Abstract
Rolandic epilepsy (RE) is designated an idiopathic epilepsy syndrome, and hence no lesional abnormalities are expected on MRI exam. Recent reports suggest that MRI abnormalities are not only common, but may be specific for temporal lobe epilepsy, and lateralized to the side of EEG discharges. However, no controlled study has been performed to test the hypothesis of association between MRI abnormalities and Rolandic epilepsy. We performed an unmatched case-control study to test the hypothesis of association between MRI abnormalities and Rolandic epilepsy, using 25 typical RE cases and 25 children with migraine. Two independent examiners rated the MRIs for abnormalities. Examiners were blinded to the study hypothesis and identity of case and control exams. Fifty-two percent of RE exams contained at least one abnormality: peri/hippocampal abnormality (1 case), non-localized congenital malformation (7 cases), subcortical parenchymal hyperintensities (2 cases), periventricular parenchymal hyperintensities (1 case), dilated perivascular spaces (6 cases). There was no difference between the number or type of abnormalities in cases and controls. No type of abnormality lateralized to the hemisphere from which the EEG spikes emanated. The odds ratio of association between MRI abnormalities and RE was 0.87, 95% CI: 0.18–4.33 after adjusting for potential demographic and technical factors. We conclude that routine cranial MRI abnormalities are common in RE, but no more common than in controls, and not specific for RE.
Keywords: Rolandic epilepsy, MRI abnormality, case-control study, migraine, pediatric
INTRODUCTION
Benign Epilepsy of Childhood with Centrotemporal Spikes (BECTS), also known as Rolandic epilepsy (RE), is an idiopathic epilepsy syndrome and its etiology is presumed to be complex genetic (1). The designation "idiopathic" implies absence of structural, inflammatory or metabolic brain lesions (2). However, several reports have demonstrated a relatively high frequency of routine brain MRI abnormalities in RE patients (3–5). These include both specific parenchymal abnormalities, such as hippocampal asymmetry, possibly ipsilateral to the rolandic seizure focus (3, 4) and focal brain lesions (6), as well as apparently incidental abnormalities such as ventricular dilatation, dilated perivascular spaces, non-specific white matter signal hyperintensities, Arnold-Chiari malformations, and congenital cysts (3, 5).
The etiological significance of such findings is not entirely clear because the incidence of these types of abnormalities in the general pediatric population is not well known, although they are probably fairly common (7). Moreover, because routine MRIs are evaluated by visual inspection, the possible role of observer bias in concluding that an association exists between Rolandic epilepsy and MRI abnormalities is also unknown. Image quality and other technical factors may further confound a potential association. Hence to test the hypothesis that RE patients exhibit excess imaging abnormalities on brain MRI, we compared the prevalence of MRI abnormalities in Rolandic epilepsy patients against controls. If the hypothesis were true, then we would predict (i) the prevalence of abnormalities in RE cases to exceed that in controls; (ii) that there would be some specificity to the types of imaging abnormality in RE; and (iii) that these abnormalities would tend to be ipsilateral to the hemisphere from which RE discharges emanate.
METHODS
Design
This was an unmatched case-control study with one-to-one ratio of cases and controls. Observer bias was controlled by blinding the neuroradiologists to the study hypothesis and to the identity of case and control MRI exams. We measured potential confounding variables including age and sex of subjects, and technical factors relating to the imaging quality, quantity and MR pulse sequences.
Materials
We obtained the brain MRI examinations of 25 RE patients and 25 pediatric control patients of similar age and demographic background who presented with recurrent headache. We obtained approval for the study from Lifespan Institutional Review Board, Rhode Island Hospital.
Eligibility
RE CASES
Children with Rolandic epilepsy were selected from a genetic linkage study ascertained from the eastern USA. Their phenotypes were rigorously evaluated for inclusion. Cases had to have a typical history of orofacial seizures with overall normal developmental milestones and interictal EEG showing centrotemporal sharp waves (CTS). Age of onset was between 3 and 12 years. Cases were rejected if they had only secondarily generalized seizures, even if their EEG demonstrated CTS. Three cases had the typical evolution of orofacial seizures ending with impaired consciousness, while six had alternating episodes of simple partial seizures with preserved consciousness and simple partial seizures with impaired consciousness. Cases were also rejected if other afebrile seizure types preceded the onset of Rolandic epilepsy. A summary of the clinical characteristics of the cases is shown in Table 1.
Table 1.
Clinical characteristics of Rolandic epilepsy cases.
| Number | 25 |
| Age of onset (years) first seizure, median (range) | 7.6 (2.0–10.8) |
| Handedness, left (%) | 23 (92) |
| Seizure lateralization, body side, n (%) | |
| Left | 11 (44) |
| Right | 8 (32) |
| Variable | 4 (16) |
| Uncertain | 2 (8) |
| EEG lateralization, hemisphere | |
| Left | 8 (36) |
| Right | 12 (50) |
| Both left and right foci observed | 5 (14) |
| Lifetime seizure total, n (%) | |
| ≤10 | 19 (76) |
| >10 | 6 (24) |
| Seizure spread, n (%) | |
| Face only | 7 (28) |
| Face and upper limb | 10 (40) |
| Face, UL and LL | 8 (32) |
| Seizure duration, n (%) | |
| ≤ 30 secs | 6 (24) |
| 30–60 secs | 6 (24) |
| > 1min | 13 (52) |
| Seizure related to sleep | 24 (96) |
| Ever treated with antiepileptic drugs, n(%) | 17 (68) |
| History of speech maturation problem, n(%) | 7 (28) |
| History of developmental reading difficulty, n (%) | 13 (52) |
CONTROLS
Control children were selected from the same referring pediatric neurology clinics. Children with migraine were chosen as controls, because they were likely to have had MRI scans as part of their routine evaluation. Migraine was diagnosed clinically by a board certified pediatric neurologist, on the basis of number, duration and characteristics of recurring headache, relief by sleep and associated somatic features (8). Children with migraine chosen as controls had to have no suspected structural, inflammatory or metabolic cause for their headache, and no prior history of epileptic seizure, and were required to have normal developmental milestones.
Assessment
MRI exams were evaluated independently by two board certified radiologists with certificates of additional qualification in neuroradiology (JLB, JR). Two reviewers were used because of the necessarily subjective manner in which minor abnormalities are interpreted by visual inspection. Importantly, the evaluators were blinded both to the disease status of the subjects, and to the study hypothesis, until the end of the study.
Imaging quality
Imaging was assessed subjectively for gross image quality, including artifacts due to patient motion and magnetic susceptibility artifacts due to the presence of orthodontic appliances. Additionally, objective data about each subject’s imaging was also collected, including the number of examinations, and the pulse sequences acquired for each exam. For example, high resolution T2-weighted coronal imaging through the temporal lobes is not commonly acquired on routine brain MRI exams, but is commonly performed when there is clinical evidence of epilepsy, because it provides more detailed evaluation of the hippocampal formations and mesial temporal structures that may have pathology in individuals with epilepsy. Additionally, high-resolution T1-weighted pulse sequences that afford high contrast between gray and white matter are potentially helpful for identifying “blurring” of the gray matter – white matter interface, which has been purported to be associated with RE (3). Exams were scored according to the pulse sequences performed on a scale from 10 to 16, with exams following a dedicated seizure protocol receiving a higher score. For example, exams with only sagittal T1, axial T2, axial FLAIR, axial GRE and DWI were rated as 10, while those with additional coronal T2, coronal FLAIR, axial MPRAGE were rated as 16. Exams with protocols in between these limits were scored as intermediate, according to the sequences available.
Classification
The two neuroradiologists independently evaluated the presence of (i) hippocampal and perihippocampal abnormalities (including atrophy, sclerosis, and peri-hippocampal lesions or cysts); (ii) congenital malformations (eg arachnoid cysts, pineal cysts, and Arnold-Chiari malformations); (iii) subcortical white matter and (iv) periventricular white matter parenchymal hyperintensities; (v) dilated perivascular spaces and (vi) cortical and sulcal morphologic abnormalities. These categories were created to reflect the types of abnormalities reported to be associated with epilepsy in the literature (3, 5).
Statistical analysis
We calculated inter-rater agreement (kappa scores) for abnormalities in each category between the two neuroradiologists (9). Items which had discrepant scores were resolved through consensus. Consensus scores were used for subsequent analyses. We computed odds ratios of association between overall abnormalities and case status, controlling for differences in quality and other possible confounders such as age, sex and number of studies. Adjusted odds ratios were computed using multiple logistic regression, implemented in Stata 8.2 (10).
RESULTS
The imaging quality and quantity of exams did not differ significantly between groups (Table 2). RE cases were slightly older at time of examination than controls, and there were also more males in the case group. Inter-rater agreement was excellent for most classes of abnormality, ranging from kappa of 0.79 to 0.98 (Table 3). There was more disagreement about the classification of dilated perivascular spaces, a normal variation, than other “true” abnormalities (kappa 0.79). All discrepancies were successfully resolved through consensus.
Table 2.
Comparison of demographic factors and technical aspects of exams in cases and controls.
| IMAGE QUALITY | Cases | Controls |
|---|---|---|
| Number | 25 | 25 |
| Median age in years at time of imaging, (range) | 8.3 (2.6–14.3) | 12.8 (2.3–17.3) |
| Male sex (%) | 19 (76) | 14 (56) |
| Sequence quality, mean score | 12.8 | 10.8 |
| Median number of studies (range) | 1 (1 –2) | 1 (1 –5) |
| Motion/orthodontic artefact (%) | 2 (8) | 8 (26) |
Table 3.
Inter-rater agreement between two neuroradiologists on scoring of abnormalities found in the 50 routine cranial MRI films studied.
| ABNORMALITIES | Kappa |
|---|---|
| Peri/Hippocampal abnormality | 0.98 |
| Non-localized congenital malformations | 0.93 |
| Subcortical parenchymal hyperintensities | 0.96 |
| Periventricular parenchymal hyperintensities | 0.95 |
| Dilated perivascular spaces | 0.79 |
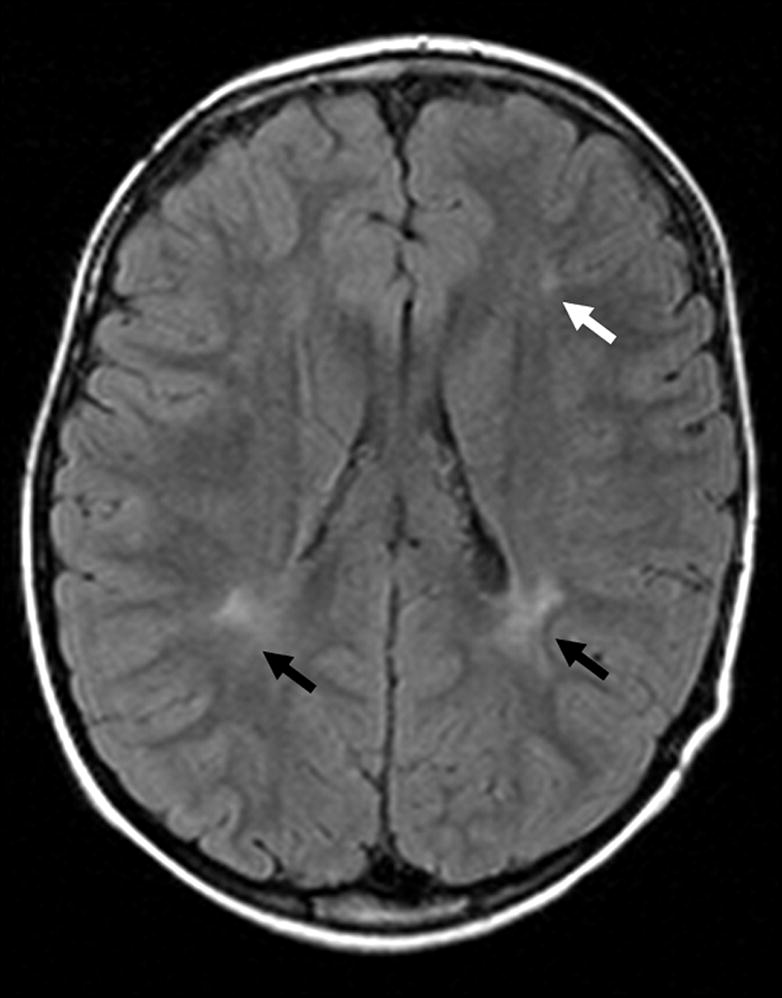
Fifty-two percent of examinations contained at least one abnormality (Table 4). There was no difference in the frequency of any category of abnormality between cases and controls. Hippocampal abnormalities included peri-hippocampal cysts and hippocampus or temporal horn size asymmetry. Congenital malformations included arachnoid cysts (Figure 1), mega cisterna magna, Chiari I malformations, pineal cysts, and neuroepithelial cysts (Figure 2). Dilated perivascular spaces were defined as CSF intensity foci exceeding punctate size and found in characteristic locations in periventricular and perilenticular parenchyma (Figure 3). Subcortical (Figure 4) and periventricular (Figure 4, 5) white matter hyperintensities were most commonly identified on FLAIR images, with corroboration on T2-weighted images when equivocal. Additionally, there was no evidence of lateralization of abnormalities in RE cases towards the side of EEG discharges: one hippocampal abnormality was contralateral to the side of discharges; one of seven congenital malformations was ipsilateral to the side of discharges, the rest of the malformations were midline; none of the three white matter hyperintensities corresponded to the side of EEG discharge. The distribution of abnormalities with regard to side, midline or bilateral, was similar in cases and controls.
Table 4.
Comparison of abnormalities in cases and controls, consensus scoring used.
| ABNORMALITIES | Cases | Controls |
|---|---|---|
| Number | 25 | 25 |
| Peri/Hippocampal abnormality (%) | 1 (4) | 0 |
| Non-localized congenital malformation (%) | 7 (28) | 2 (8) |
| Subcortical parenchymal hyperintensities (%) | 2 (8) | 4 (16) |
| Periventricular parenchymal hyperintensities (%) | 1 (4) | 5 (20) |
| Dilated perivascular spaces (%) | 6 (24) | 5 (20) |
| Total any one or more abnormality (%) | 13 (52) | 13 (52) |
Figure 1.
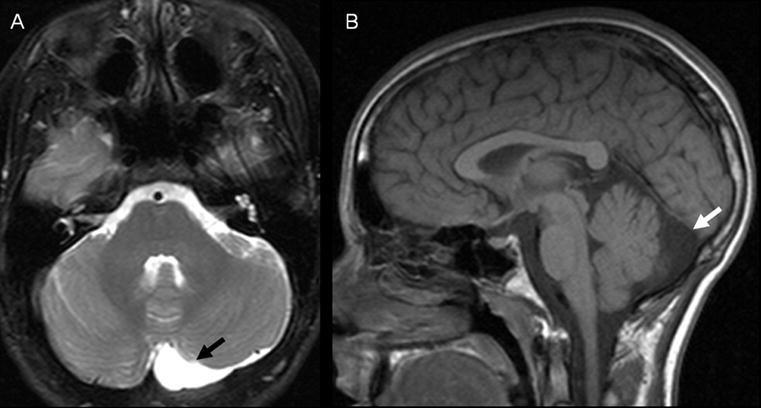
Figure 2.

Figure 3.
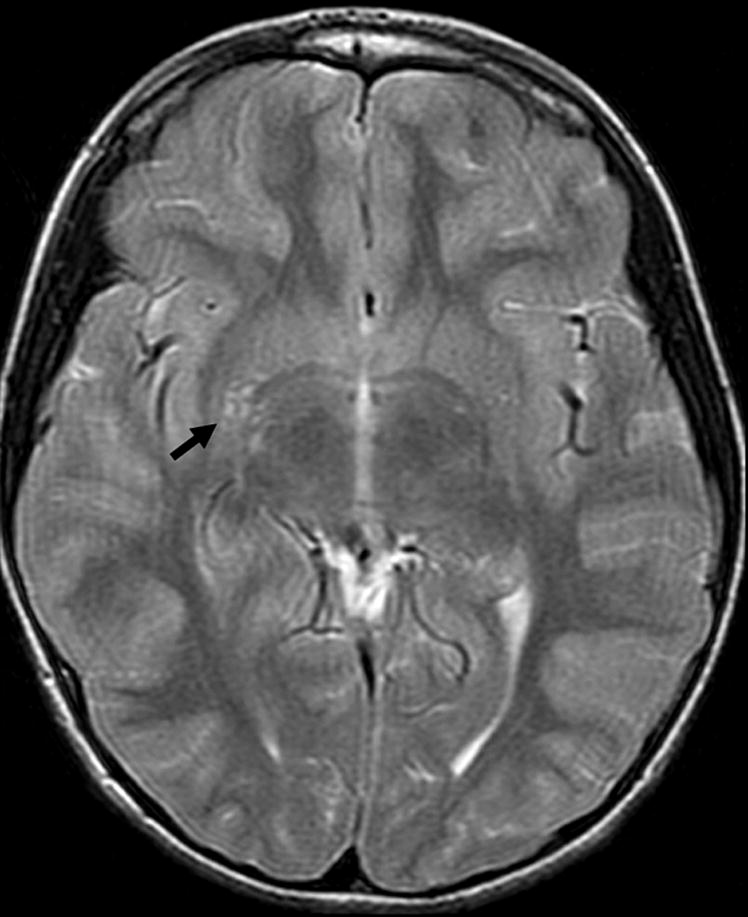
Figure 4.

Figure 5.
The crude odds ratio for association between RE cases and any MRI abnormality was 1.00 (95% CI: 0.33–3.03). After controlling for the potential confounding effect of demographic and imaging-related variables, there were still no significant overall differences in the frequency of abnormalities between groups (adjusted Odds Ratio 0.87, 95% CI: 0.18–4.33).
DISCUSSION
This is the first controlled study to test the hypothesis that RE cases exhibit an excess of abnormalities on routine cranial MRI. There was a high frequency of abnormalities (52% overall) with no excess of abnormalities in RE cases compared to controls. We found no evidence that existing MRI abnormalities lateralized to the hemisphere from which Rolandic discharges predominantly emanated. Our results do not support an association between any particular type of abnormality and RE. We also demonstrated high inter-observer reliability between the two neuroradiologists rating the MRI abnormalities.
Abnormalities in the cranial neuroimaging of Rolandic epilepsy patients have been noted before, in 15% of mostly CT exams (5) and in 60% (10/18) of MRI exams (3). The significance of these abnormalities is debated, with some authors believing that these findings represent subtle clues to a developmental etiology, and other authors ascribing no clinical importance. In the MRI study of 18 RE cases (3), hippocampal abnormalities or subcortical hyperintensities were found ipsilateral to the spike lateralization. Examiners were blinded to the patients’ diagnoses, but no controls were included. Because of the known association between hippocampal sclerosis and temporal lobe epilepsy, and the possibility that subcortical hyperintensities represented areas of focal demyelination or dysmyelination, the authors suggested an association between reported abnormalities and Rolandic epilepsy, of possible etiological significance (3). A later study of 71 RE cases, in which seizure outcome was compared to initial neuroimaging findings, demonstrated that abnormalities such as ventricular enlargement, hippocampal atrophy and congenital malformations did not influence the prognosis of RE and were not consistently ipsilateral to the spike lateralization (5). However, it is possible that some abnormalities were not detected in the second study because most (72%) of the exams consisted of CT imaging, and not MRI. Differences in classification between the two studies is also possible because the detection of abnormalities depends on the subjective judgement of the examiner, as well as technical aspects of the exam. In any case, the etiological and prognostic significance of these abnormalities is hard to judge in the absence of appropriate control data.
The findings of our case-control study demonstrate that RE cases may exhibit a wide variety of non-localizing abnormalities and that these abnormalities are common on routine MRI exams. The overall frequency and type of cranial MRI abnormalities are similar among control children with migraine. Among RE cases, we found a comparable frequency of focal parenchymal signal hyperintensities and congenital malformations as reported previously in MRI studies of this population (3). Neither type of abnormality lateralized to the hemisphere from which EEG spikes predominantly emanated. Interestingly, though, we found a much lower frequency (4%) of hippocampal abnormality than reported previously (60%) (3). The discrepancy may be partly due to the subjective diagnosis of hippocampal asymmetry or atrophy. Another possibility is that pulse sequences were optimized for assessing hippocampal abnormalities in the previous study (3), while our study relied on existing MRI exams, of varying pulse sequence protocols, which did not necessarily include dedicated high resolution coronal T2/FLAIR images.
Possible sources of error in our findings include observer bias, selection bias of controls, confounding by demographic or technical differences between the exams of cases and controls, and lack of sensitivity of routine MRI to pathological abnormalities reflecting developmental disturbances. We addressed observer bias by blinding the neuroradiologists to the diagnoses, and we controlled for variation in assessment by using consensus classification. We found excellent inter-rater agreement for all classes of abnormalities with the possible exception of dilated perivascular spaces, which are arguably a variation of normal. We ascribe the slightly worse kappa score for perivascular spaces to a lack of a priori agreement on the definition of “dilated”; perivascular spaces are a relatively common finding on brain MRI, and are therefore often not mentioned in neuroradiology reports. Consensus was easily reached on perivascular spaces when it was agreed to use punctate (1 mm) appearance as a size threshold.
We also took into account potential differences in sample characteristics and technical quality between the exams of RE cases and controls. While age and sex distribution differed somewhat between cases and controls, there was no statistical evidence for a confounding effect from these variables. Similarly, technical quality was comparable between the two groups.
The possible role of selection bias among controls, and of the sensitivity of neuroimaging modality, must also be considered. One potential reason for finding no difference in the prevalence of MRI abnormalities among RE cases and migraine controls is that the abnormalities relevant to idiopathic epilepsy are also relevant to the pathology of migraine. The frequency of congenital malformations and white matter abnormalities in our pediatric migraine controls is about the same as that found among migraine patients in a retrospective survey of pediatric headache MRIs (11). The overall frequency of abnormalities in their study did not significantly differ between children with migraine and children with tension type headache (11). It seems unlikely therefore, that MRI abnormalities in migraine and epilepsy are specifically alike in nature or number. This suggests that our choice of control group was appropriate and not a source of selection bias. This conclusion is further supported by MRI data from healthy children. Kim et al (7) studied the prevalence of incidental findings on brain MRI in a healthy pediatric population (younger than 18 years, 90% ages 7–17 years) undergoing research studies of language, spatial discrimination, and higher cognitive function. They found a 21% overall prevalence of incidental findings not including dilated perivascular spaces, similar to the 23% in our findings when the same classification is used, and similar to 18% of abnormalities found in healthy adults (12).
Lastly, we note that despite our results, there is a logical basis for seeking brain MRI abnormalities in idiopathic epilepsy. In idiopathic generalized epilepsies, neuropathological studies have shown increased dystopic neurons in subcortical white matter (13), and quantitative MRI has demonstrated increased mesial frontal cortical grey matter (14), supporting a pathological basis for subtle cerebral structural abnormality in the idiopathic epilepsies. Although comparable studies have not been performed specifically in Rolandic epilepsy, it is possible that anatomical and functional abnormalities exist at the cellular level that are not discernable on routine brain MRI. Such abnormalities might reflect the hypothesized cortical maturational impairment thought to underlie perisylvian regional excitability (15), but this has yet to be tested using voxel-based analysis or in-vivo molecular imaging techniques.
In summary, we have found no evidence, from routine cranial MRI exams, for an excess number, type or localization of abnormalities in Rolandic epilepsy compared to migraine controls. We conclude that white matter abnormalities, congenital malformations and variations in perivascular space dimensions are relatively common, non-lateralizing, and have no pathological significance in typical Rolandic epilepsy patients.
Acknowledgments
This study was sponsored by the Partnership for Pediatric Epilepsy Research, Epilepsy Foundation; and through the generous support of the Charles L. Shor Foundation for Epilepsy Research, Inc; and the National Institutes for Neurological Disorders and Stroke NS047530-R01. Our thanks to the physicians who referred cases to the Multicenter Genetic Study of Rolandic Epilepsy: Steven L Kugler MD, Steven M Wolf MD, William D Brown MD, David E Mandelbaum MD PhD, Murray Engel MD, and John Gaitanis MD. Thanks to Kieran Hartsough for administrative help with this study.
ABBREVIATIONS
- BECTS
Benign Epilepsy of Childhood with Centrotemporal Spikes
- RE
Rolandic epilepsy
- JME
Juvenile Myoclonic epilepsy
- IGE
Idiopathic generalized epilepsy
- T2
transverse relaxation time
Footnotes
DISCLOSURES
Authors have no conflicts of interest relating to the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bali B, Kugler SL, Pal DK. Genetic influence on Rolandic epilepsy. Ann Neurol. 2005 Feb 24;57(3):464–5. doi: 10.1002/ana.20399. [DOI] [PubMed] [Google Scholar]
- 2.Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30(4):389–99. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 3.Lundberg S, Eeg-Olofsson O, Raininko R, Eeg-Olofsson KE. Hippocampal asymmetries and white matter abnormalities on MRI in benign childhood epilepsy with centrotemporal spikes. Epilepsia. 1999 Dec;40(12):1808–15. doi: 10.1111/j.1528-1157.1999.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 4.Eeg-Olofsson O, Lundberg S, Raininko R. MRI in rolandic epilepsy. Epileptic Disord. 2000;2(Suppl 1):S51–3. [PubMed] [Google Scholar]
- 5.Gelisse P, Corda D, Raybaud C, Dravet C, Bureau M, Genton P. Abnormal neuroimaging in patients with benign epilepsy with centrotemporal spikes. Epilepsia. 2003 Mar;44(3):372–8. doi: 10.1046/j.1528-1157.2003.17902.x. [DOI] [PubMed] [Google Scholar]
- 6.Shevell MI, Rosenblatt B, Watters GV, O'Gorman AM, Montes JL. Pseudo-BECRS": intracranial focal lesions suggestive of a primary partial epilepsy syndrome. Pediatr Neurol. 1996 Jan;14(1):31–5. doi: 10.1016/0887-8994(95)00252-9. [DOI] [PubMed] [Google Scholar]
- 7.Kim BS, Illes J, Kaplan RT, Reiss A, Atlas SW. Incidental findings on pediatric MR images of the brain. AJNR Am J Neuroradiol. 2002 Nov–Dec;23(10):1674–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Maytal J, Young M, Shechter A, Lipton RB. Pediatric migraine and the International Headache Society (IHS) criteria. Neurology. 1997 Mar;48(3):602–7. doi: 10.1212/wnl.48.3.602. [DOI] [PubMed] [Google Scholar]
- 9.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 10.StataCorp. Stata Statistical Software: Release 8.2. College Station, TX; Stata Corporation: 2003. [Google Scholar]
- 11.Schwedt TJ, Guo Y, Rothner AD. "Benign" imaging abnormalities in children and adolescents with headache. Headache. 2006 Mar;46(3):387–98. doi: 10.1111/j.1526-4610.2006.00371.x. [DOI] [PubMed] [Google Scholar]
- 12.Katzman GL, Dagher AP, Patronas NJ. Incidental findings on brain magnetic resonance imaging from 1000 asymptomatic volunteers. JAMA. 1999;282(1):36–9. doi: 10.1001/jama.282.1.36. [DOI] [PubMed] [Google Scholar]
- 13.Meencke H-J, Janz D. Neuropathological findings in primary generalized epilepsy: a study of eight cases. Epilepsia. 1984;25(1):8–21. doi: 10.1111/j.1528-1157.1984.tb04149.x. [DOI] [PubMed] [Google Scholar]
- 14.Woermann FG, Free SL, Koepp MJ, Sisodiya SM, Duncan JS. Abnormal cerebral structure in juvenile myoclonic epilepsy demonstrated with voxel-based analysis of MRI. Brain. 1999;122(Pt 11):2101–8. doi: 10.1093/brain/122.11.2101. [DOI] [PubMed] [Google Scholar]
- 15.Doose H, Neubauer BA, Petersen B. The concept of hereditary impairment of brain maturation. Epileptic Disord. 2000;2(Suppl 1):S45–9. [PubMed] [Google Scholar]


