Abstract
Cholinergic efferent fibers modify hair cell responses to mechanical stimulation. It is hypothesized that calcium entering the hair cell through a nicotinic receptor activates a small-conductance (SK), calcium-activated potassium channel to hyperpolarize the hair cell. The calcium signal may be amplified by calcium-induced calcium release from the synaptic cisternae. Pharmacological tests of these ideas in the intact cochlea have been technically difficult because of the complex and fragile structure of the mammalian inner ear. We turned to the Xenopus laevis lateral line organ, whose simplicity and accessibility make it a model for understanding hair cell organ function in a relatively intact system. Drugs were applied to the inner surface of the skin while monitoring the effects of efferent stimulation on afferent fiber discharge rate. Efferent effects were blocked by antagonists of SK channels including apamin (EC50 = 0.5 μM) and dequalinium (EC50 = 12 μM). The effect of apamin was not enhanced by co-administration of phenylmethylsulfonyl fluoride, a proteolysis inhibitor. Efferent effects were attenuated by ryanodine, an agent that can interfere with calcium-induced calcium release, although relatively high (mM) concentrations of ryanodine were required. Fluorescent cationic styryl dyes, 4-di-2-asp and fm 1– 43, blocked efferent effects, although it was not possible to observe specific entry of the dye into the base of hair cells. These pharmacological findings in the Xenopus lateral line organ support the hypothesis that effects of efferent stimulation are mediated by calcium entry through the nicotinic receptor via activation of SK channels and suggest the generality of this mechanism in meditating cholinergic efferent effects.
INTRODUCTION
Almost every inner ear organ has an efferent innervation that can modify its response to mechanical stimulation. The primary efferent transmitter, acetylcholine, acts through an ionotropic nicotinic receptor containing alpha 9 and 10 subunits (Elgoyhen et al. 1994, 2001) to hyperpolarize the hair cell (Art et al. 1982; Flock and Russell 1976; Holt et al. 2001; Housley et al. 1990). Calcium entering through the receptor will activate calcium-activated (KCa) potassium channels (Art et al. 1984; Blanchet et al. 1996; Fuchs and Murrow 1992; Housley and Ashmore 1991) to produce the hyperpolarization. This calcium signal may also be amplified by calcium-induced calcium release from the synaptic cisternae (Evans et al. 2000; Lioudyno et al. 2004; Sridhar et al. 1997), an endoplasmic reticulum-like structure in the hair cell that is in very close apposition to the efferent synapse.
Several lines of evidence suggest that the KCa channels responsible for efferent effects are small conductance potassium (SK) channels. SK2 channels have been cloned from inner ear tissue (Dulon et al. 1998; Nie et al. 2004) and are localized in the basolateral membrane of hair cells at the efferent terminals (Oliver et al. 2000). Biophysical properties of the acetylcholine and efferent induced outward currents are consistent with SK channels (Fuchs and Murrow 1992; Housley and Ashmore 1991; Nenov et al. 1996; Oliver et al. 2000; Yamamoto et al. 1997; Yuhas and Fuchs 1999). Apamin and dequalinium, blockers of SK channels (Strobaek et al. 2000), block whole cell clamp responses of hair cells to acetylcholine or to potassium-induced depolarization of efferent terminals (Glowatzki and Fuchs 2000; Holt et al. 2001; Nenov et al. 1996; Oliver et al. 2000; Yamamoto et al. 1997; Yoshida et al. 1994; Yuhas and Fuchs 1999).
Evidence is also accumulating for a mechanism by which calcium entering through the cholinergic ionotropic receptor can be amplified through calcium-induced calcium release, presumably from the subsynaptic cisternae. In the guinea pig, responses to electrical stimulation of efferent nerves and to local application of acetylcholine are affected by drugs that alter calcium-induced calcium release. Drugs, such as cyclopiazonic acid, that inhibit calcium uptake into endoplasmic reticulum-like structures, can enhance slow effects of efferent stimulation (Sridhar et al. 1997) and responses to applied acetylcholine (Murugasu and Russell 1996). Ryanodine, an agent that can both enhance and block calcium-induced calcium release, depending on concentration and endogenous activity (Coronado et al. 1994; Rousseau et al. 1987), can both enhance (at 50 μM; Sridhar et al. 1997) and block (at 100 μM; Evans et al. 2000; Murugasu and Russell 1996) cholinergic action in the cochlea. More recently, Lioudyno et al. (2004) have shown the presence of type 1 ryanodine receptors at the base of the cochlea and that ryanodine can alter the amplitude of acetylcholine-induced currents in outer hair cells from excised apical turns of the rat cochlea.
These views of the efferent cellular mechanisms have proven difficult to confirm in the inner ear in vivo, in part because of the technical difficulties of delivering drugs and assessing efferent effects in this complex structure. For example, while apamin blocks the increased potassium conductance with acetylcholine application in recordings from hair cells, it does not block the effects of cholinergically mediated effects of electrical stimulation of olivocochlear efferents in the intact cochlea (Bobbin and LeBlanc 1999; Yoshida et al. 2001). The ability to observe specific effects of many cell-signal specific agents, such as ryanodine, sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) inhibitors, and ion channel blockers is compromised by the myriad of cellular processes that must function unimpeded to produce a synchronized compound action potential in response to shaped tone bursts. Pharmacological agents can begin to have more global effects on cochlear function at concentrations that are just beginning to affect efferent function (Bobbin 2002; Hendricson and Guth 2002; Lelli et al. 2003; Sridhar et al. 1997).
One approach that has been taken to overcome these problems is to excise cochlear turns and study these responses in vitro, which has the distinct advantage of allowing biophysical analysis of the hair cell’s response to cholinergic stimulation. Access of the preparation to drugs is greatly facilitated compared with the in vivo cochlea. The disadvantage is that efferent receptors must be stimulated with exogenous acetylcholine or by potassium-mediated depolarization of efferent terminals, and the cochlea, which is exquisitely sensitive to small changes in its complex environment, is removed from this network. However, the excised cochlea has proven highly valuable in examining fundamental cellular mechanisms in efferent stimulation, showing efferent action is blocked by SK channel blockers (Glowatzki and Fuchs 2000; Oliver et al. 2000), and, most recently, that ryanodine and calcium storage drugs can attenuate the amplitude of cholinergic responses in outer hair cells (Lioudyno et al. 2004).
Given that the fundamental elements needed for these proposed mechanisms (alpha 9/10 nicotinic receptors, a subsynaptic cisternae, and KCa channels), are constituent to many hair cells, we approached a test of these ideas, and the generality of the phenomenon, in the lateral line organ of the African clawed frog, Xenopus laevis, whose simplicity has long made it a model for understanding hair cell function. The accessibility of the hair cell synapse to drugs, and the ability to monitor single-fiber, afferent discharge for hours, make it ideal for pharmacological analysis. The anatomy of the Xenopus lateral line organ allows one to monitor activity in one or two afferent nerve fibers while electrically stimulating efferent fibers (Flock and Russell 1976; Harris and Flock 1967; Harris and Milne 1966; Russell 1971b; Sewell and Starr 1991). The organ comprises a series of “stitches” arranged in rows along the body wall. Each stitch contains 3–10 neuromasts, each of which contains 10–30 hair cells as well as supporting cells. Each neuromast has a cupula that projects outward into the aquatic environment. The whole organ can be easily placed in vitro after removing the piece of skin that contains the neuromasts and its nerve trunk. A single stitch is innervated by two large myelinated afferent fibers. It is possible to place the whole nerve trunk on a wire electrode, destroy all branches of the nerve trunk except to one stitch, and record activity from two single fibers. While it is often possible to separate the two spikes when their amplitudes are significantly different, we usually monitored activity in two to four nerve fibers from two stitches simultaneously to reduce the variability in discharge rate normally seen in spontaneous afferent fiber discharge over time. Physiological responses monitored in this isolated preparation (Bailey and Sewell 2000; Harris and Flock 1967; Kroese et al. 1978; Murray and Capranica 1973; Pabst 1976; Strelioff and Honrubia 1978) are similar to those monitored in vivo (Russell 1971a,b; Shelton 1971).
We have examined the ability of drugs applied to the inner surface of the skin to alter the effects of efferent stimulation on afferent fiber discharge rate. The cholinergic pharmacology of efferent block in the lateral line organ (Russell 1971a; Sewell and Starr 1991) is similar to that in hair cells of the cochlea, indicating that the same nicotinic cholinergic receptor is likely involved. Efferent fibers are electrically stimulated, and afferent activity monitored at distances relatively remote from the hair cell and its synapses, leaving intact any potentially labile or delicate mechanisms and approaching a more physiological situation. With this preparation, we have examined two elements of cell signaling following efferent activation of cholinergic receptor. Our demonstration that suppression is attenuated by the SK channel blockers, dequalinium and apamin, validate in afferent neural discharge patterns observations made in recordings from hair cells. Block of efferent effects by ryanodine raises the possibility of a general role of calcium-induced calcium release in efferent stimulation.
METHODS
We have previously described in detail many of the methods used in this analysis (Bailey and Sewell 2000; Dawkins and Sewell 2004; Sewell and Starr 1991); some of the description below is modified from those sources. Postmetamorphic X. laevis used in this study ranged from 2.6 to 4.1 cm (nose to vent) [3.2 ± 0.43 (SD) cm]. While smaller animals were desirable for drug penetration to the basolateral surface of the hair cell, we found it difficult to generate effects of efferent stimulation in frogs smaller than 3 cm. Data are presented from 38 preparations in which efferent activity was successfully elicited with electrical stimulation of the lateral line nerve.
Frogs were obtained from Nasco (Fort Atkinson, WI) and housed at room temperature in deionized water containing 1 mM added calcium chloride. Each frog was anesthetized by chilling to near 0°C and decapitated. A piece of skin containing the middle-lateral row of stitches was removed and placed inner surface up on a piece of moistened filter paper. The skin was rinsed with an artificial perilymph solution containing sodium chloride (120 mM), potassium chloride (3.5 mM), calcium chloride (2.3 mM), and glucose (5.5 mM), buffered with HEPES (20 mM) and adjusted to pH 7.5 with sodium hydroxide (total Na+ 130 mM). Perfusion of the inner surface of the skin allowed diffusion of the drugs to the basolateral surface of the sensory organs. Diffusion time from the inner surface of the skin to the hair cell region typically ranged from 60 to 200 s, depending on the size of the animal. Diffusion times are estimated on the basis of the time required for zero calcium solutions, applied at the inner surface of the skin, to increase afferent discharge rate.
We stimulated efferent fibers with a method similar to that described in Sewell and Starr (1991). Efferent fibers were electrically stimulated at the nerve trunk distal to the stitch from which we were monitoring afferent activity. The distal nerve trunk was pulled up into a plastic cone containing a chloride-silver stimulating electrode. A stimulus return was placed on the skin distal to the stimulating electrode.
Nerve activity was monitored by dissecting from the inner surface of the skin about 1 cm of the nerve trunk innervating the middle lateral row of stitches. The freed proximal end of the nerve trunk was sucked into a plastic cone filled with artificial perilymph solution and containing a chlorided silver wire electrode. The recording electrode was attached to a ×10 gain, low noise, AC-coupled, lab-built amplifier and then to a DL Instruments (Ithaca, NY) model 1201 amplifier. The DL Instruments amplifier allowed gating of the input during efferent shocks to reduce stimulus artifact. The electrode was lifted from the surface of the skin. The observed monophasic action potentials were amplified ~1,000-fold and monitored on an oscilloscope. The signal-to-noise ratio was optimized by analog filtering (high-pass, corner frequency of 300 Hz and low-pass, corner frequency of 1,000 Hz). A Schmitt trigger device was used to determine the occurrence of action potentials, which were counted with a microprocessor.
The timing of individual action potentials was determined to microsecond resolution relative to the stimulus pulse with a Tucker-Davis Technologies event timer. Timing of stimulus and gating pulses were controlled by computer with LabView (National Instruments) software. Stimuli were monophasic positive pulses of 140-μs duration at 10–80 V in trains of 10 pulses, 3 ms apart. Trains were presented approximately once per second. Impedance of the electrode-nerve complex was about 5 MOhm, giving stimulus currents of about 10 μamps (at 50 V). Time 0 for poststimulus time histograms (PSTHs) was placed at the time of the last stimulus pulse.
Measurement of drug effects required an automated quantification of efferent action as a function of time before, during, and after injection of pharmacological agents. For this, we created PSTHs of discharge following the stimulus train (e.g., Fig. 1B) by measuring spike times for 100 sequential presentations of the stimulus. (With perfusion of ryanodine, which lowered afferent discharge rate, it was sometimes necessary to extend the times of acquisition of the PSTH to 300 s to reduce variability associated with the decreased rate.) From these PSTHs, baseline discharge rate was calculated for the time period 450–600 ms (data not shown) after the start of the efferent stimulus pulse train. Discharge rate in each bin was divided by the baseline rate to give a relative increase or decrease in discharge for each bin.
Fig. 1. Responses of afferent fibers to electrical stimulation of efferent fibers.

A: 1st 4 traces show responses of 2 nerve fibers from 1 stitch, monitored simultaneously, to a train of 10 electrical shocks, 140 μs each, 3 ms apart, delivered to the nerve trunk distal to the monitored stitch. Bottom trace superimposes the top 4 traces to show reduced probability of afferent discharge following efferent stimulation. Afferent discharge was monitored in the lateral line nerve trunk proximal to the stitch monitored. Spontaneous discharge was reduced for tens of milliseconds following the shocks. Reduction in discharge was often followed by a small increase in discharge probability. Amplitude of the action potentials monitored with this approach were generally 100 μV or smaller (those depicted here ~80 μV). B: poststimulus time histograms (PSTHs) were generated by recording discharge times of each spike relative to the end of the stimulus train for 100 such stimulus trains. Gray bars on the PSTHs represent the period when shocks are being applied. To separate the suppressive from excitatory effects, each bin was normalized relative to baseline discharge measured 450–600 ms after the start of the 30-ms shock train and plotted in black (rate less than baseline) or gray (rate greater than baseline). PSTHs are only plotted out to 400 ms after the end of the spike train in this and subsequent figures, although data were routinely taken out to 700 ms. Atropine at 30 μM blocked most of the efferent response, and the effects of all drugs on efferent action were normalized to those of 30 μM atropine in the same preparation. Perfusion with solutions containing low calcium (nominally 0 mM) also reduced the efferent response. C: time course and measurement of drug action. Here PSTHs are created from overlapping 100-s periods, and an index of efferent suppression was calculated (see Methods) to quantify the changes, as a function of time, of efferent suppression during drug perfusion. Index is constructed such that 100% suppression of afferent discharge with efferent stimulation would give a value of 1, and 0% suppression would give a value of 0.
Quantifying the change in efferent suppression during drug perfusion was complicated by the afterexcitation, which occurred following the efferent suppression, but not at any fixed point in time. In fact, attenuating the suppression usually decreased the time of onset of the afterexcitation. Simply averaging the discharge rate following the shock train did not reflect the drug-induced changes in efferent suppression. Instead we calculated an index of efferent suppression from the PSTH.
The index of efferent suppression was calculated at times from 50 to 200 ms following the onset of the 30-ms shock train. The first 20 ms after the shocks ended were ignored to prevent inclusion of artifact that was occasionally present. To eliminate contributions of the after excitation, any bin in the PSTH showing a relative increase in rate was reassigned a value of 1 (equal to the baseline rate). We then subtracted the average value of bins from 50 to 150 ms after the start of the PSTH from 1 to give an index of efferent suppression. Complete suppression during the entire 150-ms measurement period following the stimulus (this never actually occurred) would have produced an index of efferent suppression of 1. This is equivalent to a 100% suppression of spontaneous rate during the period. Complete abolition of the suppression following efferent stimulus, for example by turning the efferent stimulus off, produced an index of efferent suppression of around 0.05. This small offset in the absence of an efferent effect is the result of reassigning bins with rates above the baseline to a value equal to baseline.
To quantify effects of the drug, PSTHs were created every second from the previous 100 stimulus trains (~1 s/train). From each of these PSTHs, we calculated the index of efferent suppression and plotted the index as a function of time. An example is given in Fig. 1C. From plots such as those in Fig. 1C, we calculated the change in efferent index associated with drug injection by comparing the index before drug injection to that at the peak of the drug effect. We then calculated a percentage change in the index of efferent suppression associated with drug injection.
In all cases, the percentage change in the index of efferent suppression for any particular drug was compared with the percentage change in index of efferent suppression seen with application of 30 μM atropine, which we considered to represent the change in suppression index associated with efferent block.
A sigmoidal curve was fit to the concentration response curves with a least-squares fit of the following equation
where D is concentration of drug, and y is the percentage block of suppression. The EC50 is the concentration at which the response is blocked by 50%. The factor n defines the steepness of the curve and is equivalent to the Hill coefficient.
Drugs were obtained from Sigma, dissolved in the balanced salt solution, placed in aliquots, and frozen until needed. Because phenylmethylsufonyl fluoride (PMSF) has a very short (~0.5 h) half-life in aqueous solution, aliquots from a stock solution of 0.2 M PMSF in absolute ethanol were added to apamin-containing solutions moments before administration. Stocks of PMSF in ethanol were stored at −15°C and used within 3 wk.
RESULTS
Electrical stimulation of efferent fibers produces suppression with an afterexcitation of spontaneous afferent discharge
Afferent nerve fibers innervating hair cells discharge “spontaneously”; that is, they discharge in the absence of mechanical stimulation because the hair cell is slightly depolarized at rest and releasing neurotransmitter. Electrical stimulation of efferent nerve fibers in the lateral line organ suppresses this spontaneous discharge, and this suppression is followed by a brief increase in the probability of discharge. This response pattern is shown in Fig. 1A, where we have plotted afferent spikes before and after a brief efferent stimulus train. After recording the time of occurrence of each of these action potentials relative to the stimulus train, we constructed PSTHs of the efferent response (Fig. 1B). The relative suppressive and excitatory phases are easily observed when each bin of the PSTH is plotted relative to the baseline. As has been previously shown (Bobbin et al. 1985; Russell 1971a; Sewell and Starr 1991), these effects of efferent stimulation in this organ were attenuated by a variety of cholinergic blockers now known to be capable of blocking the alpha9/10 nicotinic cholinergic receptor (an example of block by atropine is shown in Fig. 1B). To measure the effects of pharmacological agents on efferent action, we quantified the suppression as a function of time by creating overlapping ongoing PSTHs and extracting an index of efferent suppression (see Methods for details). An example is plotted in Fig. 1C, in which efferent effects are reversibly blocked by perfusion with 30 μM atropine. In all experiments, the ability of 30 μM atropine to block or attenuate efferent shocks was ascertained to ensure that the suppression noted was indeed cholinergically mediated.
As has been previously reported (Russell 1971a), we noted that the effects of efferent stimulation were very sensitive to calcium concentration in the perfusate. For example, initial experiments used 1.5 mM calcium chloride, but we were able to more consistently show efferent effects with 2.3 mM calcium. Figure 1, B and C, shows reversible attenuation of efferent action by perfusion with solutions containing low (nominally 0 mM) calcium concentration. Afferent discharge rate increased when calcium concentration was lowered, as we and others have previously described (Dawkins and Sewell 2004; Drescher and Drescher 1987a,b; Guth and Drescher 1990; Russell 1971a; Sewell 1990).
Drugs that block SK channels block efferent actions
In isolated hair cells, the outward current following application of acetylcholine is blocked by agents known to block SK channels, including apamin (Nenov et al. 1996; Yoshida et al. 1994; Yuhas and Fuchs 1999). SK channel blockers, dequalinium and apamin, block the outward current following efferent terminal stimulation in the excised cochlea (Glowatzki and Fuchs 2000; Oliver et al. 2000). We have examined the ability of these agents to block efferent effects in the lateral line organ where dosage can be reasonably well controlled and multiple doses can be applied at the same synapse.
Apamin produced a concentration-dependent attenuation of efferent action (Fig. 2B). An example at 1 μM apamin is shown in Fig. 2A. At 1 μM, apamin produced a block of efferent suppression that was more than one-half of that produced by atropine (Fig. 2B). The effects of apamin were typically very long-lasting and did not always reverse over the course of an experiment. Dequalinium also blocked efferent effects in the lateral line organ. The attenuation of efferent effects by dequalinium was concentration-dependent (Fig. 3), with an EC50 of 16 μM. The sigmoidal curve fit (Fig. 3B) suggests that most, if not all, of the effects of efferent stimulation were blocked by dequalinium. Like apamin, dequalinium produced a very long-lasting block of efferent action. Neither apamin nor dequalinium produced consistent alterations in afferent discharge rate.
Fig. 2. Apamin attenuated both suppression and afterexcitation following efferent shocks.

A: responses from all animals tested are summarized (means and SE) in the dose–response curve. B: concentration–response curve for effects of apamin. Each data point is normalized relative to the amount of block produced, in the same preparation, by 30 μM atropine, which, for this set of data, was 86.5%. A sigmoidal curve, fit to the data, indicated an EC50 of 0.5 μM. Number of injections at each concentration was as follows: 10 nM, n = 3; 100 nM, n = 5; 1 μM, n = 5; 100 μm, n = 1.
Fig. 3. Dequalinium attenuated the effects of efferent stimulation in a concentration-dependent manner.

A: examples of changes in individual histograms. B: dose–response curve responses from all animals tested, where means and SE are plotted. Efferent block for each injection was measured relative to the block, in the same preparation, by 30 μM atropine, which, for this set of experiments, was 61%. A sigmoidal curve fit to the concentration–response data suggests an EC50 of 12 μM. Number of injections at each concentrations were 1 μM, n = 3; 3 μM, n = 1; 10 μM, n = 4; 30 μM, n = 4; 100 μM, n =2. Both cases at 100 μM showed 100% block; therefore no error bars are shown.
The EC50 of 16 μM for dequalinium is in line with an analysis of dequalinium block of SK channels cloned from the cochlea and expressed in human embryonic kidney (HEK) cells (EC50 of 6 μM) (Nie et al. 2004), considering the amount of tissue the drug must diffuse through to reach the efferent synapse. This raises the question of why relatively high concentrations of apamin are required to block efferent effects in the lateral line organ (EC50 of 800 nM) compared with that required to block cochlear SK channels expressed in HEK cells (EC50 0.6 nM) (Nie et al. 2004). We considered the possibility that extracellular proteolytic enzymes may destroy apamin, a 4,000-MW peptide, before it reaches the efferent synapse. To test this, we combined apamin with PMSF, a proteolysis inhibitor with specificity for serine and cysteine containing proteolytic enzymes. PMSF alone, at concentrations of 20 and 200 μM, produced a small, reversible decrease in afferent discharge rate, but only with the first application. At 200 μM, there was also a small enhancement of efferent action, consistent with its known ability to inhibit acetylcholinesterase (Skau and Shipley 1999; Turini et al. 1969). However, it did not produce a marked change in the effects of apamin. We also administered apamin in combination with albumin (0.2 mg/ml), which can compete with apamin in proteolysis. Albumin did not alter the potency of apamin in blocking efferent action.
We found, as Russell (1971b) earlier noted, no strong correlation between the amount of suppression and the amount of afterexcitation following efferent stimulation. Both the excitation and suppression were blocked by atropine (Fig. 1, B and C), indicating both are produced by cholinergic stimulation. Both of the SK channel blockers, apamin and dequalinium, attenuated the excitatory phases (Figs. 2A and 3A) over similar dose ranges for attenuation of the suppressive phase. The afterexcitation was usually relatively small and often varied with time, independent of any drug applied. This made it difficult to determine if there was a correlation amount of attenuation of the suppression and afterexcitation with SK channel blockers. However, it is clear that the afterexcitation did not become larger after application of SK channel blockers, as might be expected if cholinergic activation produced the suppression via SK channels and the excitation via a mechanism independent of the SK channels. The results with apamin and dequalinium thus indicate that the afterexcitation is a consequence of the suppression.
Ryanodine attenuated the effects of efferent stimulation
The efferent synapse displays an unusual structure on the hair cell (postsynaptic) side of the synapse called a subsynaptic cisternae. This is an endoplasmic reticulum-like structure, which closely apposes the synapse and defines a limited (10–20 nm thick) cytosolic space between the postsynaptic membrane and the cisternae. This structure is suggested to play a role in amplifying calcium signals via calcium-induced calcium release following entry of calcium through the synapse (Evans et al. 2000; Lioudyno et al. 2004; Sridhar et al. 1997). We have examined several agents known to alter release of calcium from the endoplasmic reticulum.
Ryanodine can bind to receptors on the endoplasmic reticulum to alter calcium-induced calcium release. Ryanodine suppressed the effects of efferent stimulation (Fig. 4) with a nearly complete block of efferent effects at 3 mM. Concentrations of ryanodine that blocked efferent effects also reduced spontaneous discharge rate, with a nearly complete block of afferent discharge at 3 mM (Fig. 4C).
Fig. 4. Ryanodine attenuates efferent effects.
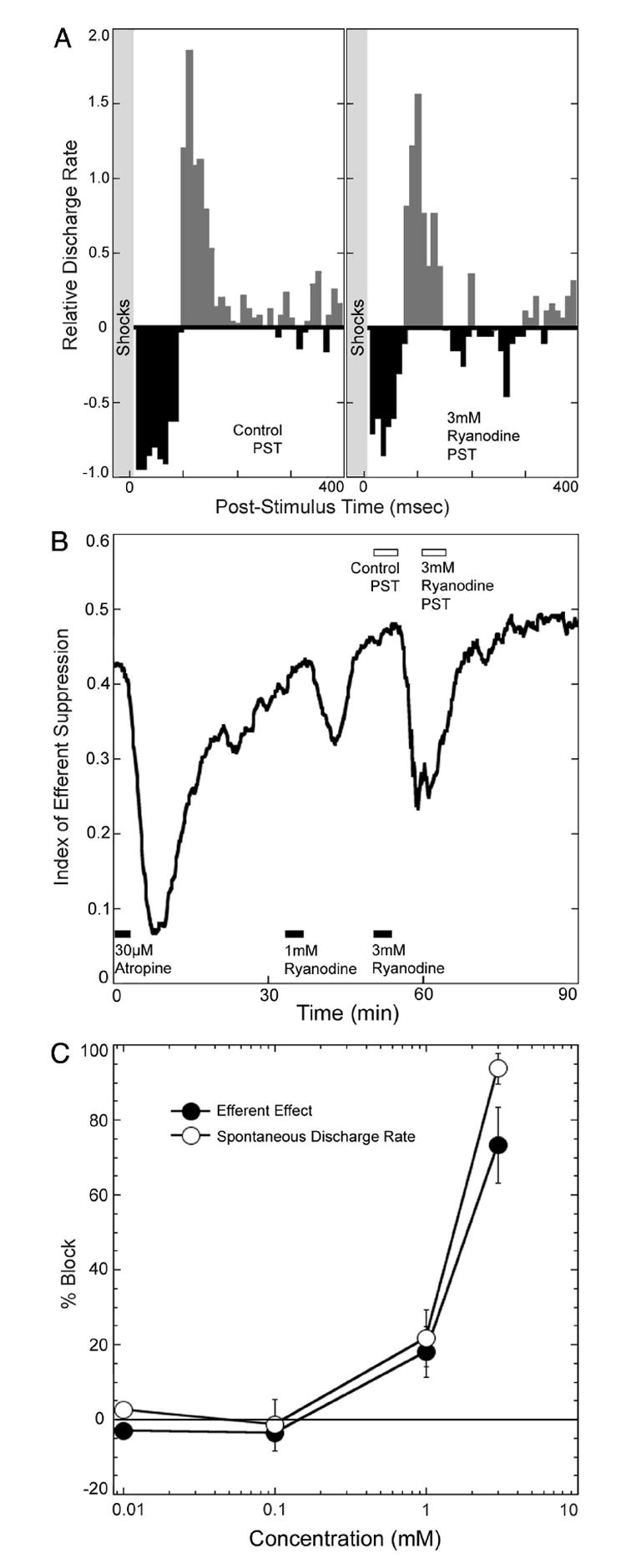
A: effects on the PSTH of perfusion with ryanodine. B: time course of attenuation of efferent suppression and comparison of the block with that of 30 μM atropine. Times of drug perfusion are indicated with filled boxes at the bottom. Times during which the PSTHs, shown in A, were taken are indicated with open boxes at the top. C: ryanodine decreased both the efferent effects and spontaneous discharge rate in afferent fibers over similar concentration ranges. Both the spontaneous discharge rate and the efferent effect were nearly completely blocked at concentrations of 3 mM. Responses from all animals tested are summarized in these dose–response curves (mean and SE). Efferent effects are expressed as a percentage of the block seen with 30 μM atropine, which in this set of experiments was 79.3%. The number of injections at each concentration was as follows: 10 μM, n = 2; 100 μM, n = 3; 1 mM, n = 7; 3 mM, n = 3. Data in A and B represent responses in PSTHs generated over 300-s time periods. This was done to overcome the variability associated with the decreases in afferent discharge rate.
Cyclopiazonic acid inhibits SERCA, an enzyme involved in uptake of calcium into the sarcoplasmic and endoplasmic reticulum. In the cochlea, cyclopiazonic acid enhanced slow effects of efferent stimulation without altering fast effects (Sridhar et al. 1997). In the lateral line organ, cyclopiazonic acid did not alter efferent effects at concentrations ranging from 1 to 100 μM, suggesting, as one might expect, that efferent effects in the lateral line organ are comparable with fast effects in the mammalian cochlea. While cyclopiazonic acid was ineffective in altering the effects of efferent stimulation, concentrations >30 μM did reduce afferent discharge rate. Dantrolene sodium, which can attenuate the release of calcium from sarcoplasmic reticulum, did not alter efferent effects at concentrations of ≤100 μM.
Cationic styryl dyes block efferent effects
Several cationic styryl dyes have been used to selectively label hair cells when applied to the apical surface of the hair cell epithelium (Balak et al. 1990; Flock et al. 1998; Gale et al. 2001; Le Calvez and Ulfendahl 2000; Meyers et al. 2003; Nishikawa and Sasaki 1996; Seiler and Nicolson 1999; Si et al. 2003). One hypothesis is that these dyes enter the hair cell via the transduction channels, which are nonspecific cation channels. Because the molecules are much larger than most permeant cations, they are thought to block transduction as they move slowly through the channels. Because the alpha9/10 nicotinic receptor forms a nonspecific cation channel and is known to be susceptible to block by the cationic aminoglyco-side antibiotics (Blanchet et al. 2000; Rothlin et al. 2003; Yoshida et al. 1999), we tested the ability of some of the cationic styryl dyes, specifically 4-di-2-asp and fm 1–43, to block efferent action. 4-di-2-asp reversibly blocked efferent effects at concentrations of between 30 and 100 μM (Fig. 5). Similarly, fm 1–43 also attenuated efferent actions (data not shown), but required concentrations higher than 100 μM. Both dyes also reduced afferent discharge rate. Recovery of afferent rate with 4-di-2-asp was relatively fast (see Fig. 5), but recovery following fm 1–43 took several hours.
Fig. 5. Styryl dyes can block the effects of efferent stimulation.

A: PSTHs obtained before and during infusion with 100 μM 4-di-2-asp. B: time course of attenuation of efferent effects (top) and decrease in spontaneous discharge rate (bottom) are indicated. Similar results were observed with fm 1– 43, except that the effects took much longer to reverse.
We also examined the hair cells with fluorescent microscopy following block to determine if the dyes may have entered the hair cell via these cholinergic ionotropic channels. Because the inner surface of the skin was perfused, the dye did not have access to the transduction channels, through which it is known to enter. Examination of surface preparations of the tissue with fluorescent microscopy showed distribution of the dye throughout the tissue of the skin, including hair cells, making it difficult to discern any specific labeling of efferent synapses.
DISCUSSION
We have shown, in an intact hair cell organ, that efferent effects can be blocked by agents known to block SK channels. This supports the generally held hypothesis, derived from work on isolated hair cells and excised organ of Corti preparations, that calcium enters through the nicotinic receptor to activate SK channels, which hyperpolarize the cell. The demonstration of these actions in the amphibian lateral line organ indicates that these efferent mechanisms may be a general feature of efferent action. The attenuation of efferent effects with ryanodine are consistent with the idea that one role of the subsynaptic cisternae is to amplify the calcium signal through calcium-induced calcium release. The ability of cationic styryl dyes to block efferent stimulation suggests the possibility that these dyes might be used as visual markers for efferent stimulation similar to their use to indicate transduction in hair cells.
SK channels and efferent signaling
We have shown that apamin can attenuate the effects of efferent stimulation. However, the concentrations required to block efferent effects (EC50 of 800 nM) are significantly higher than those normally needed to block SK channels (generally picomolar to low nanomolar concentrations) (Strobaek et al. 2000). SK2 channels, cloned from the cochlea and expressed in HEK cells, are sensitive to apamin, being blocked with an EC50 of 600 pM (Nie et al. 2004). We suggest that the insensitivity of efferent action to apamin may be because the SK channels that produce effects of efferent stimulation are located deep within the efferent synapse in close proximity to the efferent receptors and may thus be relatively inaccessible to this large (4,000 MW) peptide. Attempts to block proteolytic enzymes with PMSF did not enhance apamin’s potency, suggesting that proteolytic degradation may not be a dominant factor. The ability of PMSF to reduce afferent discharge rate and to enhance efferent action (presumably by its known action to inhibit acetylcholinesterase) indicates that the drug is active and likely reaching the synapse. It is possible then that other mechanisms, such as uptake of the peptide, are available and protect the SK channels associated with the efferent synapse from exposure to apamin.
The concentrations of apamin required to block efferent effects in the amphibian lateral line organ are comparable with those required in isolated hair cells (Nenov et al. 1996; Yamamoto et al. 1997; Yoshida et al. 1994; Yuhas and Fuchs 1999). In the intact mammalian cochlea, the effects of efferent stimulation are highly resistant to block by apamin, being attenuated only at concentrations as high as 100 μM. Even then, only the slow effects of efferent stimulation are susceptible (Yoshida et al. 2001). It is possible that if uptake mechanisms do exist to prevent apamin access to the efferent synapse, then those mechanisms may be more active in the in vivo mammalian cochlea than in the lateral line organ.
Dequalinium, a much smaller molecule, blocks efferent action at reasonable concentrations. The EC50 of 16 μM was higher than that seen for SK channels cloned from the cochlea and expressed in HEK cells (EC50 of 6 μM; Nie et al. 2004), but this is likely due to the diffusional distance required to reach the synapse after perfusion at the inner surface of the skin. One caution in interpreting the effects of dequalinium is that many agents that block SK channels also block nicotinic cholinergic receptors. Two SK channel blockers, tetraethyl ammonium and tubocurare (Park 1994), are known to block the alpha9/10 nicotinic receptor (Blanchet et al. 1996; Elgoyhen et al. 1994). Dequalinium can block ganglionic nicotinic receptors at micromolar concentrations (Dunn 1993), although its action on the alpha9/10 receptor is not determined.
Calcium signaling and amplification
The effects of efferent stimulation are highly sensitive to changes in extracellular calcium concentrations, such that we had to increase the calcium concentration in the perfusate to 2.3 mM from our normal level of 1.5 mM. This increased the probability of observing efferent effects. This finding is consistent with observations that the alpha9 nicotinic receptor is permeable to calcium and regulated by divalent cation concentrations (Weisstaub et al. 2002), although many other possibilities for the effects of calcium on efferent action in the lateral line organ (discussed in Russell 1971a).
Calcium entering via the activated cholinergic receptor is the trigger for efferent action. The role of the subsynaptic cisternae in this process is debatable. Its similarity to endoplasmic reticulum posits a role in calcium release. Martin and Fuchs (1992) approached the issue mathematically, determining that the amount of calcium entering via the acetylcholine receptor is sufficient to activate the KCa channels responsible for hyperpolarization without additional calcium released from the cisternae. They suggest that the subsynaptic cisternae may serve a role to constrain calcium entering via the acetylcholine channel to a region near the synapse. On the other hand, the possibility that intracellular calcium release may amplify this calcium signal in efferent action has been raised based on the ability of ryanodine to modify cholinergic efferent action. The ryanodine receptor in endoplasmic reticulum mediates calcium-induced calcium release. Ryanodine acts as an agonist for the ryanodine receptor at low concentrations and blocks at higher (near millimolar) concentrations (Coronado et al. 1994; Rousseau et al. 1987). Relevant findings include 1) the ability of ryanodine to enhance effects of efferent stimulation in the mammalian cochlea at concentrations of around 30 μM (Sridhar et al. 1997); 2) ryanodine’s ability at 100 μM to block responses in isolated hair cells to cholinergic agonists (Evans et al. 2000); 3) ryanodine’s ability to block acetylcholine’s effects on basilar membrane motion (Murugasu and Russell 1996); and 4) ryanodine’s ability to enhance and then block the effects of potassium-induced efferent stimulation in the excised organ of Corti preparation (Lioudyno et al. 2004).
The reduction of efferent action by ryanodine in the lateral line organ suggests that this amplification mechanism may be generally applicable to efferent action in hair cells, although the high concentrations of ryanodine needed to block raise questions of specificity of the effect. The concentrations required were generally higher than those needed for action in isolated outer hair cells (Evans et al. 2000), where 0.1 mM ryanodine produced an 19% block of the acetylcholine evoked potassium current in cells that responded to ryanodine. It is also higher than in excised organ of Corti preparations (Lioudyno et al. 2004), where 100 μM ryanodine produced a 30% block of the efferent-evoked response. We required 1 mM ryanodine to produce a similar block of 20%. Given that the ryanodine must diffuse through the tissue of the frog skin to act and that these were relatively large frogs, a 10-fold difference in sensitivity is not surprising.
We note the ability of ryanodine to block afferent activity over concentration ranges similar to those that blocked efferent effects. It as been suggested that a calcium-induced calcium release process may be involved in afferent transmitter release from the hair cell (Kennedy and Meech 2002; Lelli et al. 2003). Concentrations of ryanodine required to block afferent transmission in the lateral line organ were comparable with those required to block afferent transmission in the labyrinth (Kennedy and Meech 2002), although higher than that required to reduce transmitter release from the semi-isolated hair cells of the neonatal mouse (Kennedy and Meech 2002; Lelli et al. 2003), where drug access is presumably better. This mechanism likely accounts for the decrease in spontaneous rate we observed in the lateral line organ.
That afferent transmitter release and efferent transmitter action are blocked in the lateral line organ at similar concentrations of ryanodine would argue for similar ryanodine-sensitive components to be active in both processes. The action of ryanodine on calcium-induced calcium release is complex. Ryanodine at submicromolar concentrations can interact with the ryanodine receptor to put the receptor in a subconductance state that can alter its interaction with calcium, but concentrations of 0.5 mM can be required to block responses mediated through the ryanodine receptor (Nagasaki and Fleischer 1988; Protasi et al. 2004).
Other drugs that alter calcium signaling in the cell can influence some of the less prominent effects of efferent stimulation in the mammalian cochlea. SERCA inhibitors, cyclopiazonic acid and thapsigargin, enhance the slow effects of efferent stimulation (Sridhar et al. 1997). Consistent with the interpretation that slow effects are produced by action in the subsurface cisternae, which are not present in hair cells of the lateral line organ, we did not observe changes in the effects of efferent stimulation with cyclopiazonic acid. The inability to block efferent effects with SERCA inhibitors does raise the issue of whether the subsynaptic cisterna is repleted using SERCA enzymes. This is an issue we probably cannot resolve from our data. First, it is not clear that we are delivering concentrations of agents for a sufficient length of time to deplete the reservoir. In this regard, a negative result does not allow a deduction. Second, it is not clear just what proportion of the efferent response is due to unamplified calcium signal via the receptor and how much is due to calcium-induced calcium release.
Cationic styryl dyes block efferent action
Many of the cationic styryl dyes, originally developed for monitoring endocytosis, can be selectively taken up by hair cells when applied to the apical surface of the cell. Examples include fm 1–43, daspei, and 4-di-2-asp (Balak et al. 1990; Flock et al. 1998; Gale et al. 2001; Le Calvez and Ulfendahl 2000; Meyers et al. 2003; Nishikawa and Sasaki 1996; Seiler and Nicolson 1999; Si et al. 2003). While there is considerable debate regarding the mechanism by which the dyes are selectively taken up by the hair cell, some have suggested that they enter through the transduction channel, which is a nonspecific cation channel. If this is the case, it may be possible to label efferent synapses with these dyes by applying dyes to the efferent synapse while stimulating efferent fibers. Toward this end, an initial goal is to determine if the dyes are blocking efferent stimulation and we have shown that at least two of them, fm 143 and 4-di-2-asp, do block efferent action. However, the lateral line organ, in which the sensory cells are deeply embedded in a complex tissue (skin), did not prove an ideal venue to test whether hair cells are specifically labeled because of the broad spectrum of cells throughout the skin that take up these dyes. It is possible that these dyes can serve as a visualized marker for efferent activation in a less complex tissue, such as the organ of Corti.
Generality of efferent mechanisms
Different hair cell organs likely share key features of efferent signaling mechanisms. The primary efferent feedback system in most of these organs uses acetylcholine acting via an ionotropic nicotinic receptor, containing alpha9 and alpha10 subunits (Elgoyhen et al. 1994, 2001; Glowatzki and Fuchs 2000; Katz et al. 2004; Morley and Simmons 2002). A subsynaptic cisternae on the hair cell is a dominant feature of efferent synaptic morphology. Pharmacological profiles of efferent transmission and signaling are common to most of these systems analyzed. A parsimonious hypothesis for the mechanism of efferent signaling is that a calcium signal, triggered by calcium entry via the nicotinic receptor and amplified by calcium-induced calcium release from the subsynaptic cisternae, activates SK channels to induce potassium exit from the cell and subsequent hyperpolarization. Consistent with this hypothesis, whole cell recordings from isolated hair cells and those in excised cochlea have shown block of cholinergic responses with the SK channel blockers, apamin and dequalinium (Glowatzki and Fuchs 2000; Holt et al. 2001; Nenov et al. 1996; Oliver et al. 2000; Yoshida et al. 1994; Yuhas and Fuchs 1999). A weak link in this hypothesis has been, until now, the inability to observe a block of the action of electrical stimulation of efferent neurons with apamin (Bobbin and LeBlanc 1999; Yoshida et al. 2001). The resistance of the mammalian cochlea is perplexing, but may be related to uptake or other mechanisms that protect the efferent synapse from access by this large peptide. Attenuation of electrical efferent stimulation by apamin and dequalinium in the lateral line organ provide additional support for the argument that SK channels are indeed involved.
Acknowledgments
We thank I. Stefanov-Wagner, F. Cardarelli, and C. Scarpino for engineering support and M. C. Brown, J. Guinan, and S. Heller for discussions and criticisms of early versions of the manuscript.
GRANTS This work was supported by National Institute of Deafness and Other Communicative Disorders Grant DC-00767 to W. F. Sewell and by a grant from the Raine Foundation for Medical Research to R. Dawkins.
References
- Art JJ, Crawford AC, Fettiplace R, Fuchs PA. Efferent regulation of hair cells in the turtle cochlea. Proc R Soc Lond B Biol Sci. 1982;216:377–384. doi: 10.1098/rspb.1982.0081. [DOI] [PubMed] [Google Scholar]
- Art JJ, Fettiplace R, Fuchs PA. Synaptic hyperpolarization and inhibition of turtle cochlear hair cells. J Physiol. 1984;356:525–550. doi: 10.1113/jphysiol.1984.sp015481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey GP, Sewell WF. Calcitonin gene-related peptide suppresses hair cell responses to mechanical stimulation in the Xenopus lateral line organ. J Neurosci. 2000;20:5163–5169. doi: 10.1523/JNEUROSCI.20-13-05163.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balak KJ, Corwin JT, Jones JE. Regenerated hair cells can originate from supporting cell progeny: evidence from phototoxicity and laser ablation experiments in the lateral line system. J Neurosci. 1990;10:2502–2512. doi: 10.1523/JNEUROSCI.10-08-02502.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet C, Erostegui C, Sugasawa M, Dulon D. Acetylcholine-induced potassium current of guinea pig outer hair cells: its dependence on a calcium influx through nicotinic-like receptors. J Neurosci. 1996;16:2574–2584. doi: 10.1523/JNEUROSCI.16-08-02574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet C, Erostegui C, Sugasawa M, Dulon D. Gentamicin blocks ACh-evoked K+ current in guinea-pig outer hair cells by impairing Ca2+ entry at the cholinergic receptor. J Physiol. 2000;525:641–654. doi: 10.1111/j.1469-7793.2000.t01-1-00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobbin RP. Caffeine and ryanodine demonstrate a role for the ryanodine receptor in the organ of Corti. Hear Res. 2002;174:172–182. doi: 10.1016/s0378-5955(02)00654-8. [DOI] [PubMed] [Google Scholar]
- Bobbin RP, Bledsoe SC, Jr, Winbery S, Ceasar G, Jenison GL. Comparative actions of GABA and acetylcholine on the Xenopus laevis lateral line. Comp Biochem Physiol C. 1985;80:313–318. doi: 10.1016/0742-8413(85)90062-3. [DOI] [PubMed] [Google Scholar]
- Bobbin RP, LeBlanc CS. Apamin reduces but does not abolish the effects of contralateral suppression of cubic dpoaes. In: Berlin CI, editor. The Efferent Auditory System. San Diego, CA: Singular Publishing Group; 1999. pp. 61–72. [Google Scholar]
- Coronado R, Morrissette J, Sukhareva M, Vaughan DM. Structure and function of ryanodine receptors. Am J Physiol. 1994;266:C1485–C1504. doi: 10.1152/ajpcell.1994.266.6.C1485. [DOI] [PubMed] [Google Scholar]
- Dawkins R, Sewell WF. Afferent synaptic transmission in a hair cell organ: pharmacological and physiological analysis of the role of the extended refractory period. J Neurophysiol. 2004;92:1105–1115. doi: 10.1152/jn.01107.2003. [DOI] [PubMed] [Google Scholar]
- Drescher DG, Drescher MJ. Calcium and magnesium dependence of spontaneous and evoked afferent neural activity in the lateral-line organ of Xenopus laevis. Comp Biochem Physiol A. 1987a;87:305–310. doi: 10.1016/0300-9629(87)90126-5. [DOI] [PubMed] [Google Scholar]
- Drescher DG, Drescher MJ. Spontaneous neural activity of a mechanoreceptive system is undiminished by replacement of external calcium with equimolar magnesium in the presence of EGTA. Life Sci. 1987b;40:1371–1377. doi: 10.1016/0024-3205(87)90327-4. [DOI] [PubMed] [Google Scholar]
- Dulon D, Luo L, Zhang C, Ryan AF. Expression of small-conductance calcium-activated potassium channels (SK) in outer hair cells of the rat cochlea. Eur J Neurosci. 1998;10:907–915. doi: 10.1046/j.1460-9568.1998.00098.x. [DOI] [PubMed] [Google Scholar]
- Dunn PM. Ganglion-blocking activity of dequalinium in frog and rat sympathetic ganglia in vitro. Eur J Pharmacol. 1993;230:335–339. doi: 10.1016/0014-2999(93)90570-8. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci USA. 2001;98:3501–3506. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MG, Lagostena L, Darbon P, Mammano F. Cholinergic control of membrane conductance and intracellular free Ca2+ in outer hair cells of the guinea pig cochlea. Cell Calcium. 2000;28:195–203. doi: 10.1054/ceca.2000.0145. [DOI] [PubMed] [Google Scholar]
- Flock A, Russell I. Inhibition by efferent nerve fibres: action on hair cells and afferent synaptic transmission in the lateral line canal organ of the burbot Lota lota. J Physiol. 1976;257:45–62. doi: 10.1113/jphysiol.1976.sp011355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock A, Scarfone E, Ulfendahl M. Vital staining of the hearing organ: visualization of cellular structure with confocal microscopy. Neuroscience. 1998;83:215–228. doi: 10.1016/s0306-4522(97)00335-7. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Murrow BW. Cholinergic inhibition of short (outer) hair cells of the chick’s cochlea. J Neurosci. 1992;12:800–809. doi: 10.1523/JNEUROSCI.12-03-00800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J Neurosci. 2001;21:7013–7025. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea. Science. 2000;288:2366–2368. doi: 10.1126/science.288.5475.2366. [DOI] [PubMed] [Google Scholar]
- Guth SL, Drescher DG. Effects of divalent cations on the frequency of spontaneous action potentials from the lateral line organ of Xenopus laevis. Brain Res. 1990;508:76–84. doi: 10.1016/0006-8993(90)91120-6. [DOI] [PubMed] [Google Scholar]
- Harris GG, Flock A. Spontaneous and evoked activity from the Xenopus laevis lateral line. In: Cahn P, editor. Lateral Line Detectors. Bloomington, IN: Indiana University Press; 1967. pp. 135–162. [Google Scholar]
- Harris GG, Milne DC. Input-output characteristics of the lateral-line sense organs of Xenopus laevis. J Acoust Soc Am. 1966;40:32–42. doi: 10.1121/1.1910060. [DOI] [PubMed] [Google Scholar]
- Hendricson AW, Guth PS. Transmitter release from Rana pipiens vestibular hair cells via mGluRs: a role for intracellular Ca(++) release. Hear Res. 2002;172:99–109. doi: 10.1016/s0378-5955(02)00519-1. [DOI] [PubMed] [Google Scholar]
- Holt JC, Lioudyno M, Athas G, Garcia MM, Perin P, Guth PS. The effect of proteolytic enzymes on the alpha9-nicotinic receptor-mediated response in isolated frog vestibular hair cells. Hear Res. 2001;152:25–42. doi: 10.1016/s0378-5955(00)00225-2. [DOI] [PubMed] [Google Scholar]
- Housley GD, Ashmore JF. Direct measurement of the action of acetylcholine on isolated outer hair cells of the guinea pig cochlea. Proc R Soc Lond B Biol Sci. 1991;244:161–167. doi: 10.1098/rspb.1991.0065. [DOI] [PubMed] [Google Scholar]
- Housley GD, Norris CH, Guth PS. Cholinergically-induced changes in outward currents in hair cells isolated from the semicircular canal of the frog. Hear Res. 1990;43:121–133. doi: 10.1016/0378-5955(90)90221-a. [DOI] [PubMed] [Google Scholar]
- Katz E, Elgoyhen AB, Gomez-Casati ME, Knipper M, Vetter DE, Fuchs PA, Glowatzki E. Developmental regulation of nicotinic synapses on cochlear inner hair cells. J Neurosci. 2004;24:7814–7820. doi: 10.1523/JNEUROSCI.2102-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy HJ, Meech RW. Fast Ca2+ signals at mouse inner hair cell synapse: a role for Ca2+-induced Ca2+ release. J Physiol. 2002;539:15–23. doi: 10.1113/jphysiol.2001.013171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroese AB, Van der Zalm JM, Van den Bercken J. Frequency response of the lateral-line organ of Xenopus laevis. Pfluegers. 1978;375:167–175. doi: 10.1007/BF00584240. [DOI] [PubMed] [Google Scholar]
- Le Calvez S, Ulfendahl M. An in vitro preparation to access cellular and neuronal components in the mouse inner ear. J Neurocytol. 2000;29:645–652. doi: 10.1023/a:1010831303845. [DOI] [PubMed] [Google Scholar]
- Lelli A, Perin P, Martini M, Ciubotaru CD, Prigioni I, Valli P, Rossi ML, Mammano F. Presynaptic calcium stores modulate afferent release in vestibular hair cells. J Neurosci. 2003;23:6894–6903. doi: 10.1523/JNEUROSCI.23-17-06894.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioudyno M, Hiel H, Kong J, Katz E, Waldman E, Parameshwaran-Iyer S, Glowatzki E, Fuchs PA. A “synaptoplasmic cistern” mediates rapid inhibition of cochlear hair cells. J Neurosci. 2004;24:11160–11164. doi: 10.1523/JNEUROSCI.3674-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AR, Fuchs PA. The dependence of calcium-activated potassium currents on membrane potential. Proc R Soc Lond B Biol Sci. 1992;250:71–76. doi: 10.1098/rspb.1992.0132. [DOI] [PubMed] [Google Scholar]
- Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley BJ, Simmons DD. Developmental mRNA expression of the alpha10 nicotinic acetylcholine receptor subunit in the rat cochlea. Brain Res Dev Brain Res. 2002;139:87–96. doi: 10.1016/s0165-3806(02)00514-x. [DOI] [PubMed] [Google Scholar]
- Murray MJ, Capranica RR. Spike generation in the lateral-line afferents of Xenopus laevis, evidence favoring multiple sites of initiation. J Comp Physiol. 1973;87:1–20. [Google Scholar]
- Murugasu E, Russell IJ. The role of calcium on the effects of intraco-chlear acetylcholine perfusion on basilar membrane displacement in the basal turn of the guinea pig cochlea. Auditory Neurosci. 1996;2:363–376. doi: 10.1523/JNEUROSCI.16-01-00325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki K, Fleischer S. Ryanodine sensitivity of the calcium release channel of sarcoplasmic reticulum. Cell Calcium. 1988;9:1–7. doi: 10.1016/0143-4160(88)90032-2. [DOI] [PubMed] [Google Scholar]
- Nenov AP, Norris C, Bobbin RP. Acetylcholine response in guinea pig outer hair cells. II. Activation of a small conductance Ca(2+)-activated K+ channel. Hear Res. 1996;101:149–172. doi: 10.1016/s0378-5955(96)00143-8. [DOI] [PubMed] [Google Scholar]
- Nie L, Song H, Chen MF, Chiamvimonvat N, Beisel KW, Yamoah EN, Vazquez AE. Cloning and expression of a small-conductance Ca(2+)-activated K+ channel from the mouse cochlea: coexpression with alpha9/alpha10 acetylcholine receptors. J Neurophysiol. 2004;91:1536–1544. doi: 10.1152/jn.00630.2003. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Sasaki F. Internalization of styryl dye FM1-43 in the hair cells of lateral line organs in Xenopus larvae. J Histochem Cytochem. 1996;44:733–741. doi: 10.1177/44.7.8675994. [DOI] [PubMed] [Google Scholar]
- Oliver D, Klocker N, Schuck J, Baukrowitz T, Ruppersberg JP, Fakler B. Gating of Ca2+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron. 2000;26:595–601. doi: 10.1016/s0896-6273(00)81197-6. [DOI] [PubMed] [Google Scholar]
- Pabst A. Number and location of the sites of impulse generation in the lateral line afferents of Xenopus laevis. J Comp Physiol. 1976;114:51–67. [Google Scholar]
- Park YB. Ion selectivity and gating of small conductance Ca(2+)-activated K+ channels in cultured rat adrenal chromaffin cells. J Physiol. 1994;481:555–570. doi: 10.1113/jphysiol.1994.sp020463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protasi F, Shtifman A, Julian FJ, Allen PD. All three ryanodine receptor isoforms generate rapid cooling responses in muscle cells. Am J Physiol Cell Physiol. 2004;286:C662–C670. doi: 10.1152/ajpcell.00081.2003. [DOI] [PubMed] [Google Scholar]
- Rothlin CV, Lioudyno MI, Silbering AF, Plazas PV, Casati ME, Katz E, Guth PS, Elgoyhen AB. Direct interaction of serotonin type 3 receptor ligands with recombinant and native alpha 9 alpha 10-containing nicotinic cholinergic receptors. Mol Pharmacol. 2003;63:1067–1074. doi: 10.1124/mol.63.5.1067. [DOI] [PubMed] [Google Scholar]
- Rousseau E, Smith JS, Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am J Physiol. 1987;253:C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Russell IJ. The pharmacology of efferent synapses in the lateral-line system of Xenopus laevis. J Exp Biol. 1971a;54:643–658. doi: 10.1242/jeb.54.3.643. [DOI] [PubMed] [Google Scholar]
- Russell IJ. The role of the lateral-line efferent system in Xenopus laevis. J Exp Biol. 1971b;54:621–641. doi: 10.1242/jeb.54.3.621. [DOI] [PubMed] [Google Scholar]
- Seiler C, Nicolson T. Defective calmodulin-dependent rapid apical endocytosis in zebrafish sensory hair cell mutants. J Neurobiol. 1999;41:424–434. [PubMed] [Google Scholar]
- Sewell WF. Synaptic potentials in afferent fibers innervating hair cells of the lateral line organ in Xenopus laevis. Hear Res. 1990;44:71–81. doi: 10.1016/0378-5955(90)90023-i. [DOI] [PubMed] [Google Scholar]
- Sewell WF, Starr PA. Effects of calcitonin gene-related peptide and efferent nerve stimulation on afferent transmission in the lateral line organ. J Neurophysiol. 1991;65:1158–1169. doi: 10.1152/jn.1991.65.5.1158. [DOI] [PubMed] [Google Scholar]
- Shelton PMJ. The structure and function of the lateral line system in larval Xenopus laevis. J Exp Zool. 1971;178:211–232. doi: 10.1002/jez.1401780207. [DOI] [PubMed] [Google Scholar]
- Si F, Brodie H, Gillespie PG, Vazquez AE, Yamoah EN. Developmental assembly of transduction apparatus in chick basilar papilla. J Neurosci. 2003;23:10815–10826. doi: 10.1523/JNEUROSCI.23-34-10815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skau KA, Shipley MT. Phenylmethylsulfonyl fluoride inhibitory effects on acetylcholinesterase of brain and muscle. Neuropharmacology. 1999;38:691–698. doi: 10.1016/s0028-3908(98)00205-6. [DOI] [PubMed] [Google Scholar]
- Sridhar TS, Brown MC, Sewell WF. Unique postsynaptic signaling at the hair cell efferent synapse permits calcium to evoke changes on two time scales. J Neurosci. 1997;17:428–437. doi: 10.1523/JNEUROSCI.17-01-00428.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelioff D, Honrubia V. Neural transduction in Xenopus laevis lateral line system. J Neurophysiol. 1978;41:432–444. doi: 10.1152/jn.1978.41.2.432. [DOI] [PubMed] [Google Scholar]
- Strobaek D, Jorgensen TD, Christophersen P, Ahring PK, Olesen SP. Pharmacological characterization of small-conductance Ca(2+)-activated K(+) channels stably expressed in HEK 293 cells. Br J Pharmacol. 2000;129:991–999. doi: 10.1038/sj.bjp.0703120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turini P, Kurooka S, Steer M, Corbascio AN, Singer TP. The action of phenylmethylsulfonyl fluoride on human acetylcholinesterase, chymotyrpsin and trypsin. J Pharmacol Exp Ther. 1969;167:98–104. [PubMed] [Google Scholar]
- Weisstaub N, Vetter DE, Elgoyhen AB, Katz E. The alpha9alpha10 nicotinic acetylcholine receptor is permeable to and is modulated by divalent cations. Hear Res. 2002;167:122–135. doi: 10.1016/s0378-5955(02)00380-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kakehata S, Yamada T, Saito T, Saito H, Akaike N. Effects of potassium channel blockers on the acetylcholine-induced currents in dissociated outer hair cells of guinea pig cochlea. Neurosci Lett. 1997;236:79–82. doi: 10.1016/s0304-3940(97)00749-0. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Liberman MC, Brown MC, Sewell WF. Fast, but not slow, effects of olivocochlear activation are resistant to apamin. J Neurophysiol. 2001;85:84–88. doi: 10.1152/jn.2001.85.1.84. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Liberman MC, Brown MC, Sewell WF. Gentamicin blocks both fast and slow effects of olivocochlear activation in anesthetized guinea pigs. J Neurophysiol. 1999;82:3168–3174. doi: 10.1152/jn.1999.82.6.3168. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Shigemoto T, Sugai T, Ohmori H. The role of inositol trisphosphate on ACh-induced outward currents in bullfrog saccular hair cells. Brain Res. 1994;644:90–100. doi: 10.1016/0006-8993(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Yuhas WA, Fuchs PA. Apamin-sensitive, small-conductance, calcium-activated potassium channels mediate cholinergic inhibition of chick auditory hair cells. J Comp Physiol [A] 1999;185:455–462. doi: 10.1007/s003590050406. [DOI] [PubMed] [Google Scholar]


