Abstract
Background and Objectives
Magnesium sulfate (MgSO4) is well-known as an antagonist of n-methyl-d-aspartate receptors and was used for intrathecal analgesia a century ago. However, the effects of MgSO4 combined with local anesthetics (LAs) on peripheral nerves are unclear. We tested the hypothesis that MgSO4 could be used as an adjuvant to prolong and intensify conduction block by amide-type LAs in a rat sciatic nerve block model. Further, the mechanism of possible synergy between LAs and MgSO4 was investigated in whole-cell mode patch-clamp experiments.
Methods
Sciatic nerves were exposed to 2%/73.9mM lidocaine, 0.25%/7.7mM bupivacaine, and 0.5%/15.4mM ropivacaine, with or without addition of 1.25%, 2.5%, or 5% MgSO4/50.7 mM, and nerve block characteristics were assessed. To elucidate the LA-MgSO4 interaction, voltage-dependent inactivation curves were determined in cultured rat GH3 cells expressing neuronal Na+ channels.
Results
Unexpectedly, the addition of MgSO4 overall significantly shortened the duration of blockade by lidocaine, bupivacaine, and ropivacaine. The steady-state inactivation of Na+ channels in the presence of 300 μM lidocaine was almost unchanged by the addition of 10 mM MgSO4, indicating that MgSO4 does not affect the potency of lidocaine toward the inactivated Na+ channel.
Conclusions
MgSO4 coadministered with amide-type LAs shortened the duration of sciatic nerve blockade in rats. Therefore, it does not seem to be useful as an adjuvant for peripheral nerve blockade. The mechanism of this observed antagonism is unclear, but appears to be independent of the action of LAs and MgSO4 at the LA receptor within the Na+ channel.
Keywords: Local Anesthetics, Magnesium Sulfate, Sciatic Nerve, Sodium Channel
Introduction
It has been demonstrated in a rat sciatic nerve block model that the effects of local anesthetics (LAs) can be prolonged and intensified by coadministering other compounds with local anesthetic properties such as tetrodotoxin (TTX), saxitoxin (STX), and ephedrine.1-3 Ideally, combining LAs with a synergistic adjuvant would allow the dose of LA to be reduced while increasing block efficacy and decreasing adverse effects such as systemic toxicity and local neurotoxicity. As TTX and STX are not used clinically, and ephedrine is likely to cause adverse cardiovascular effects at dosages necessary for efficient peripheral block, we explored the effects of magnesium sulfate (MgSO4) as an adjuvant to clinically applied LAs.
MgSO4 is a n-methyl-d-aspartate (NMDA) receptor antagonist,4 which is relevant to nociceptive transmission. Indeed, the local anesthetic properties of MgSO4 were first demonstrated more than a century ago,5 followed by a series of intrathecal applications in humans that achieved varying degrees of anesthesia and paralysis.6 These reports were confirmed by in vivo rat studies suggesting that MgSO4 alone elicits sensory and motor blockade.7-9 Recent reports suggest that coadministration of MgSO4 with lidocaine for intravenous regional anesthesia results in shorter onset times and longer block duration.10
We therefore hypothesized that the addition of MgSO4 to amide-type LAs would prolong sciatic nerve blockade (assessed by motor, sensory, and proprioceptive blockade) in rats. Furthermore, we sought to identify the mechanism of interaction between MgSO4 and lidocaine as the prototype LA at neuronal Na+ channels by whole-cell patch-clamp recordings.
Methods
Drugs
MgSO4 was purchased from Sigma Chemical Co., St. Louis, MO. Lidocaine, bupivacaine, and ropivacaine (HCl form) were gifts from AstraZeneca, Inc., Waltham, MA. For sciatic nerve blockade, MgSO4, lidocaine, bupivacaine, and ropivacaine were dissolved in 0.9% sodium chloride. The addition of MgSO4 insignificantly changed the pH of the final solution, e.g., from 6.61 (2% lidocaine) to 6.73 (2% lidocaine combined with 5% MgSO4), from 5.71 (0.25% bupivacaine) to 5.89 (0.25% bupivacaine combined with 5% MgSO4), and from 4.99 (0.5% ropivacaine) to 5.48 (0.5% ropivacaine combined with 5% MgSO4). Upon local injection, the relatively low pH of these pure solutions is likely to be buffered quickly by the tissue fluid, which has a pH of 7.4. Furthermore, no precipitation was detectable macro- or microscopically (1000x magnification).
For the electrophysiology recordings, lidocaine and MgSO4 were dissolved in external solution (bath solution)
All drugs were freshly prepared the morning of the experiments, at the concentrations specified.
Subfascial Sciatic Nerve Injections
The animal experimental protocol was approved by the Standing Committee on Animals of Mackay Memorial Hospital, Taipei, Taiwan. Male Sprague-Dawley rats were purchased from BioLASCO (Taipei, Taiwan) and kept in animal housing facilities with controlled relative humidity (20%-30%), at room temperature (24°C), and in a 12-hour (6:00 AM-6:00 PM) light-dark cycle. Rats were handled before the procedures to familiarize them with the experimental environment and to minimize stress-induced analgesia. At the time of injections, animals weighed 250-300 grams and showed no signs of neurobehavioral impairment. The experimenter was blinded to the drug and concentration used. For subfascial sciatic nerve blockade, rats were anesthetized by inhalation of 1-2% isoflurane, and the sciatic nerves were exposed by lateral incision of the thighs and division of the superficial fascia and muscle. A volume of 0.2 mL of the test dose was injected with a 30-G needle attached to a tuberculin syringe directly beneath the clear fascia surrounding the nerve, but outside the perineurium, proximal to the sciatic bifurcation. The test doses comprised three groups of LAs, consisting of 2% lidocaine, 0.25% bupivacaine, or 0.5% ropivacaine, which were administered alone or in combination with 1.25%, 2.5%, or 5% of MgSO4, respectively. Similarly, we studied the administration of 1% lidocaine, 0.125% bupivacaine, or 0.25% ropivacaine, alone or in combination with 5% of MgSO4 (n = 8 per group). Of note, all combinations were prepared at a double concentration and mixed in a ratio of 1:1, in order to yield the stated final concentration. In addition, we injected MgSO4 alone at a concentration of 5%, 10%, or 20% (n = 8 per group). The superficial muscle layer was sutured with 4-0 silk, and the wound was closed as described elsewhere.3;11
Neurobehavioral Examination
We evaluated proprioception, motor function, and nociception as for previous reports.3;11 Initially, rats were examined before injection, 15 min after drug administration, and at 15-min intervals until complete recovery.
Briefly, proprioception was evaluated by assessing a hopping response, graded as 0 indicating normal or baseline, 1 = minimally impaired, 2 = moderately impaired, and 3 = severely impaired. The animal’s hind limbs were placed on the observation bench, the front half of the animal was lifted off the ground, and the animal’s body was moved laterally. The animal then continuously hopped with the weight-bearing limb in the direction of movement to compensate for imbalance.
We evaluated motor function by holding the rat upright with the hind limb extended so that the distal metatarsus and toes supported the animal’s weight; the extensor postural thrust was recorded as the gram force applied to a digital platform balance (Ohaus Lopro, Fisher Scientific, Florham Park, NJ). The reduction in this force, representing reduced extensor muscle contraction due to motor blockade, was calculated as a percentage of the control force (preinjection control value range was 145 to 165 grams). The obtained percentage value was assigned a score: 0 = no block or baseline; 1 = minimal block, force between preinjection control value of 100% and 50% thereof; 2 = moderate block, force between 50% of the preinjection control value and 20 grams (∼20 grams representing the approximate weight of the flaccid limb); 3 = complete block, force ≤ 20 grams.
We evaluated nociception by the withdrawal reflex or vocalization to pinch of a skin fold over the lateral metatarsus (cutaneous pain) and of the distal phalanx of the fifth toe (deep pain). We graded the combination of withdrawal reflex and vocalization on a scale of 0 to 3 and repeated the examination three times; the average was used. Grading was performed as above on a scale of 0 to 3, where 0 indicates no block or baseline, 1 = minimal block, 2 = moderate block, and 3 = complete block.
For comparison of groups, we defined the complete block time (CBT) as time from injection to the first signs of recovery and the complete recovery time (CRT) as time from injection to the time of complete recovery of function.
Whole-Cell Patch Clamp
The whole-cell configuration of the patch-clamp technique was used to record macroscopic Na+ currents at room temperature (22 ± 1°C). Pipette electrodes were fabricated with a tip resistance ranging from 0.8 to 1.2 mV. Command voltages were controlled by pCLAMP software (Axon Instruments, Inc., Foster City, CA) and delivered by a List-EPC7 patch-clamp amplifier (List-Electronic, Darmstadt/Eberstadt, Germany). Data were filtered at 5 kHz, sampled at 50 kHz, collected, and stored with pCLAMP software. Leak and capacitance currents were subtracted by P/-4 protocol. Pipette electrodes were filled with an internal solution containing 100 mM NaF, 30 mM NaCl, 10 mM EGTA, and 10 mM HEPES titrated with CsOH to pH 7.2. The external (bath) solution consisted of 85 mM choline Cl, 65 mM NaCl, 2 mM CaCl2, and 10 mM HEPES titrated with tetramethylammonium-hydroxide to pH 7.4. Whole-cell recordings can be maintained for more than 1 h in this preparation with little or no run-down of the Na+ current.
Cell Culture
Rat clonal pituitary GH3 cells were purchased from the American Type Culture Collection (Rockville, MD). Cells were split twice a week and maintained in Dulbecco modified Eagle medium (Hyclon Labs, Logan, UT) supplemented with taurin (1%), penicillin-streptomycin (1%), HEPES (20 mM), and heat-inactivated fetal bovine serum (10%), as described previously.12 The 35-mm culture dishes in which the cells were grown also were used as recording chambers.
Statistical Analysis
Non-parametric one-way analysis of variance (Kruskal-Wallis test) was used to test for overall mean differences in proprioceptive, motor, and nociceptive CBT and CRT values among different MgSO4 concentrations with each of the three LAs. If the test showed significance (p value < 0.05), the post-hoc test of Bonferroni adjustment was performed. There are four (k) MgSO4 concentrations to perform multiple comparisons, hence pairwise comparison demonstrated significance if the p value was less than 0.004 (α/k(k-1) = 0.05/4(3) = 0.004).
Results
Rat Sciatic Nerve Blockade
Addition of MgSO4 to either 2% lidocaine (fig. 1), 0.25% bupivacaine (fig. 2), or 0.5% ropivacaine (fig. 3) shortened the sciatic nerve block duration, at a higher degree/significance level with increasing MgSO4 concentrations (table 1). For example, the mean CBT of proprioception was significantly shortened when 2.5% or 5% MgSO4 was added to LAs, but not when 1.25 % MgSO4 was added to bupivacaine. MgSO4(5%, 10% or 20%) injected alone did not elicit a discernible rat sciatic nerve blockade. Similarly, 1% lidocaine, 0.125% bupivacaine, or 0.25% ropivacaine, in combination with 5% of MgSO4, elicited significantly shorter block than when administered alone (data not shown).
Fig. 1.
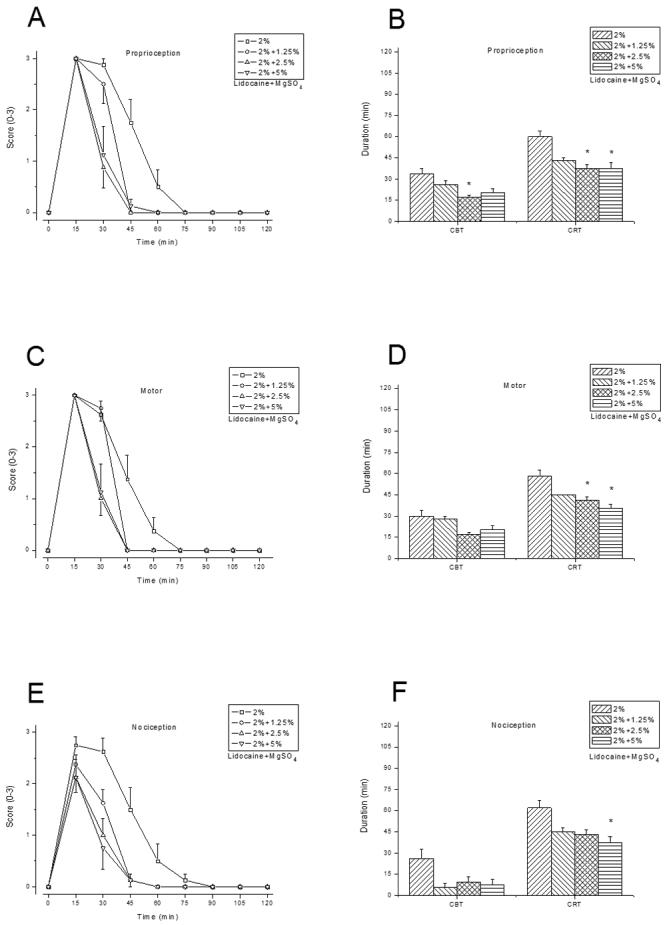
Rat sciatic nerve blockade by 2% lidocaine combined with 0%, 1.25%, 2.5%, or 5% MgSO4 (n = 8/group). Time courses of proprioceptive, motor, and nociceptive blockade by lidocaine combined with MgSO4 at various concentrations are shown in (A), (C), and (E), respectively. A score of 0 indicates no block or baseline, and a score of 3 indicates complete blockade. The complete blockade time (CBT) and complete recovery time (CRT) in minutes for proprioceptive, motor, and nociceptive function are shown in (B), (D), and (F). Data are presented as mean ± SEM. * P < 0.004 (lidocaine alone vs. lidocaine with MgSO4 added).
Fig. 2.
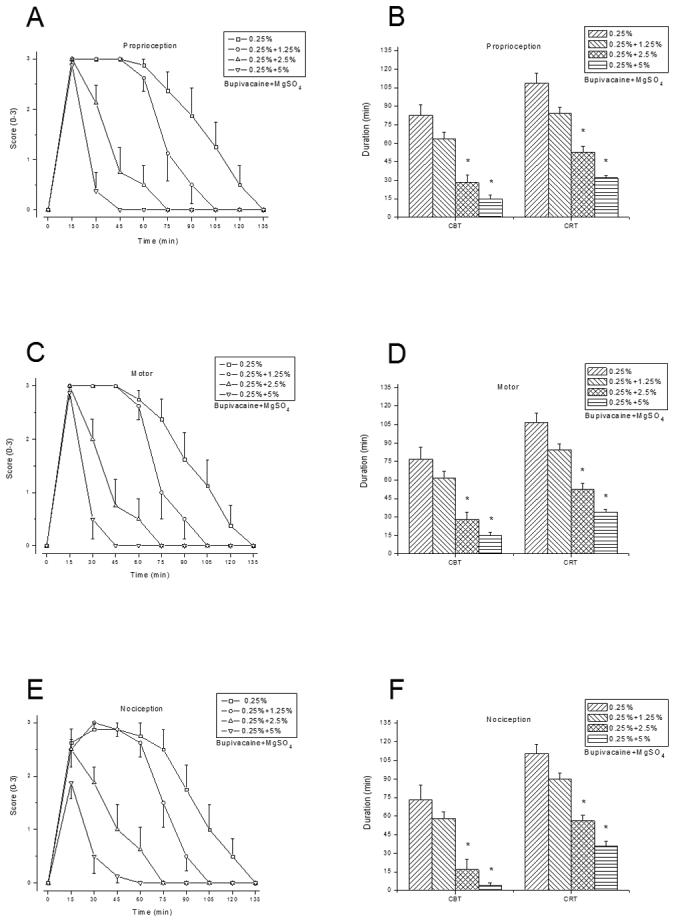
Rat sciatic nerve blockade by 0.25% bupivacaine combined with 0%, 1.25%, 2.5%, or 5% MgSO4 (n = 8/group). Time courses of block (A), (C), and (E), CBT and CRT (B), (D), and (F), as outlined in figure 1. Data are presented as mean ± SEM. * P < 0.004 (bupivacaine alone vs. bupivacaine with MgSO4 added).
Fig. 3.
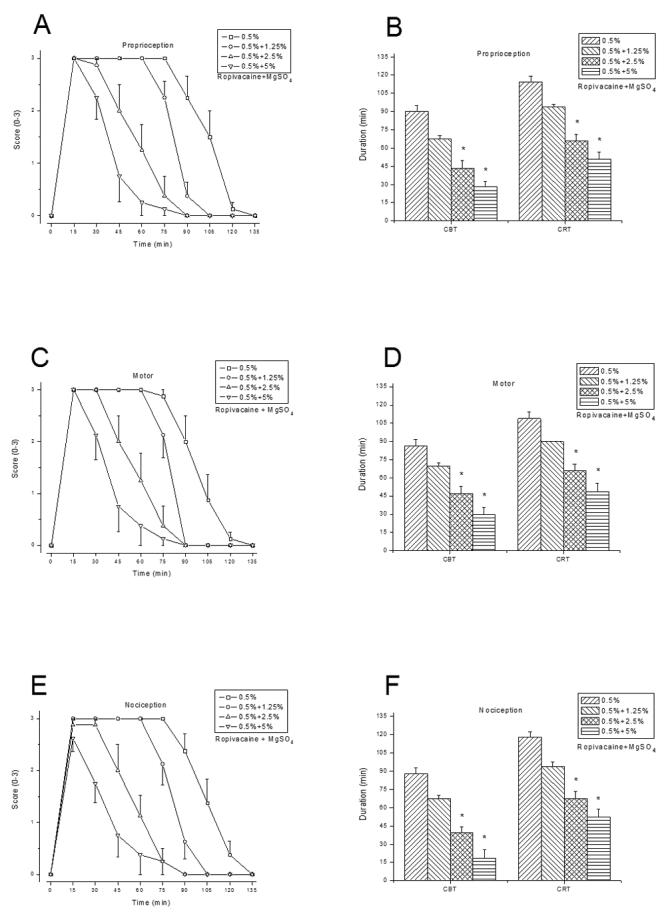
Rat sciatic nerve blockade by 0.5% ropivacaine combined with 0%, 1.25%, 2.5%, or 5% MgSO4 (n = 8/group). Time courses of block (A), (C), and (E), CBT and CRT (B), (D), and (F), as outlined in figure 1. Data are presented as mean ± SEM. * P < 0.004 (ropivacaine alone vs. ropivacaine with MgSO4 added).
Table 1.
Kruskal-Wallis one-way ANOVA and pairwise comparisons
| Local anesthetic | Function | Blockade time | Hc Statistics | p-value |
p-value (pairwise comparisons* between different concentrations of magnesium sulfate) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0% v.s. 1.25% | 0% v.s. 2.5% | 0% v.s. 5% | 1.25% v.s. 2.5% | 1.25% v.s. 5% | 2.5% v.s. 5% | |||||
| 2% Lidocaine | Proprioception | CBT | 12.759 | 0.005 | 0.925 | 0.002 | 0.029 | 0.078 | 0.735 | 1.000 |
| CRT | 14.899 | 0.002 | 0.023 | 0.002 | 0.004 | 1.000 | 0.859 | 1.000 | ||
| Motor | CBT | 11.134 | 0.011 | 1.000 | 0.017 | 0.217 | 0.021 | 0.253 | 1.000 | |
| CRT | 17.036 | 0.001 | 0.065 | 0.003 | <0.001 | 1.000 | 0.038 | 0.525 | ||
| Nociception | CBT | 7.773 | 0.051 | 0.066 | 0.257 | 0.111 | 1.000 | 1.000 | 1.000 | |
| CRT | 12.820 | 0.005 | 0.074 | 0.028 | 0.001 | 1.000 | 0.686 | 1.000 | ||
| 0.25% Bupivacaine | Proprioception | CBT | 23.417 | <0.001 | 0.697 | <0.001 | <0.001 | 0.001 | <0.001 | 0.379 |
| CRT | 26.490 | <0.001 | 0.030 | <0.001 | <0.001 | 0.005 | <0.001 | 0.001 | ||
| Motor | CBT | 22.647 | <0.001 | 1.000 | <0.001 | <0.001 | 0.001 | <0.001 | 0.391 | |
| CRT | 25.994 | <0.001 | 0.044 | <0.001 | <0.001 | 0.001 | <0.001 | 0.005 | ||
| Nociception | CBT | 19.745 | <0.001 | 1.000 | <0.001 | <0.001 | 0.006 | <0.001 | 1.000 | |
| CRT | 25.554 | <0.001 | 0.145 | <0.001 | <0.001 | <0.001 | <0.001 | 0.016 | ||
| 0.5% Ropivacaine | Proprioception | CBT | 24.249 | <0.001 | 0.019 | <0.001 | <0.001 | 0.004 | <0.001 | 0.203 |
| CRT | 24.640 | <0.001 | 0.024 | <0.001 | <0.001 | <0.001 | <0.001 | 0.423 | ||
| Motor | CBT | 22.403 | <0.001 | 0.165 | <0.001 | <0.001 | 0.014 | <0.001 | 0.228 | |
| CRT | 24.206 | <0.001 | 0.032 | <0.001 | <0.001 | 0.002 | <0.001 | 0.364 | ||
| Nociception | CBT | 25.884 | <0.001 | 0.009 | <0.001 | <0.001 | <0.001 | <0.001 | 0.091 | |
| CRT | 24.652 | <0.001 | 0.008 | <0.001 | <0.001 | 0.002 | <0.001 | 0.432 | ||
Bonferroni adjustment shows significance with p-value < 0.004.
Voltage-dependent inactivation of Na+ channels by MgSO4
This experiment was designed to determine the steady-state (h∞) inactivation of Na+ channels when lidocaine alone, MgSO4 alone, or the combination was added to the bath solution.
The addition of 300 μM lidocaine to the bath solution produced a 4.8 mV left shift of the steady-state inactivation curve (fig. 4A), which is typical for LAs. However, the addition of 10 mM MgSO4 to the bath solution produced no shift of the inactivation curve (fig. 4B), and the addition of 300 μM lidocaine combined with 10 mM MgSO4 to the bath solution had the same effect as lidocaine alone, indicating the absence of a synergistic interaction (fig. 4C).
Fig. 4.
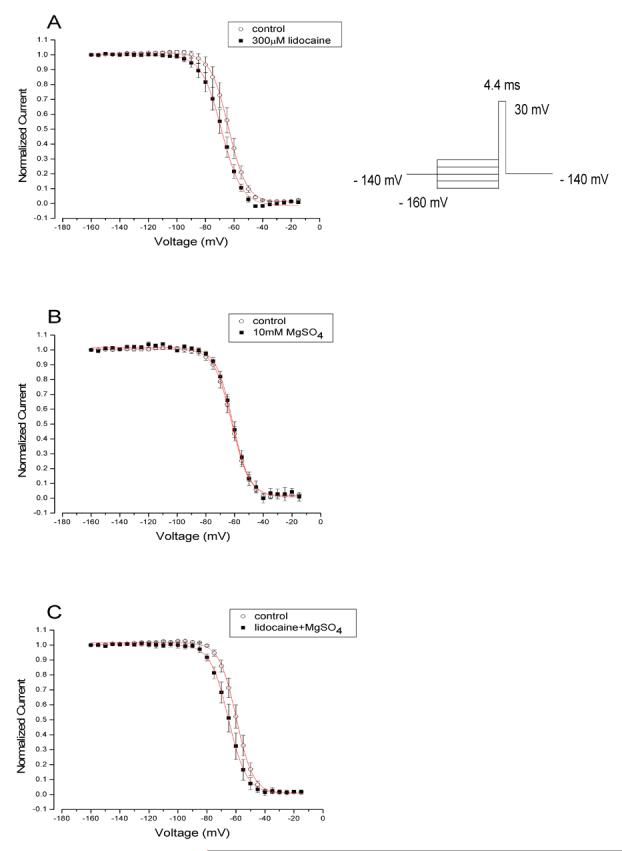
Na+ current inhibition by lidocaine alone, lidocaine combined with MgSO4, and MgSO4 alone (n = 5 cells/group; data are presented as mean ± SEM). The respective pulse protocol is inserted above the representative tracings. Conditioning prepulses ranging in amplitude from -160 to -15 mV were applied. Na+ currents were evoked by the delivery of the test pulse to -30 mV. Pulse protocol is inserted on the top of the figure.
(A) Normalized Na+ current in the absence (control) or presence of 300 μM lidocaine was plotted against conditioning prepulse potential. Data were fitted well with a Boltzmann function. The average V0.5 value (50% availabilities) and KE (a slope factor) values for the fitted Boltzmann functions were -63.8 ± 0.14 mV and 6.3 ± 0.13 for control and -68.7 ± 0.24 mV and 7.0 ± 0.21 for lidocaine, respectively.
(B) Voltage-dependent block of Na+ channels by 10 mM MgSO4 alone. No change in inactivation occurred. The average V0.5 value (50% availabilities) and KE (a slope factor) values for the fitted Boltzmann functions were -61.5 ± 0.23 mV and 5.9 ± 0.2 for control and -62.0 ± 0.13 mV and 6.1 ± 0,13 for MgSO4, respectively.
(C) Voltage-dependent block of Na+ channels by 300 μM lidocaine combined with 10 mM MgSO4. The average V0.5 value (50% availabilities) and KE (a slope factor) values for the fitted Boltzmann functions were -59.8 ± 0.16 mV and 5.8 ± 0.1 for control and lidocaine combined with MgSO4, respectively, and -65.2 ± 0.14 mV and 6.2 ± 0.1, respectively.
Discussion
The principal result of the present study is that, unexpectedly, MgSO4 coadministered with LAs significantly shortened the duration of sciatic nerve block in vivo and did not affect the steady-state inactivation shift induced by lidocaine in vitro. We further note that attenuation of conduction block was similar for all three LAs tested, and that MgSO4 injected at concentrations up to 20% did not elicit peripheral nerve blockade in vivo.
The mechanism by which MgSO4 attenuates LA-induced block is unclear. We found no evidence in our data of a direct action of MgSO4 with LAs at the LA binding sites of Na+ channels. However, an interaction between the divalent cation Mg2+ and the Na+ channel blockers TTX and STX has been conjectured, presumably due to conformational changes in binding sites.13;14
Magnesium divalent cations have been shown to affect the negative surface charge near neuronal Na+ channels.15 Our data are also in contrast to a previous investigation that found an enhancement of block induced by LAs (lidocaine, benzocaine, and QX-572) by magnesium in isolated frog sciatic nerves in vitro.16 We found neither an enhancing effect of MgSO4 on lidocaine-induced nerve block, nor a shift of the inactivation curve in neuronal Na+ channels by MgSO4 at a concentration of 10 mM. This apparent discrepancy in results may be explained by the difference in species and experimental setup (isolated desheated frog nerve versus our in vivo rat sciatic nerve block model). Of note, lidocaine, benzocaine, and QX-572 were found to have different effects depending on the type of stimulation, e.g., frequency-dependent block (response to repetitive stimulation) with benzocaine did not show a change when the concentration of magnesium was changed from 3mM to 10 mM and even 20 mM, or with lidocaine when the concentration of magnesium was changed from 3mM to 10 mM.16 Also, the attenuating effect of MgSO4 upon sciatic nerve block was evident when added to any of three amide-type LAs and at different concentrations of MgSO4, and we therefore tested along converging lines of evidence.
Another possible contribution to the effect of MgSO4 in shortening sciatic block duration in vivo could involve local vaso-dilatation in the perineural injection compartment. As MgSO4 most likely vasodilates the tissues around the injection site, it will accelerate systemic uptake of LA, thereby shortening block duration. A dramatic example of the influence of changes in regional blood flow in the perineural musculature on sciatic block durations has been elegantly shown using different formulations: Coinjection of epinephrine with TTX, a naturally occurring Na+ channel blocker, prevented TTX-induced increases in perisciatic muscle blood flow and thereby prolonged block.17 We note that any type of mechanism that involves actions of magnesium on the pharmacokinetics of LA entry into the nerve or on LA systemic uptake and distribution would be consistent with our demonstration of an effect of MgSO4 on rat sciatic block duration in vivo and our inability to demonstrate effects of MgSO4 on Na+ channel inactivation in the patch clamp experiments in vitro. Also, it should be considered that MgSO4 has multiple electrophysiological properties, e.g., it acts on potassium channels and calcium channels as well as NMDA18 receptors, so that the magnitude of each single mechanism may be different under distinct circumstances.
Two clinical studies assessed the efficacy of a combination of MgSO4 with lidocaine for intravenous regional anesthesia. In a collective of chronic pain patients, lidocaine combined with MgSO4 provided a more thorough and long-lasting block than lidocaine alone.19 Similarly, Turan et al. also demonstrated superior block characteristics when MgSO4 was used as an adjuvant to lidocaine in elective surgical patients.10 These results differ from ours, probably due to the antagonistic action of MgSO4 on NMDA receptors. Therefore, peripheral NMDA receptors may contribute to a synergistic effect in wound infiltration 20;21, although the clinical relevance of this interaction remains unclear. However, in our studies the lack of effect of MgSO4 appears to be the product of non-interaction with Na+ channels, and peripheral nerves do not contain NMDA receptors, at least not where the drug was injected, next to the nerve trunk. In agreement with our results, Lee et al. found no enhancement of brachial plexus nerve blockade when applying the NMDA antagonist ketamine combined with ropivacaine.22
Furthermore, it has been reported that MgSO4 was successfully used to resuscitate a patient suffering from an overdose of the tricyclic antidepressant amitriptyline, in itself a potent Na+ channel blocker.23 Similarly, bupivacaine-induced toxicity in the central nervous system and heart can be attenuated by MgSO4.24;25 These studies also provide some evidence for an antagonism between MgSO4 and Na+ channel blockers.
In conclusion, MgSO4 coadministered with amide LAs significantly shortens the duration of sciatic nerve blockade in rats. We suggest that MgSO4 is not a useful adjuvant in peripheral nerve blockade. The mechanism of this observed antagonism is independent of an antagonistic interaction of either lidocaine, bupivacaine, or ropivacaine with MgSO4 at the Na+ channel.
Acknowledgments
Identification of Financial Source: This study was supported by Mackay Memorial Hospital (research grant no. MMH95116 to YCH) and National Institutes of Health, Bethesda, MD (research grant no. GM64051 to PG).
Portions of this paper were presented at the Annual Meeting of American Society of Anesthesiologists, Chicago, October 14-18, 2006.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Kohane DS, Smith SE, Louis DN, Colombo G, Ghoroghchian P, Hunfeld NG, Berde CB, Langer R. Prolonged duration local anesthesia from tetrodotoxin-enhanced local anesthetic microspheres. Pain. 2003;104:415–421. doi: 10.1016/s0304-3959(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 2.Barnet CS, Tse JY, Kohane DS. Site 1 sodium channel blockers prolong the duration of sciatic nerve blockade from tricyclic antidepressants. Pain. 2004;110:432–438. doi: 10.1016/j.pain.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 3.Hung YC, Kau YC, Zizza AM, Edrich T, Zurakowski D, Myers RR, Wang GK, Gerner P. Ephedrine blocks rat sciatic nerve in vivo and sodium channels in vitro. Anesthesiology. 2005;103:1246–1252. doi: 10.1097/00000542-200512000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 5.Meltzer SJ, Auer J. Physiological and pharmacological studies on magnesium salts. III. Narcotizing effect of magnesium salts upon nerve fibers. Am J Physiol. 1906;16:233–251. [Google Scholar]
- 6.Houbold HA, Meltzer SJ. Spinal anesthesia by magnesium sulfate. A report of seven operations performed under its influence. JAMA. 1906;46:647–650. [Google Scholar]
- 7.Bahar M, Chanimov M, Grinspun E, Koifman I, Cohen ML. Spinal anaesthesia induced by intrathecal magnesium sulphate. Anaesthesia. 1996;51:627–633. doi: 10.1111/j.1365-2044.1996.tb07843.x. [DOI] [PubMed] [Google Scholar]
- 8.Ishizaki K, Sasaki M, Karasawa S, Obata H, Nara T, Goto F. The effect of intrathecal magnesium sulphate on nociception in rat acute pain models. Anaesthesia. 1999;54:241–246. doi: 10.1046/j.1365-2044.1999.00741.x. [DOI] [PubMed] [Google Scholar]
- 9.Chanimov M, Cohen ML, Grinspun Y, Herbert M, Reif R, Kaufman I, Bahar M. Neurotoxicity after spinal anaesthesia induced by serial intrathecal injections of magnesium sulphate. An experimental study in a rat model. Anaesthesia. 1997;52:223–228. doi: 10.1111/j.1365-2044.1997.034-az0057.x. [DOI] [PubMed] [Google Scholar]
- 10.Turan A, Memis D, Karamanlioglu B, Guler T, Pamukcu Z. Intravenous regional anesthesia using lidocaine and magnesium. Anesth Analg. 2005;100:1189–1192. doi: 10.1213/01.ANE.0000145062.39112.C5. [DOI] [PubMed] [Google Scholar]
- 11.Kau YC, Hung YC, Zizza AM, Zurakowski D, Greco WR, Wang GK, Gerner P. Efficacy of lidocaine or bupivacaine combined with ephedrine in rat sciatic nerve block. Reg Anesth Pain Med. 2006;31:14–18. doi: 10.1016/j.rapm.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Cota G, Armstrong CM. Sodium channel gating in clonal pituitary cells. The inactivation step is not voltage dependent. J Gen Physiol. 1989;94:213–232. doi: 10.1085/jgp.94.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grissmer S. Effect of various cations and anions on the action of tetrodotoxin and saxitoxin on frog myelinated nerve fibers. Pflugers Arch. 1984;402:353–359. doi: 10.1007/BF00583935. [DOI] [PubMed] [Google Scholar]
- 14.Lonnendonker U. Use-dependent block with tetrodotoxin and saxitoxin at frog Ranvier nodes. II. Extrinsic influence of cations. Eur Biophys J. 1991;20:143–149. doi: 10.1007/BF01561136. [DOI] [PubMed] [Google Scholar]
- 15.Hille B, Woodhull AM, Shapiro BI. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975;270:301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- 16.Akutagawa T, Kitahata LM, Saito H, Collins JG, Katz JD. Magnesium enhances local anesthetic nerve block of frog sciatic nerve. Anesth Analg. 1984;63:111–116. [PubMed] [Google Scholar]
- 17.Masuda T, Cairns BE, Sadhasivam S, Dunning PS, Berde CB. Epinephrine prevents muscle blood flow increases after perineural injection of tetrodotoxin. Anesthesiology. 2004;101:1428–1434. doi: 10.1097/00000542-200412000-00025. [DOI] [PubMed] [Google Scholar]
- 18.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 19.Tramer MR, Glynn CJ. Magnesium Bier’s block for treatment of chronic limb pain: a randomised, double-blind, cross-over study. Pain. 2002;99:235–241. doi: 10.1016/s0304-3959(02)00105-7. [DOI] [PubMed] [Google Scholar]
- 20.Li J, McRoberts JA, Nie J, Ennes HS, Mayer EA. Electrophysiological characterization of N-methyl-D-aspartate receptors in rat dorsal root ganglia neurons. Pain. 2004;109:443–452. doi: 10.1016/j.pain.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Tverskoy M, Oren M, Vaskovich M, Dashkovsky I, Kissin I. Ketamine enhances local anesthetic and analgesic effects of bupivacaine by peripheral mechanism: a study in postoperative patients. Neurosci Lett. 1996;215:5–8. doi: 10.1016/s0304-3940(96)12922-0. [DOI] [PubMed] [Google Scholar]
- 22.Lee IO, Kim WK, Kong MH, Lee MK, Kim NS, Choi YS, Lim SH. No enhancement of sensory and motor blockade by ketamine added to ropivacaine interscalene brachial plexus blockade. Acta Anaesthesiol Scand. 2002;46:821–826. doi: 10.1034/j.1399-6576.2002.460711.x. [DOI] [PubMed] [Google Scholar]
- 23.Citak A, Soysal DD, Ucsel R, Karabocuoglu M, Uzel N. Efficacy of long duration resuscitation and magnesium sulphate treatment in amitriptyline poisoning. Eur J Emerg Med. 2002;9:63–66. doi: 10.1097/00063110-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Solomon D, Bunegin L, Albin M. The effect of magnesium sulfate administration on cerebral and cardiac toxicity of bupivacaine in dogs. Anesthesiology. 1990;72:341–346. doi: 10.1097/00000542-199002000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Okutomi T, Saito M, Matsumoto Y, Shimizu M, Fukuoka M, Hoka S. Altered bupivacaine pharmacokinetics by MgSO4 in rats. Can J Anaesth. 2004;51:93–94. doi: 10.1007/BF03018565. [DOI] [PubMed] [Google Scholar]


