0000164273.57823.26. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–96. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 7.Aurigemma GP, Zile MR, Gaasch WH. Contractile behavior of the left ventricle in diastolic heart failure: with emphasis on regional systolic function. Circulation. 2006;113:296–304. doi: 10.1161/CIRCULATIONAHA.104.481465. [DOI] [PubMed] [Google Scholar]
- 8.van Heerebeek L, Borbely A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJ, Linke WA, Laarman GJ, Paulus WJ. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–73. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 9.Melenovsky V, Borlaug B, Rosen B, Hay I, Ferrucci L, Morell C, Lakatta E, Najjar S, Kass D. Cardiovascular features of heart failure with preserved ejection fraction versus non-failing hypertensive left ventricular hypertrophy in the urban Baltimore community. J Am Coll Card. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–34. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 12.Kelly RP, Ting C-T, Yang T-M, Liu C-P, Maughan WL, Chang M-S, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 13.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 14.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. Journal of the American College of Cardiology. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 15.Klotz S, Hay I, Dickstein ML, Yi GH, Wang J, Maurer MS, Kass DA, Burkhoff D. Single-beat estimation of end-diastolic pressure-volume relationship: a novel method with potential for noninvasive application. Am J Physiol Heart Circ Physiol. 2006;291:H403–12. doi: 10.1152/ajpheart.01240.2005. [DOI] [PubMed] [Google Scholar]
- 16.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 17.Pakhomov SV, Buntrock J, Chute CG. Prospective recruitment of patients with congestive heart failure using an ad-hoc binary classifier. J Biomed Inform. 2005;38:145–53. doi: 10.1016/j.jbi.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. American Journal of Cardiology. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- 19.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. Journal of the American College of Cardiology. 2003;41:1036–43. doi: 10.1016/s0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 20.Chemla D, Hebert JL, Coirault C, Zamani K, Suard I, Colin P, Lecarpentier Y. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol. 1998;274:H500–5. doi: 10.1152/ajpheart.1998.274.2.H500. [DOI] [PubMed] [Google Scholar]
- 21.Senzaki H, Chen CH, Kass DA. Single-beat estimation of end-systolic pressure-volume relation in humans. A new method with the potential for noninvasive application. Circulation. 1996;94:2497–506. doi: 10.1161/01.cir.94.10.2497. [DOI] [PubMed] [Google Scholar]
- 22.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–62. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 23.Maurer MS, El Khoury Rumbarger L, King DL. Ventricular volume and length in hypertensive diastolic heart failure. J Am Soc Echocardiogr. 2005;18:1051–7. doi: 10.1016/j.echo.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol. 2005;289:H501–12. doi: 10.1152/ajpheart.00138.2005. [DOI] [PubMed] [Google Scholar]
- 25.Liu C-P, Ting C-T, Lawrence W, Maughan WL, Chang M-S, Kass DA. Diminished contractile response to increased heart rate in intact human left ventricular hypertrophy: Systolic versus diastolic determinants. Circulation. 1993;88:1893–1906. doi: 10.1161/01.cir.88.4.1893. [DOI] [PubMed] [Google Scholar]
- 26.Borbely A, van der Velden J, Papp Z, Bronzwaer JG, Edes I, Stienen GJ, Paulus WJ. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–81. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 27.Oh JK, Hatle L, Tajik AJ, Little WC. Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol. 2006;47:500–6. doi: 10.1016/j.jacc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 28.Chen HH, Lainchbury JG, Senni M, Bailey KR, Redfield MM. Diastolic heart failure in the community: clinical profile, natural history, therapy, and impact of proposed diagnostic criteria. J Card Fail. 2002;8:279–87. doi: 10.1054/jcaf.2002.128871. [DOI] [PubMed] [Google Scholar]
- 29.Vasan RS, Levy D. Defining diastolic heart failure: a call for standardized diagnostic criteria. Circulation. 2000;101:2118–21. doi: 10.1161/01.cir.101.17.2118. [DOI] [PubMed] [Google Scholar]
- 30.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–50. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 31.Lopez B, Gonzalez A, Querejeta R, Larman M, Diez J. Alterations in the pattern of collagen deposition may contribute to the deterioration of systolic function in hypertensive patients with heart failure. J Am Coll Cardiol. 2006;48:89–96. doi: 10.1016/j.jacc.2006.01.077. [DOI] [PubMed] [Google Scholar]
- 32.Little WC, Zile MR, Kitzman DW, Hundley WG, O’Brien TX, Degroof RC. The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. J Card Fail. 2005;11:191–5. doi: 10.1016/j.cardfail.2004.09.010. [DOI] [PubMed] [Google Scholar]


