Abstract
Acute activation of the hypothalamic-pituitary-adrenal (HPA) axis releases glucocorticoids to maintain homeostasis, whereas prolonged exposure to elevated glucocorticoids has deleterious effects. Due to the potential benefits of limiting stress-induced glucocorticoid secretion, the present study uses drinking in dehydrated rats as a model to delineate mechanisms mobilized to rapidly inhibit HPA activity during stress. Using Fos expression as an indicator of neuronal activation, the effect of a single or repeated episode of dehydration-induced drinking on the activity of magnocellular and parvocellular neurons in the paraventricular nucleus (PVN) of the hypothalamus was examined. Adult male rats underwent a single episode or repeated (six) episodes of water restriction and were sacrificed before or after drinking water in the AM. Plasma osmolality, vasopressin (AVP), adrenocorticotropic hormone (ACTH) and corticosterone were elevated by water restriction and reduced after drinking in both models. Fos expression was elevated in AVP-positive magnocellular PVN neurons and AVP- and corticotropin releasing hormone (CRH)-positive parvocellular PVN neurons after water restriction. Fos expression was reduced in magnocellular AVP neurons after both models of restriction-induced drinking. In contrast, Fos expression did not change in AVP and CRH parvocellular neurons after a single episode of restriction-induced drinking, but was reduced after repeated episodes of restriction-induced drinking. These data indicate that drinking-induced decreases in glucocorticoids in dehydrated rats involve multiple factors including reduction in magnocellular release of vasopressin and reduction in parvocellular neuronal activity. The differential inhibition of PVN parvocellular neurons after repeated rehydration may reflect a conditioned response to repeated stress reduction.
Keywords: Fos, stress, stress relief, glucocorticoids, ACTH, vasopressin, corticotropin-releasing hormone, dehydration, rehydration, magnocellular neurons, parvocellular neurons
INTRODUCTION
Significant progress has been made in understanding the neural circuitry that supports stress-induced activation of the hypothalamic-pituitary-adrenal (HPA) axis and secretion of glucocorticoids (for recent reviews, see (Herman et al., 2003; Pecoraro et al., 2006). The HPA axis is comprised of parvocellular neurons in the medial division of the paraventricular nucleus (mpPVN) of the hypothalamus that synthesize corticotropin releasing hormone (CRH) and arginine vasopressin (AVP) that are released into the portal blood system of the pituitary. Together, CRH and AVP act synergistically to stimulate the secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary (Antoni, 1986). Plasma ACTH in turn stimulates the synthesis and secretion of glucocorticoids from the adrenal gland (Hall, 2001). Increases in glucocorticoids following stress are critical for supporting metabolic, cardiovascular, immunologic and neural responses induced by threats to tissue viability and survival (Tsigos and Chrousos, 2002). Reducing glucocorticoids is equally important, since prolonged or repeated exposure to elevated glucocorticoids results in severe detrimental consequences including depression, hypertension, osteoporosis, immunosuppression and metabolic disease (Chrousos and Gold, 1998). Understanding mechanisms that could be initiated to limit the stress response would be beneficial. However, less attention has been directed toward examining the reduction of HPA axis activity from an elevated state.
Classically, inhibition of the HPA axis is thought to occur through glucocorticoid negative feedback (Keller-Wood and Dallman, 1984); however, rapid inhibition of the HPA axis also may occur through modulating neural activity in pathways that project to the PVN, independent of hormonal feedback (Bali et al., 2005). To begin to delineate inhibitory pathways to the PVN which when activated may reduce stress, we have utilized a model in rats that demonstrates rapid decreases in HPA activity from an elevated state (Wotus and Engeland, 2003, Wotus et al., 2007). Disturbances in fluid homeostasis activate the HPA axis as reflected by increases in plasma ACTH and corticosterone (Aguilera et al., 1993; Wotus et al., 2007) and increased Fos protein expression in parvocellular PVN neurons (Gottlieb et al., 2006; Stocker et al., 2004; Wotus et al., 2007). Ingestion of fluid after repeated water restriction results in an immediate reduction of plasma corticosterone, the primary glucocorticoid in rodents (Gray et al., 1978; Heybach and Vernikos-Danellis, 1979; Wilkinson et al., 1982; Wotus and Engeland, 2003). Water restriction differs from most models of dehydration in that animals are rehydrated daily. In rats restricted to drinking for 30 min daily in the PM for 6 days, plasma osmolality, ACTH, corticosterone and vasopressin are increased during the day prior to rehydration and are reduced following drinking for 15 min (Wotus et al., 2007). We have used labeling for Fos and phenotypic markers to define neuronal sub-populations within the PVN that respond to drinking after repeated water restriction (Wotus et al., 2007). A 6-day period of restriction in the PM induces Fos expression in AVP-positive posterior magnocellular PVN (pmPVN) neurons, AVP-positive mpPVN neurons, but not CRH-positive mpPVN neurons. Restriction-induced drinking reduces Fos expression in AVP magnocellular and parvocellular neurons, but does not change Fos expression in CRH parvocellular neurons (Wotus et al., 2007). Collectively, these data show that during water restriction, rats undergo daily episodes of dehydration followed by rehydration. Furthermore, these data demonstrate that water restriction represents a model in which the HPA axis is activated and rapidly inhibited daily. Whereas previous studies have examined the effect of rehydration following repeated water restriction (Gray et al., 1978; Heybach and Vernikos-Danellis, 1979; Wilkinson et al., 1982; Wotus and Engeland, 2003), no studies have compared the HPA response to rehydration after a single and a repeated episode of dehydration.
Numerous studies show that the responsiveness of the HPA axis changes in response to repeated stress. For example, repeated exposure to a stressor can result in attenuated ACTH and corticosterone responses (Hauger et al., 1990; Li and Sawchenko, 1998; Bhatnagar et al., 2002), and reduced Fos mRNA (Watanabe et al., 1994; Bonaz and Rivest, 1998) and protein (Viau and Sawchenko, 2002) expression in PVN neurons. These data suggest that repeated activation induces functional changes in the HPA axis. In contrast, there have been few studies addressing how repeated inhibition affects HPA activity. We are not aware of any studies that directly examine mechanisms that mediate inhibition of the HPA axis after an acute versus a repeated stress. It is possible that repeated episodes of dehydration followed by rehydration, which repeatedly activate and inhibit the HPA axis, respectively, alter the sensitivity of parvocellular PVN neurons to inhibitory stimuli resulting in rapid suppression of neural activity when drinking ensues. The present study is designed to test the hypothesis that repeated episodes of restriction-induced drinking, but not a single episode of restriction-induced drinking, inhibits parvocellular PVN neurons. In the first set of experiments, we used double-label immunohistochemistry for Fos and CRH or Fos and AVP to examine subpopulations of PVN neurons following a single 23.5h episode of water restriction-induced drinking or repeated episodes of water restriction-induced drinking. In the second set of experiments, we exposed rats to a single 47.5h episode of water restriction-induced drinking to determine whether a longer duration of water restriction prior to a single episode of restriction-induced drinking would affect Fos expression in PVN neurons. In each model we measured plasma osmolality, AVP, and hematocrit to reflect the level of dehydration, and plasma ACTH and corticosterone to reflect the activity of the HPA axis.
As noted above, our previous work assessing HPA responses to dehydration and rehydration utilized a PM water restriction model (Wotus et al., 2003; Wotus and Engeland 2003; Wotus et al., 2007); rats had access to water in the PM, just before lights out. The present experiments use an AM water restriction model in which rats receive access to water in the AM, shortly after lights on. This change in protocol was implemented for a number of reasons. First, the circadian rhythm of corticosterone is at its nadir in the AM allowing increases in HPA activity caused by water restriction to be readily detected. Second, the majority of experiments that examine the effects of a single episode of dehydration on the HPA axis are performed in the AM (Heybach and Vernikos-Danellis, 1979; Doell et al., 1981; Wilkinson et al., 1982; Kiss et al., 1994; Aguilera et al., 1993). In comparing a single to repeated episodes of dehydration in the AM, our goal was to directly compare our data to earlier results and at the same time, to expand our model of water restriction so that mechanisms mediating inhibition of the HPA axis could be further delineated.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (175-200 g; Charles River, Wilmington, MA) were housed two per cage under a 12-h light, 12-h dark cycle (lights on at 0530h) with food and water available ad libitum prior to initiation of the water restriction schedule. Experiments were initiated at least 2 to 3 days after arrival. Animals were maintained and cared for in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Experimental procedures were approved by the University of Minnesota Animal Care and Use Committee.
Experiment 1: Effect of a single 23.5h episode of water restriction followed by rehydration versus repeated 23.5h episodes of water restriction and rehydration on HPA hormones
The initial experiment was performed to compare a single episode versus a repeated episode of water restriction-induced drinking on inhibition of the HPA axis. Water restriction consisted of 23.5h water deprivation followed by 30 min access to water. Water was removed starting 3h after light onset (0830h; day 0) and was returned the following day 2.5h after light onset (0800h; day 1); control rats received water ad libitum. All rats had access to food at all times. To examine the effects of a single episode of restriction-induced drinking on HPA hormones, control rats (AL; n=6) and a group of water restricted rats (WR; n=6) were sacrificed by decapitation on day 1 at 0800h; another group of water restricted rats was given water and sacrificed after 15 min of drinking (WR+water; n=6). To examine the effects of repeated episodes of restriction-induced drinking on HPA hormones, the remaining water restricted rats were allowed access to water for 30 min each day, 2.5h after light onset, for a period of 6 days. On day 6, control rats (AL; n=6) and a group of water restricted rats (WR; n=6) were sacrificed by decapitation at 0800h; another group of water-restricted rats were sacrificed after 15 min of drinking (WR +Water; n=6). Trunk blood was collected in tubes containing disodium EDTA (2 mg/ml of blood) and used for the measurement of hematocrit (Hct). Plasma was aliquoted for assay of osmolality (Posmol) by vapor pressure osmometry, and for measurement of ACTH, corticosterone, and AVP by RIA.
Experiment 2: Effect of a single 23.5h episode of water restriction followed by rehydration versus repeated 23.5h episodes of water restriction and rehydration on Fos expression in PVN neurons
A second experiment was performed to examine the effects of a single episode and a repeated episode of water restriction-induced drinking on Fos expression in PVN neurons. Water restriction consisted of 23.5h water deprivation followed by 30 min access to water as described in experiment 1. To examine the effects of a single episode of rehydration on Fos expression in PVN neurons, control rats (AL; n=4) and a group of water restricted rats (WR; n=6) were sacrificed by decapitation on day 1 at 0800h; another group of water restricted rats was given water for 30 min and sacrificed 1h after drinking for 15 min (WR+water; n=6). To examine the effects of repeated episodes of rehydration on Fos expression in PVN neurons, the remaining water restricted rats were allowed access to water for 30 min each day, 2.5h after light onset, for a period of 6 days. On day 6, control rats (AL; n=2) and a group of water restricted rats (WR; n=6) were sacrificed by decapitation at 0800h; another group of water-restricted rats were sacrificed 1h after drinking for 15 min (WR+Water; n=6). Brains were rapidly removed and fixed for subsequent immunohistochemical analysis of Fos and AVP or CRH immunoreactivity.
Experiment 3: Effect of a single 47.5h episode of water restriction followed by rehydration on HPA hormones
The next experiment was performed to determine if a 47.5h episode of water restriction-induced drinking results in inhibition of the HPA axis. Water restriction consisted of 47.5h water deprivation followed by 30 min access to water. Water was removed starting 3h after light onset (0830h; day 0) and was returned 47.5h later, 2.5h after light onset (0800h; day 2); control rats received water ad libitum. All rats had access to food at all times. Control rats (AL; n=6) and a group of water-restricted rats (WR; n=6) were sacrificed by decapitation on day 2 at 0800h; another group of water-restricted rats was given water and sacrificed after 15 min of drinking (WR+Water; n=6). Trunk blood was collected and processed as described for experiment 1.
Experiment 4: Effect of a single 47.5h episode of water restriction followed by rehydration on Fos expression in PVN neurons
The final experiment was performed to examine the effects of a 47.5h episode of water restriction-induced drinking on Fos expression in PVN neurons. Water restriction consisted of 47.5h water deprivation followed by a 30 min access to water as described in experiment 3. Control rats (AL; n=4) and a group of water restricted rats (WR; n=8) were sacrificed by decapitation on day 2 at 0800h; another group of water restricted rats was given water for 30 min and sacrificed 1h after drinking for 15 min (WR+Water; n=8). Brains were rapidly removed and fixed for subsequent immunohistochemical analysis of Fos and AVP or CRH immunoreactivity.
Determination of plasma hormones
Plasma ACTH was determined by RIA with 125I labeled ACTH (DiaSorin; Stillwater, MN) as described previously (Jasper and Engeland, 1991). The intra-assay and inter-assay coefficients of variation (CVs) for ACTH were 5.5% and 4.6%, respectively. Plasma corticosterone was determined by an RIA kit (ICN Biochemical, Costa Mesa, CA). The intra-assay and inter-assay CVs for corticosterone were 7.6% and 13.3%, respectively. Plasma AVP was extracted with acetone and petroleum ether (Rose et al., 1981) and measured by RIA using 125I labeled AVP (Perkin Elmer, Boston, MA), synthetic AVP (Bachem, Torrance, CA) and a AVP antibody provided by Dr. Alan Robinson (UCLA, (Verbalis et al., 1986)). The intraassay and inter-assay CVs for AVP were 13.3% and 14.4%, respectively.
Processing of neural tissue
Brains were fixed using a modification of methods described previously (Berod et al., 1981; Khan and Watts, 2004). Briefly, brains were immersed in a near frozen 4% paraformaldehyde solution buffered with sodium acetate (0.1 M, pH 6.0) and incubated while shaking for 7h at 4°C, then transferred to a 4% paraformaldehyde solution buffered with sodium borate (0.1 M, pH 9.5) and incubated for 48h at 4°C. Brains were then transferred into 20% glycerol in phosphate buffer (0.1 M) and incubated at 4°C for 48h, after which time they were cut into segments containing the hypothalamus and frozen in OCT mounting media (Miles, Inc., Elkhart, IN) until sectioning. Brain sections containing the PVN defined using a rat stereotaxic atlas (Paxinos and Watson, 1998) were cut at 40 μm, washed, free floating, in PBS (0.1M) and stored in cryoprotectant (30% ethylene glycol and 20% glycerol in 0.05M PBS) at -20°C until the time of labeling.
Immunohistochemistry
For each animal, serial brain sections containing mpPVN and pmPVN were thawed and washed, and then blocked for 10 min in 3% H2O2 in 10% methanol. After rinsing with PBS, sections were blocked for 1h in 20% normal goat serum (NGS; Vector Laboratories; Burlingame, CA) at room temperature, then incubated for 48h at 4°C with rabbit anti-Fos primary antibody (Ab-5, Oncogene, San Diego, CA) diluted 1:160,000 in PBS-T (0.3% Triton X-100 in 0.1M PBS) with 2% NGS. Using a Vectastain ABC kit (Vector Laboratories), sections were then incubated for 1h at room temperature with goat anti-rabbit biotinylated secondary antibody, followed by 1h incubation with the avidin-biotin complex. Fos labeling was visualized using diaminobenzidine (DAB) with nickel (Vector Laboratories) resulting in a black/purple nuclear staining. Alternate sections from each brain were then double-labeled for either AVP or CRH immunoreactivity using the same procedure described for Fos labeling. Primary rabbit anti-AVP (1:80,000; Chemicon, Temecula, CA) and rabbit anti-CRH (1:160,000; generously provided by Dr. Wylie Vale, The Salk Institute, La Jolla, CA) antibodies were visualized using DAB alone, resulting in brown cytoplasmic staining. Sections were washed, mounted and dried on glass slides, dehydrated and coverslipped in DPX media (Sigma Chemical Co., St. Louis, MO).
Labeling for each of the antibodies was eliminated when the primary antibodies were omitted or preabsorbed with their cognate peptides (50 ug/ml, 4°C, overnight). In addition, when the primary antibody against Fos was omitted, only brown, cytoplasmic labeling for CRH or for AVP remained; conversely, when the primary antibody against CRH or AVP was omitted, only black/purple, nuclear labeling for Fos was observed.
Quantification of Fos labeling
Fos-positive nuclei were counted in two brain sections (4 PVN nuclei) double-labeled for Fos and AVP or Fos and CRH from each animal; the brain sections were at the mid-rostrocaudal level of the PVN that included the pmPVN and mpPVN as defined previously (Swanson and Kuypers, 1980). In the PVN, the parvocellular AVP-positive neurons were distinguished from the magnocellular neurons based on size and location. Both the number of double-labeled (Fos-positive and AVP-positive or Fos-positive and CRH-positive) cells and the total number of AVP-positive or CRH-positive cells per PVN nucleus were counted for each animal. Counts were determined by two independent observers blind to the treatment conditions and were averaged. Data were then expressed as a percentage of AVP or CRH neurons that were Fos-positive. Optical images were collected using a digital camera and computer processed using Adobe Photoshop CS.
Statistical analysis
Data are presented as means ± SEM. Statistical differences were assessed by a one-way or two-way ANOVA; when ANOVA showed a significant difference within an experiment, Fisher‘s post-hoc analysis was used to identify differences between groups. Differences were considered statistically significant when the test yielded a p < 0.05.
RESULTS
Experiment 1
Plasma osmolality, hematocrit, and AVP were elevated and body weight was decreased by a single 23.5h episode of water restriction (Single Episode, AL vs. WR; Table 1) and by six episodes of water restriction (Repeated Episode, AL vs. WR; Table 1), indicating that rats were dehydrated in both models of water restriction. Plasma osmolality, hematocrit and AVP were similar after a single and repeated water restriction (Table 1). Plasma osmolality and AVP were decreased at 15 min after drinking induced by a single (WR vs. WR+Water; Table 1) and repeated water restriction (WR vs. WR+Water; Table 1), indicating that drinking rapidly reversed dehydration in both models. Plasma ACTH and corticosterone were elevated by a single (AL vs. WR; Table 1) and repeated water restriction (AL vs. WR; Table 1). Although plasma ACTH was similar after a single and repeated water restriction, corticosterone was increased after repeated water restriction compared to a single water restriction (Single Episode-WR vs. Repeated Episode-WR; Table 1). Drinking for 15 min decreased plasma corticosterone, but not ACTH, after a single water restriction (WR vs. WR+Water; Table 1); drinking after repeated water restriction decreased plasma ACTH and corticosterone (WR vs. WR+Water; Table 1), showing a rapid reduction of HPA axis activity in both models of dehydration.
Table 1.
Body weight, plasma osmolality, hematocrit and plasma hormones in rats that had ad libitum access to water (AL; single and repeated, n=6/group), rats that had undergone water restriction (WR; single and repeated, n=6/group), and rats that had undergone water restriction and then allowed to drink for 15min (WR+Water; single and repeated, n=6/group).
| Group | Single Episode | Repeated Episode | ||||
|---|---|---|---|---|---|---|
| AL | WR | WR+Water | AL | WR | WR+Water | |
| Body Weight (g) | 224 ±4 | 207 ±1* | 211 ±2* | 272 ±6* | 232 ±4+# | 234 ±3+° |
| Plasma Osmolality (mOsm/Kg H20) | 290 ± 3.3 | 303 ± 2.4 * | 294 ± 2.0 *# | 290 ± 1.0 | 302 ± 1.0+ | 294 ± 1.0+^ |
| Hematocrit | 35 ± 0.0 | 41 ± 0.0 * | 42 ± 0.5* | 35 ± 0.5 | 41 ± 0.0 + | 37 ± 0.5+^° |
| Plasma VP (pg/mL) | 1.2 ± 0.5 | 3.0 ± 1.0 * | 1.6 ± 0.5*# | 1.0 ± 0.0 | 3.6 ± 0.1 + | 1.3 ± 0.2^ |
| Plasma ACTH (pg/mL) | 34 ± 1 | 46 ± 2 * | 43 ± 1* | 35 ± 1 | 47 ± 2 + | 42 ± 2 +^ |
| Plasma Corticosterone (ng/mL) | 7.6 ± 2.1 | 100.3 ± 20.0 * | 16.0 ± 1.5*# | 5.5 ± 2.0 | 169.0 ± 25.0+# | 60.9 ± 15.3+^° |
Values represent means ± SEM.
=p<0.05 vs Single Episode-AL
=p<0.05 vs Single Episode-WR
=p<0.05 vs Repeated Episode-AL
=p<0.05 vs Repeated Episode-WR
=p<0.05 vs Single Episode-WR+Water
Experiment 2
Changes in neural activity were assessed by monitoring Fos expression in magnocellular AVP (pmPVN), parvocellular AVP (mpPVN) and parvocellular CRH (mpPVN) neurons after water restriction and subsequent drinking (see images in Figure 1 and corresponding quantitative data in Figure 2). Both a single episode and repeated episodes of water restriction increased Fos expression in AVP magnocellular (Figure 2A), CRH parvocellular (Figure 2B) and AVP parvocellular neurons (Figure 2C) compared to ad libitum controls (AL vs. WR, Single Episode and AL vs. WR, Repeated Episode), indicating that both models of water restriction activated each neuronal phenotype in the PVN. However, Fos expression in all neuronal phenotypes was reduced after repeated water restriction (WR, Single Episode vs. WR, Repeated Episode). Drinking water after a single episode or after repeated episodes of water restriction reduced Fos expression in AVP magnocellular neurons compared to water restricted rats (WR vs. WR+Water; Figure 2A). However, the magnitude of the response was greater when rehydration occurred after repeated water restriction; Fos expression in magnocellular neurons remained elevated above ad libitum controls after a single episode of drinking (AL vs. WR+Water; Figure 2A), but was reduced to control levels after drinking in animals that had undergone repeated episodes of water restriction (AL vs. WR+Water; Figure 2A).
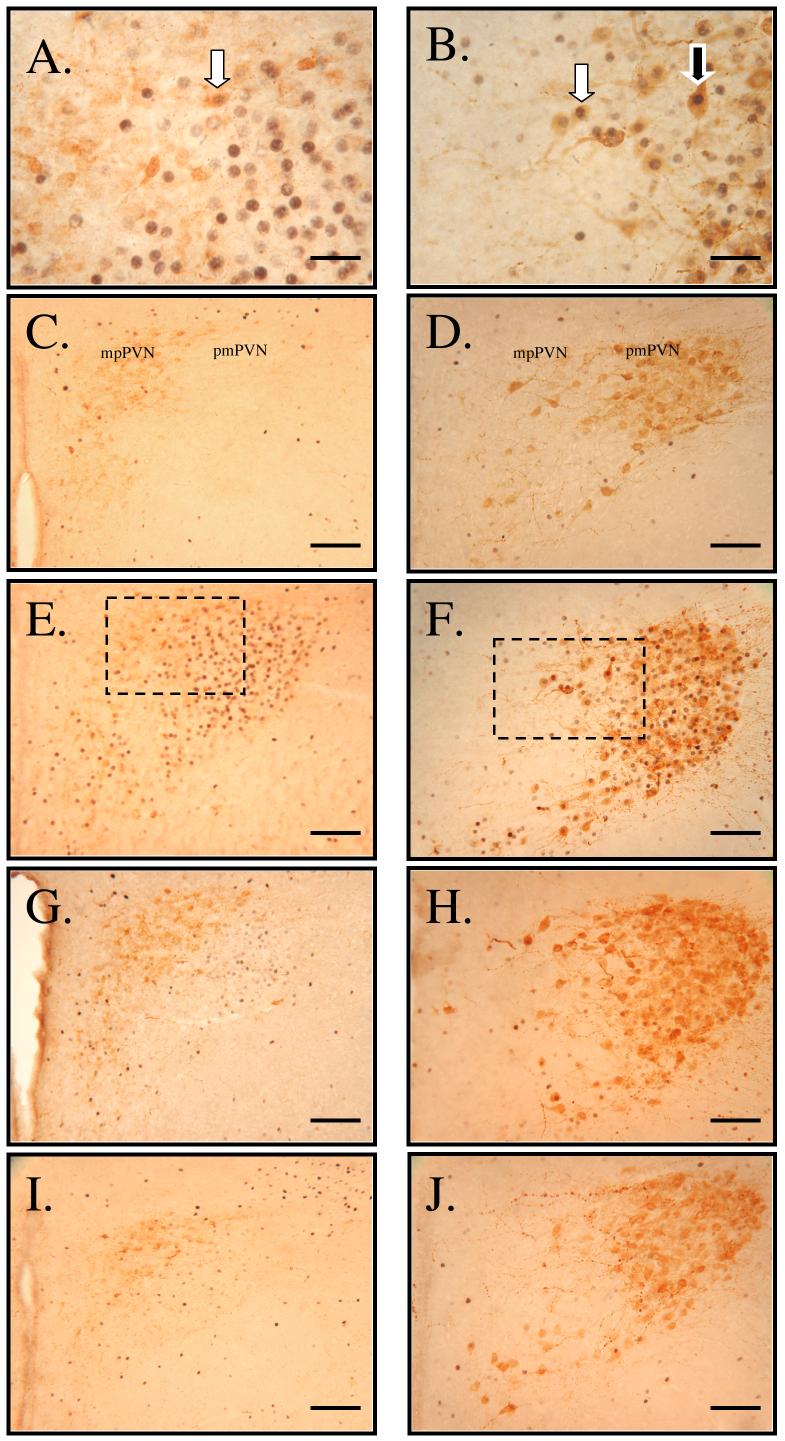
Figure 1.
Double-immunolabeling for Fos (black) and CRH (brown, left panels) or AVP (brown, right panels) in medial parvocellular (mp) and posterior magnocellular (pm) PVN neurons. High magnification of neurons double-labeled for Fos and CRH (A, area denoted by box in panel E) and for Fos and AVP (B, area denoted by box in panel F) from rats that were water restricted one time. Labeling of neurons in rats that had water ad libitum (C and D), in rats that were water restricted one time (E and F), in rats that were water restricted one time and received water for 30 min (G and H) and in rats that were water restricted six times and received water for 30 min (I and J). White arrow indicates Fos-positive parvocellular neurons phenotypically labeled for CRH (A) or AVP (B). Black arrow indicates Fos-positive magnocellular neuron labeled for AVP (B). Scale bar = 25μM (A and B); bar = 100 μM (C-J).
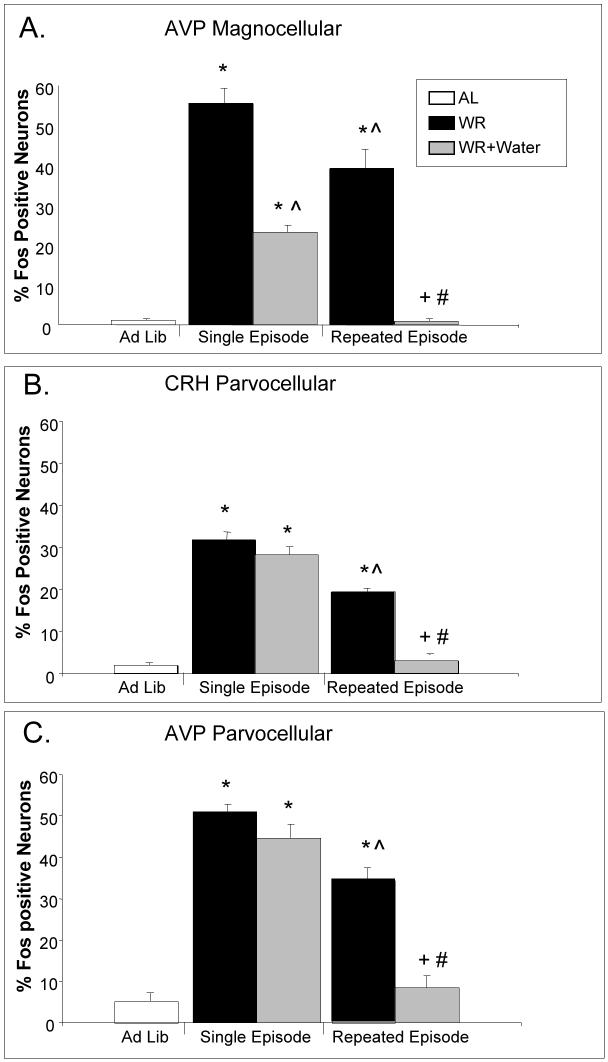
Figure 2.
The effect of single and repeated 23.5h episodes of water restriction and drinking on the percentage of Fos-positive AVP or CRH neurons in the PVN. Both a single episode and repeated episodes of water restriction increased Fos expression in AVP magnocellular (pmPVN) (A), CRH parvocellular (mpPVN) (B), and AVP parvocellular (mpPVN) neurons (C). Drinking for 15 min after a single episode of water restriction reduced Fos expression in AVP magnocellular neurons (A), but not in CRH (B) or AVP parvocellular neurons (C). Drinking after repeated episodes of water restriction reduced Fos expression in AVP magnocellular neurons (A) as well as CRH (B) and AVP parvocellular neurons (C). Values represent means ± SEM; n=6/group. * = p < 0.05 vs. AL, ^ = p < 0.05 vs. WR, Single Episode, # = p < 0.05 vs. WR+Water, Single Episode, + = p < 0.05 vs. WR, Repeated Episode.
In contrast to magnocellular neurons, rehydration for 15 min after a single episode of water restriction did not change Fos expression in CRH or AVP parvocellular neurons (WR vs. WR+Water, Single Episode; Figure 2B, 2C); however, drinking water for 15 min after repeated episodes of water restriction reduced Fos expression in CRH and AVP parvocellular neurons compared to water restricted rats (WR vs. WR+Water, Repeated Episode; Figure 2B, 2C). Furthermore, drinking water after repeated episodes of water restriction reduced Fos expression in CRH and AVP parvocellular neurons to the level observed in ad libitum controls (AL vs. WR+Water, Repeated Episode; Figure 2B, 2C). These data suggest that activity of CRH and AVP parvocellular neurons in the PVN of water-restricted rats is reduced after repeated episodes of restriction-induced drinking, but not after a single episode of restriction-induced drinking.
In experiment 2, the total number of neurons counted for each phenotype did not vary across treatment groups (Table 2). Differences in the total number of double-labeled neurons (data not shown) paralleled differences observed in the percentage of Fos-positive neurons (Fig. 2).
Table 2.
Number of hypothalamic neurons staining for CRH or AVP in each treatment condition in Experiment 1: ad libitum access to water (AL; n=6), single and repeated episodes of water restriction (WR; n=6/group), and single and repeated episodes of water restriction followed by drinking (WR+Water; n=6/group).
| Group | Ad Lib | WR | WR+Water | WR | WR+Water |
|---|---|---|---|---|---|
| Single Episode | Repeated Episode | ||||
| CRH Parvocellular | 46.4 ± 4.0 | 43.5 ± 3.7 | 50.7 ± 4.2 | 46.3 ± 4.0 | 34.6 ± 3.7 |
| AVP Parvocellular | 20.0 ± 1.4 | 19.9 ± 0.8 | 18.3 ± 1.4 | 16.4 ± 1.8 | 13.7 ± 2.0 |
| AVP Magnocellular | 71.1 ± 8.1 | 81.2 ± 8.1 | 76.8 ± 6.1 | 66.8 ± 4.6 | 69.0 ± 4.0 |
No statistical differences
Experiment 3
Plasma osmolality and AVP were elevated by a 47.5h episode of water restriction and reduced by drinking for 15 min (AL vs. WR+Water; Table 3), indicating that drinking rapidly reversed dehydration induced by the longer duration of water restriction. Plasma ACTH and corticosterone were elevated by the longer period of water restriction (AL vs. WR; Table 3). Drinking for 15 min decreased plasma ACTH and corticosterone, demonstrating that rehydration resulted in a reduction of HPA activity.
Table 3.
Plasma osmolality, hematocrit and plasma hormones in rats that had ad libitum access to water (AL; n=4), rats that had undergone a 47.5h episode of water restriction (WR; n=8), and rats that had undergone a 47.5h episode of water restriction and then allowed to drink for 15 min (WR+Water; n=8).
| Group | AL | WR | WR+Water |
|---|---|---|---|
| Plasma Osmolality (mOsm/Kg H20) | 287 ± 1.2 | 301 ± 1.3 * | 292 ± 1.1 *# |
| Plasma VP (pg/mL) | 0.4± 0.1 | 5.1 ± 0.4 * | 1.8 ± 0.5 *# |
| Plasma ACTH (pg/mL) | 16.3 ± 2.1 | 53.6 ± 5.5 * | 39.0 ± 3.1 *# |
| Plasma Corticosterone (ng/mL) | 10.0 ± 3.7 | 177.5 ± 23.5 * | 87.4 ± 10.7 *# |
Values represent means ± SEM.
=p<0.05 vs AL
=p<0.05 vs WR
Experiment 4
Changes in neural activity were assessed by monitoring Fos expression in AVP magnocellular, AVP parvocellular and CRH parvocellular neurons after water restriction and subsequent drinking. A single 47.5h episode of water restriction increased Fos expression in AVP magnocellular (Figure 3A), CRH parvocellular (Figure 3B) and AVP parvocellular neurons (Figure 3C) compared to ad libitum controls (AL vs. WR), indicating that a longer duration of water restriction activated each neuronal phenotype in the PVN. Drinking water for 15 min after a 47.5h episode of water restriction reduced Fos expression in AVP magnocellular neurons compared to water restricted rats (WR vs. WR+Water; Figure 3A); however, drinking water did not reduce Fos expression in these neurons to the level of ad libitum controls (AL vs. WR+Water; Figure 3A). Moreover, rehydration for 15 min after a 47.5h of water restriction did not change Fos expression in CRH or AVP parvocellular neurons (WR vs. WR+Water; Figure 3B, 3C). These data suggest that drinking for 15 min does not change the activity of parvocellular neurons in the PVN induced by a single 47.5h episode of water restriction.
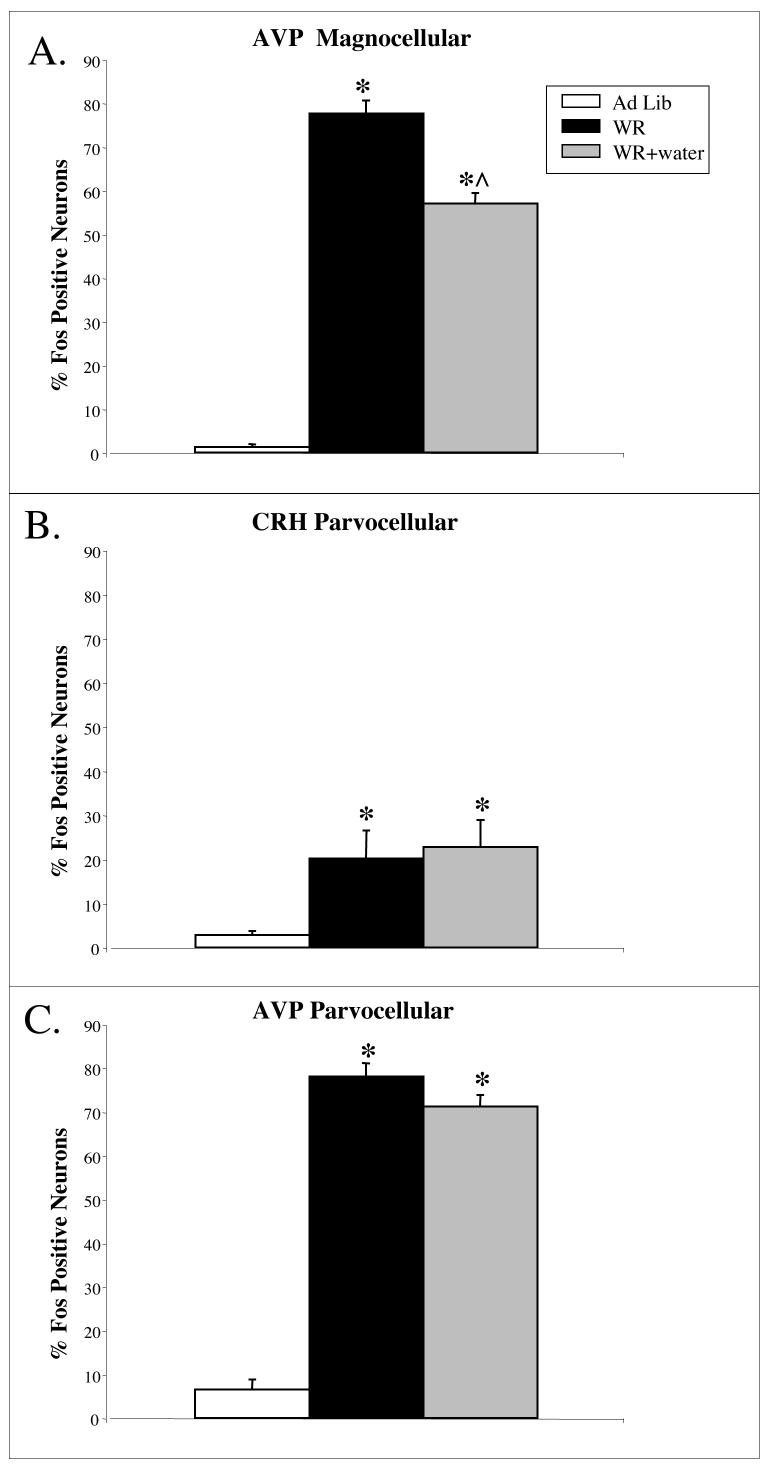
Figure 3.
The effect of a single 47.5h episode of water restriction and drinking on the percentage of Fos-positive AVP or CRH neurons in the PVN. A single 47.5h episode of water restriction increased Fos expression in AVP magnocellular (pmPVN) (A), CRH parvocellular (mpPVN) (B), and AVP parvocellular (mpPVN) neurons (C). Drinking for 15 min reduced Fos expression in AVP magnocellular neurons (A) but not in CRH (B) or AVP parvocellular neurons (C). Values represent means ± SEM; AL, n=4; WR and WR+Water, n=8. * = p < 0.05 vs. AL, ^ = p < 0.05 vs. WR.
In experiment 4, the total number of CRH and AVP parvocellular neurons counted to determine phenotypic expression of Fos did not vary across treatment groups (Table 4); in addition, the total number of AVP magnocellular neurons in 47.5h water restricted rats and water restricted rats that drank water were not different from AL controls (Table 4). For these comparisons, differences in the total number of double-labeled neurons (data not shown) paralleled differences in the percentage of Fos-positive neurons for each phenotype (Fig. 3). The number of AVP magnocellular neurons counted in 47.5h water restricted rats after rehydration was greater than the number in water restricted rats not given water (WR vs. WR+Water; Table 4); thus, the number of double-labeled neurons did not differ between groups (data not shown). Although we cannot rule out the possibility that the increase in number of AVP magnocellular neurons in this rehydrated group was a response to drinking, it is more likely due to the biological variability inherent in sampling a subset of sections collected from the PVN. However, as indicated above, there was a reduction in the percentage of AVP magnocellular neurons expressing Fos after drinking in 47.5h water restricted rats (Fig. 3), suggesting that rehydration reduces neural activity in this subpopulation of PVN neurons.
Table 4.
Number of hypothalamic neurons staining for CRH or AVP in each treatment condition in Experiment 1: rats that had ad libitum access to water (AL; n=4), rats that had undergone a 47.5h episode of water restriction (WR; n=8), and rats that had undergone a 47.5h episode of water restriction and then allowed to drink for 15 min (WR+Water; n=8).
| Group | AL | WR | WR+Water |
|---|---|---|---|
| CRH Parvocellular | 71.3 ± 8.6 | 51.4 ± 9.7 | 64.9 ± 11.3 |
| AVP Parvocellular | 14.8 ± 0.6 | 15.0 ± 1.0 | 14.0 ± 1.4 |
| AVP Magnocellular | 72.3 ± 7.5 | 69.9 ± 4.4 | 88.6 ± 4.5# |
Values represent means ± SEM.
=p<0.05 vs WR
DISCUSSION
By characterizing HPA activity in response to drinking after a single versus repeated episodes of water restriction, our experiments provide new information regarding stress reduction following a novel or recurring stressor. After overnight or six days of water restriction, plasma AVP, ACTH and corticosterone are elevated, reflecting activation of the HPA axis. After either a single or repeated episode of drinking, plasma ACTH and corticosterone decrease rapidly, reflecting a reduction in HPA activity. Previous studies have shown that HPA activity decreases after drinking induced by repeated water restriction (Gray et al., 1978; Heybach and Vernikos-Danellis, 1979; Wilkinson et al., 1982; Wotus and Engeland, 2003), but the novel finding that corticosterone decreases after a single episode of rehydration suggests that the reduction in glucocorticoids is an important component of the rehydration response. Similar to plasma AVP in which rapid decreases induced by the act of drinking likely occur to prevent over hydration after a period of dehydration (Stricker et al., 2002), a rapid reduction in glucocorticoids after drinking may be important to limit responses to dehydration. For example, maintenance of blood pressure during dehydration requires increased sympathetic activity (Brooks et al., 2004; Stocker et al., 2004). Since glucocorticoids act peripherally to increase vascular tone (Girod and Brotman, 2004) and centrally to stimulate cardiovascular areas critical for regulation of sympathetic activity (Rong et al., 1999; Scheuer et al., 2004), it may be important to reduce glucocorticoids to effectively control blood pressure during rehydration.
Since the hormonal response to a single or repeated episode of drinking are similar, it is likely that a common mechanism contributes to the rapid inhibition of plasma corticosterone observed in both models. In the present study, we found that both a single episode and repeated episodes of water restriction elevated plasma vasopressin and corticosterone. Additionally, water restriction elevated Fos expression in AVP magnocellular neurons in the PVN. Drinking after a single or repeated episode of water restriction reduced plasma hormones and Fos expression in AVP magnocellular neurons, suggesting that changes in the activity of these neurons may contribute to the reduction of plasma corticosterone when water restricted rats drink. Vasopressin can act on the pituitary to increase ACTH (Dohanics et al., 1991) or act directly at the adrenal to stimulate corticosterone secretion (Hinson et al., 1987; Guillon et al., 1995). Previous work from our laboratory supports the hypothesis that during dehydration, plasma vasopressin affects corticosterone secretion by directly acting on the adrenal (Wotus et al., 2003). Following repeated water restriction, a stronger correlation exists between changes in plasma vasopressin and corticosterone than between plasma ACTH and corticosterone after drinking; moreover, peripheral administration of vasopressin elevates corticosterone, whereas peripheral injection of an immunoneutralizing antibody against AVP reduces plasma corticosterone, but not ACTH, in water restricted rats (Wotus et al., 2003). Collectively, these data suggest that reduction in magnocellular neuronal activity contributes to decreases in plasma corticosterone following both single and repeated episodes of restriction-induced drinking.
In addition to AVP magnocellular neurons, both single and repeated episodes of water restriction in the AM increased Fos expression in AVP and CRH parvocellular neurons, suggesting that dehydration activates these neurons to drive the HPA axis. Previous studies have documented increased Fos expression in the PVN after 24 or 48h water deprivation (McKinley et al., 1994; Morien et al., 1999). Only recently have attempts been made to characterize the response in specific subpopulations of PVN neurons. For example, PVN parvocellular neurons shown by retrograde labeling to project to the rostral ventral lateral medulla and spinal cord increased Fos expression after 48h of water deprivation (Stocker et al., 2004). Together with our results, these data suggest that a single episode of water deprivation induces neuronal activation in specific subpopulations of PVN neurons. Our laboratory has recently reported data from rats that underwent repeated water restriction in the PM, just prior to the onset of the dark period (Wotus et al., 2007). Unlike AM water restriction, repeated water restriction in the PM did not increase Fos expression in CRH parvocellular PVN neurons compared to ad libitum controls. It is well established that restricting food and/or water to the AM produces a food entrained peak in corticosterone just prior to feeding which rapidly falls after nutrient ingestion (Krieger, 1974; Honma et al., 1984). The food-entrained rhythm is independent of the light-entrained circadian corticosterone rhythm, since it is not affected by ablation of the suprachiasmatic nucleus (Krieger et al., 1977). Furthermore, in addition to altering the rhythm of plasma corticosterone, food restriction in the morning alters the rhythm in CRH content in the hypothalamus. In rats that have free access to food and water, CRH hnRNA, mRNA (Watts et al., 2004), and protein content (Honma et al., 1992) in the PVN exhibit a circadian rhythm that precedes by 12h the rhythm in plasma ACTH and corticosterone. After AM food restriction, the rhythm in CRH content in the PVN shifts temporally to precede the increase in plasma corticosterone (Honma et al., 1992). In our model of AM water restriction, it is possible that restricting the availability of water to a short period in the AM, a time that nocturnal rats normally do not eat or drink, induces a water entrained corticosterone rhythm that is driven at least in part by increased activity of CRH parvocellular neurons. Because rats normally eat and drink during the PM, repeated water restriction in the PM presents no temporal disruption of water intake and thus, induces no corticosterone rhythm separate from the light-entrained circadian rhythm.
Drinking after a single episode of water restriction did not affect Fos expression in CRH or AVP parvocellular neurons, whereas drinking for 15 min after repeated water restriction reduced Fos expression in AVP and CRH parvocellular neurons. The differential responsiveness of parvocellular neurons may have resulted in part from differences in the duration of dehydration prior to drinking. To assess this possibility, rats underwent a single episode of water restriction of a longer duration (47.5h). As observed following either a single or repeated episode of water restriction, plasma hormones were elevated along with Fos expression in AVP magnocellular, AVP parvocellular, and CRH parvocellular neurons. Drinking for 15 min reduced plasma hormones and Fos expression in AVP magnocellular neurons without affecting Fos in CRH or AVP parvocellular neurons, suggesting that differential responses of parvocellular neurons result from repeated rehydration, not from the duration of dehydration. In the present experiments, brains were collected one hour after drinking for 15 min, a time chosen to reflect neuronal activity after a short period of drinking, prior to satiety. Drinking water for 2h has been shown to reduce Fos expression in both magnocellular and parvocellular PVN neurons in water deprived rats (Ji et al., 2005; Gottlieb et al., 2006), indicating that the duration of water availability may affect the neuronal response. It is possible that the initial response to dehydration-induced drinking is a rapid inhibition of PVN magnocellular neurons and with continued drinking parvocellular neurons are inhibited. The latency of the response of parvocellular PVN neurons to drinking appears to be reduced after repeated water restriction, producing a pattern of neural activity that is comparable to a dehydrated animal that has drunk to satiety.
The differential pattern of Fos expression in parvocellular PVN neurons suggests that parvocellular neurons may contribute to the rapid inhibition of corticosterone after repeated water restriction-induced drinking. Decreases in parvocellular neuronal activity, resulting in reduced stimulation of pituitary corticotrophs, would decrease plasma ACTH. Although the rapid reduction in ACTH likely would decrease adrenal secretion of corticosterone, as noted in the present study (Table 1), drinking after a single 23.5h episode of dehydration reduced corticosterone, but not ACTH. The dissociation between ACTH and corticosterone also has been observed after repeated episodes of restriction-induced drinking (Wilkinson et al., 1982; Wotus and Engeland, 2003), suggesting that ACTH-independent mechanisms also are employed to reduce plasma corticosterone when an animal drinks. In addition to putative direct adrenal effects of AVP (Wotus et al., 2003), increases in corticosterone clearance (Doell et al., 1981; Wotus and Engeland, 2003) and changes in adrenal sensitivity to ACTH mediated by adrenal neural input (Wilkinson et al., 1982) have been implicated in the response. Thus, it is likely that drinking-induced decreases in corticosterone after repeated water restriction involve multiple factors including, but not limited to, a reduction in magnocellular release of vasopressin and a reduction in parvocellular neuronal activity.
Following repeated water or food restriction, stimuli associated with nutrient presentation or the presentation of an empty water bottle elicit a transient decrease in corticosterone (Levine and Coover, 1976; Coover et al., 1977). These results suggest that previously neutral stimuli associated with the act of drinking can predict rehydration and induce a conditioned inhibition of plasma corticosterone. Conditioned stimuli have been used to predict “safety” or the imminent relief of stress as reflected by decreases in glucocorticoids. For example, the presentation of a conditioned inhibitor, an auditory stimulus used to signal the absence of foot shock, reduced plasma corticosterone and the acoustic startle reflex in rats when presented prior to a conditioned stimulus that predicts foot shock (Campeau et al., 1997). In rats presented with a conditioned inhibitor, the most prominent increase in neural activity based on c-fos mRNA expression was observed in the ventral bed nucleus of the stria terminalis (V-BNST), an area known to have inhibitory projections to the PVN (Cullinan et al., 1993). These results led to the suggestion that the V-BNST contributes to conditioned behavioral or endocrine inhibition. In a similar experiment, Rogan and associates (Rogan et al., 2005) used behavioral measures of fear to demonstrate that fear responses are reduced by the presentation of a stimulus signifying “protection” or the absence of aversive events. Furthermore, these studies showed that the lateral amygdala, a brain region intimately involved in fear conditioning and modulation of emotional memories, exhibited long term depression in mice trained to recognize the “safety signal.” Collectively these data demonstrate a precedent for a neural mechanism capable of signifying safety, protection, and perhaps, the relief of stress. Since decreases in glucocorticoids occur, it is possible that inhibition of PVN neurons is a component of the safety response. Additional work is needed to determine the contribution of subpopulations of PVN neurons and ACTH-independent mechanisms in regulating glucocorticoid responses induced by conditioned safety.
Acknowledgements
We would like to thank Rebecca Frino, Victoria Anderson-Barnes and Alexis Gerber for their technical assistance. This work was supported in part by NSF grant IOB0548584, by a grant-in-aid from the Graduate School of the University of Minnesota, by NIDA grant T32DA07097 (MMA) and by an American Association of University Women Dissertation Fellowship (CW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilera G, Lightman SL, Kiss A. Regulation of the hypothalamic-pituitary-adrenal axis during water deprivation. Endocrinology. 1993;132(1):241–248. doi: 10.1210/endo.132.1.8380375. [DOI] [PubMed] [Google Scholar]
- Antoni FA. Hypothalamic control of ACTH secretion: advances since the discovery of 41-residue corticotropin-releasing factor. Endoc. Rev. 1986;7:351–378. doi: 10.1210/edrv-7-4-351. [DOI] [PubMed] [Google Scholar]
- Bali B, Erdelyi F, Szabo G, Kovacs KJ. Visualization of stress-responsive inhibitory circuits in the GAD65-eGFP transgenic mice. Neurosci. Lett. 2005;380:20–27. doi: 10.1016/j.neulet.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol. 2002;14:403–410. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- Berod A, Hartman BK, Pujol JF. Importance of fixation in immunohistochemistry: use of formaldehyde solutions at variable pH for the localization of tyrosine hydroxylase. J. Histochem. Cytochem. 1981;29:844–850. doi: 10.1177/29.7.6167611. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Rivest S. Effect of a chronic stress on CRH neuronal activity and expression of its type 1 receptor in the rat brain. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1438–R1449. doi: 10.1152/ajpregu.1998.275.5.R1438. [DOI] [PubMed] [Google Scholar]
- Brooks VL, Freeman KL, Chow KA. Excitatory amino acids in rostral ventrolateral medulla support blood pressure during water deprivation in rats. Am. J. Physiol. (Heart Circ. Physiol.) 2004;286:H1642–1648. doi: 10.1152/ajpheart.01004.2003. [DOI] [PubMed] [Google Scholar]
- Campeau S, Falls WA, Cullinan WE, Helmreich DL, Davis M, Watson SJ. Elicitation and reduction of fear: behavioral and neuroendocrine indices and brain induction of the immediate-early gene c-fos. Neuroscience. 1997;78:1087–1104. doi: 10.1016/s0306-4522(96)00632-x. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. A healthy body in a healthy mind - and vice versa - the damaging power of “uncontrollable” stress. J. Clin. Endocrinol. Metab. 1998;83:1842–1845. doi: 10.1210/jcem.83.6.4908. [DOI] [PubMed] [Google Scholar]
- Coover GD, Sutton BR, Heybach JP. Conditioning decreases in plasma corticosterone level in rats by pairing stimuli with daily feedings. J. Comp. Physiol. Psych. 1977;91:716–726. doi: 10.1037/h0077363. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J. Comp. Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- Doell RG, Dallman MF, Clayton RB, Gray GD, Levine S. Dissociation of adrenal corticosteroid production from ACTH in water-restricted female rats. Am. J. Physiol. (Regul. Integr. Comp. Physiol.) 1981;241:R21–R24. doi: 10.1152/ajpregu.1981.241.1.R21. [DOI] [PubMed] [Google Scholar]
- Dohanics J, Hoffman GE, Verbalis JG. Hyponatremia-induced inhibition of magnocellular neurons causes stressor-selective impairment of stimulated adrenocorticotropin secretion in rats. Endocrinology. 1991;128:331–340. doi: 10.1210/endo-128-1-331. [DOI] [PubMed] [Google Scholar]
- Girod JP, Brotman DJ. Does altered glucocorticoid homeostasis increase cardiovascular risk? Cardiovascular Res. 2004;64:217–226. doi: 10.1016/j.cardiores.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Gottlieb HB, Ji LL, Jones H, Penny ML, Fleming T, Cunningham JT. Differential effects of water and saline intake on water deprivation induced c-Fos staining in the rat. Am. J. Physiol. (Reg. Integr. Comp. Physiol.) 2006;290:R1251–1261. doi: 10.1152/ajpregu.00727.2005. [DOI] [PubMed] [Google Scholar]
- Gray GD, Bergfors R, Levin R, Levine S. Comparisons of the effect of restricted morning or evening water intake on adrenocortical activity in female rats. Neuroendocrinology. 1978;25:236–246. doi: 10.1159/000122745. [DOI] [PubMed] [Google Scholar]
- Guillon G, Trueba M, Joubert D, Grazzini E, Chouinard L, Cote M, Payet MD, Manzoni O, Barberis C, Robert M, Gallo-Payet N. Vasopressin stimulates steroid secretion in human adrenal glands: comparison with angiotensin-II effect. Endocrinology. 1995;136:1285–1295. doi: 10.1210/endo.136.3.7867583. [DOI] [PubMed] [Google Scholar]
- Hall PF. Actions of corticotropin on the adrenal cortex: biochemistry and cell biology. In: McEwen BS, editor. Handbook of Physiology. Oxford University Press; New York, IV. Coping with the Environment: Neural and Endocrine Mechanisms: 2001. pp. 61–101. [Google Scholar]
- Hauger RL, Lorang M, Irwin M, Aguilera G. CRF receptor regulation and sensitization of ACTH responses to acute ether stress during chronic intermittent immobilization stress. Brain Res. 1990;532:34–40. doi: 10.1016/0006-8993(90)91738-3. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front. Neuroendocrinology. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Heybach JP, Vernikos-Danellis J. Inhibition of adrenocorticotrophin secretion during deprivation-induced eating and drinking in rats. Neuroendocrinology. 1979;28:329–338. doi: 10.1159/000122880. [DOI] [PubMed] [Google Scholar]
- Hinson JP, Vinson GP, Porter ID, Whitehouse BJ. Oxytocin and arginine vasopressin stimulate steroid secretion by the isolated perfused rat adrenal gland. Neuropeptides. 1987;10:1–7. doi: 10.1016/0143-4179(87)90083-7. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S, Hiroshige T. Feeding-associated corticosterone peak on rats under various feeding cycles. Am. J. Physiol. (Regul. Integr. Comp. Physiol.) 1984;246:R721–726. doi: 10.1152/ajpregu.1984.246.5.R721. [DOI] [PubMed] [Google Scholar]
- Honma K-I, Noe Y, Honma S, Katsuno Y, Hiroshige T. Roles of paraventricular catecholamines in feeding-associated corticosterone rhythm in rats. Am. J. Physiol. (Endo. Metab. Physiol.) 1992;262:E948–955. doi: 10.1152/ajpendo.1992.262.6.E948. [DOI] [PubMed] [Google Scholar]
- Jasper MS, Engeland WC. Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. Am. J. Physiol. (Regul. Integr. Comp. Physiol.) 1991;261:R1257–R1268. doi: 10.1152/ajpregu.1991.261.5.R1257. [DOI] [PubMed] [Google Scholar]
- Ji LL, Fleming T, Penny ML, Toney GM, Cunningham JT. Effects of water deprivation and rehydration on c-Fos and FosB staining in the rat supraoptic nucleus and lamina terminalis region. Am. J. Physiol. (Regul. Integr. Comp. Physiol.) 2005;288:R311–321. doi: 10.1152/ajpregu.00399.2004. [DOI] [PubMed] [Google Scholar]
- Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr. Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Khan AM, Watts AG. Intravenous 2-deoxy-2-glucose injection rapidly elevates levels of the phosphorylated forms of p44/42 mitogen-activated protein kinases (extracellularly regulated kinases 1/2) in rat hypothalamic parvicellular paraventricular neurons. Endocrinology. 2004;145:351–359. doi: 10.1210/en.2003-0539. [DOI] [PubMed] [Google Scholar]
- Kiss A, Jezova D, Aguilera G. Activity of the hypothalamic pituitary adrenal axis and sympathoadrenal system during food and water deprivation in the rat. Brain Res. 1994;663(1):84–92. doi: 10.1016/0006-8993(94)90465-0. [DOI] [PubMed] [Google Scholar]
- Krieger DT. Food and water restriction shifts corticosterone, temperature, activity and brain amine periodicity. Endocrinology. 1974;95:1195–1201. doi: 10.1210/endo-95-5-1195. [DOI] [PubMed] [Google Scholar]
- Krieger DT, Hauser H, Krey LC. Suprachiasmatic nuclear lesions do not abolish food-shifted circadian adrenal and temperature rhythmicity. Science. 1977;197:398–399. doi: 10.1126/science.877566. [DOI] [PubMed] [Google Scholar]
- Li HY, Sawchenko PE. Hypothalamic effector neurons and extended circuitries activated in “neurogenic” stress: a comparison of footshock effects exerted acutely, chronically, and in animals with controlled glucocorticoids levels. J Comp Neurol. 1998;393:244–266. [PubMed] [Google Scholar]
- Levine S, Coover GD. Environmental control of suppression of the pituitary-adrenal system. Physiol. Behav. 1976;17:35–37. doi: 10.1016/0031-9384(76)90265-1. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Hards DK, Oldfield BJ. Identification of neural pathways activated in dehydrated rats by means of Fos-immunohistochemistry and neural tracing. Brain Res. 1994;653:305–312. doi: 10.1016/0006-8993(94)90405-7. [DOI] [PubMed] [Google Scholar]
- Morien A, Garrard L, Rowland NE. Expression of fos immunoreactivity in rat brain during dehydration: effect of duration and timing of water deprivation. Brain Res. 1999;816:1–7. doi: 10.1016/s0006-8993(98)00828-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Dallman MF, Warnea JP, Ginsberg AB, Laugero KD, Fleur S.E.l., Houshyar H, Gomez F, Bhargava A, Akana SF. From Malthus to motive: How the HPA axis engineers the phenotype, yoking needs to wants. Progress in Neurobiology. 2006;79:247–340. doi: 10.1016/j.pneurobio.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Leon KS, Perez DL, Kandel ER. Distinct neural signatures for safety and danger in the amygdala and striatum of the mouse. Neuron. 2005;46:309–320. doi: 10.1016/j.neuron.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Rong W, Wang W, Yuan W, Chen Y. Rapid effects of corticosterone on cardiovascular neurons in the rostral ventrolateral medulla of rats. Brain Res. 1999;815:51–59. doi: 10.1016/s0006-8993(98)01090-7. [DOI] [PubMed] [Google Scholar]
- Rose JC, Meis PJ, Morris M. Ontogeny of endocrine (ACTH, vasopressin, cortisol) responses to hypotension in lamb fetuses. Am. J. Physiol. (Endo. Metab.) 1981;240:E565–561. doi: 10.1152/ajpendo.1981.240.6.E656. [DOI] [PubMed] [Google Scholar]
- Scheuer DA, Bechtold AG, Shank SS, Akana SF. Glucocorticoids act in the dorsal hindbrain to increase arterial pressure. Am. J. Physiol. (Heart Circ. Physiol.) 2004;286:H458–467. doi: 10.1152/ajpheart.00824.2003. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Cunningham JT, Toney GM. Water deprivation increases Fos immunoreactivity in PVN autonomic neurons. Am. J. Physiol. (Regul. Integr. Comp. Physiol.) 2004;287:R1172–1183. doi: 10.1152/ajpregu.00394.2004. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Keith KJ, Toney GM. Acute inhibition of the hypothalamic paraventricular nucleus decreases renal sympathetic nerve activity and arterial blood pressure in water-deprived rats. Am. J. Physiol. (Regul. Integr. Comp. Physiol.) 2004;286:R719–725. doi: 10.1152/ajpregu.00494.2003. [DOI] [PubMed] [Google Scholar]
- Stricker EM, Huang W, Sved AF. Early osmoregulatory signals in the control of water intake and neurohypophyseal hormone secretion. Physiol. Behav. 2002;76:415–421. doi: 10.1016/s0031-9384(02)00752-7. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HGJM. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and the organization projections to the pituitary, dorsal vagal complex and spinal cord as demonstrated by retrograde fluorescence labeling methods. J. Comp. Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Viau V, Sawchenko PE. Hypophysiotropic neurons of the paraventricular nucleus respond in spatially, temporally, and phenotypically differentiated manners to acute vs. repeated restraint stress: rapid publication. J Comp Neurol. 2002;445:293–307. doi: 10.1002/cne.10178. [DOI] [PubMed] [Google Scholar]
- Verbalis JG, Baldwin EF, Robinson AG. Osmotic regulation of plasma vasopressin and oxytocin after sustained hyponatremia. Am. J. Physiol. (Regul. Integr. Comp. Physiol.) 1986;250:R444–451. doi: 10.1152/ajpregu.1986.250.3.R444. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Stone E, McEwen B. Induction and habituation of c-fos and zif/268 by acute and repeated stressors. Neuroreport. 1994;5(11):1321–4. [PubMed] [Google Scholar]
- Watts AG, Tanimura S, Sanchez-Watts G. Corticotropin-releasing hormone and arginine vasopressin gene transcription in the hypothalamic paraventricular nucleus of unstressed rats: daily rhythms and their interactions with corticosterone. Endocrinology. 2004;145:529–540. doi: 10.1210/en.2003-0394. [DOI] [PubMed] [Google Scholar]
- Wilkinson C, Shinsako J, Dallman MF. Rapid decreases in adrenal and plasma corticosterone concentrations after drinking are not mediated by changes in plasma ACTH concentration. Endocrinology. 1982;110:1599–1606. doi: 10.1210/endo-110-5-1599. [DOI] [PubMed] [Google Scholar]
- Wotus C, Arnhold MM, Engeland WC. Dehydration-induced drinking results in a rapid decreases Fos expression in hypothalamic paraventricular neurons expressing vasopressin, but not corticotropin-releasing hormone. Am. J. Physiol. (Regul. Integr. Comp. Physiol.) 2007;292:1349–1358. doi: 10.1152/ajpregu.00304.2006. [DOI] [PubMed] [Google Scholar]
- Wotus C, Engeland WC. Differential regulation of adrenal corticosteroids after restriction-induced drinking in rats. Am. J. Physiol. (Regul. Integr. Comp. Physiol.) 2003;284:R183–191. doi: 10.1152/ajpregu.00027.2002. [DOI] [PubMed] [Google Scholar]
- Wotus C, Osborn JW, Nieto PA, Engeland WC. Regulation of corticosterone production by vasopressin during water-restriction and after drinking in rats. Neuroendocrinology. 2003;78:301–311. doi: 10.1159/000074883. [DOI] [PubMed] [Google Scholar]