Abstract
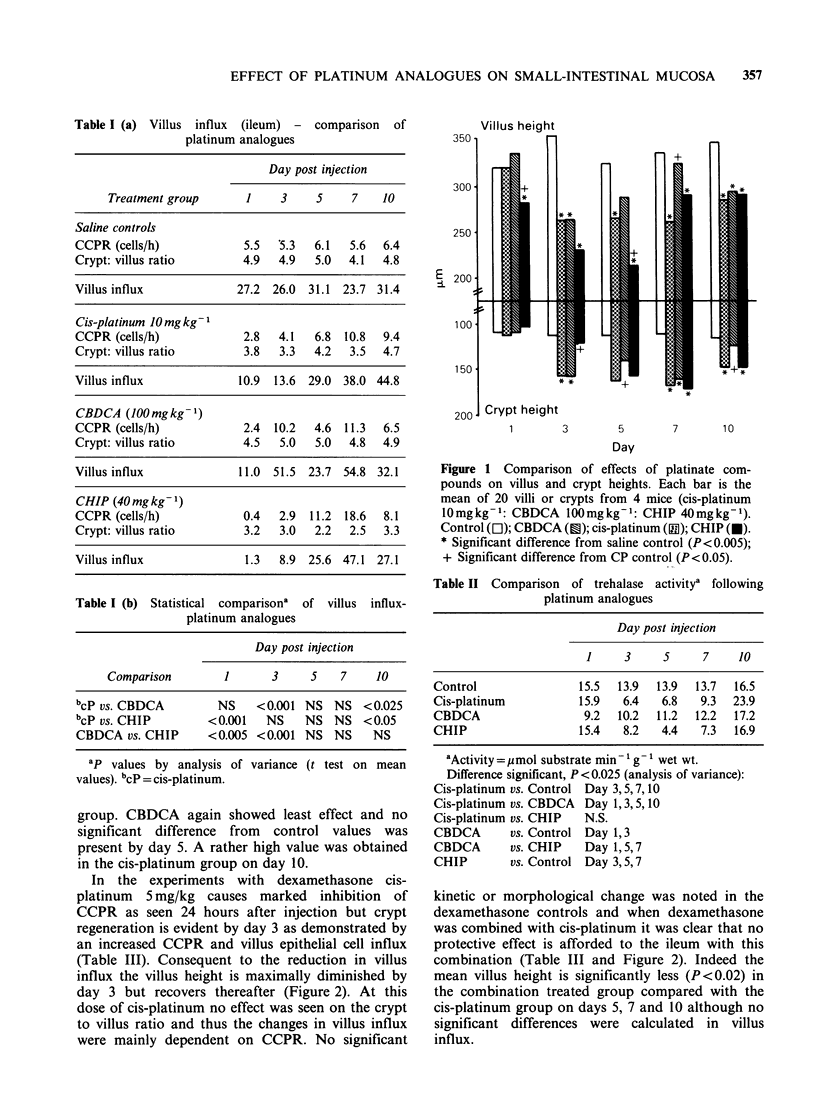
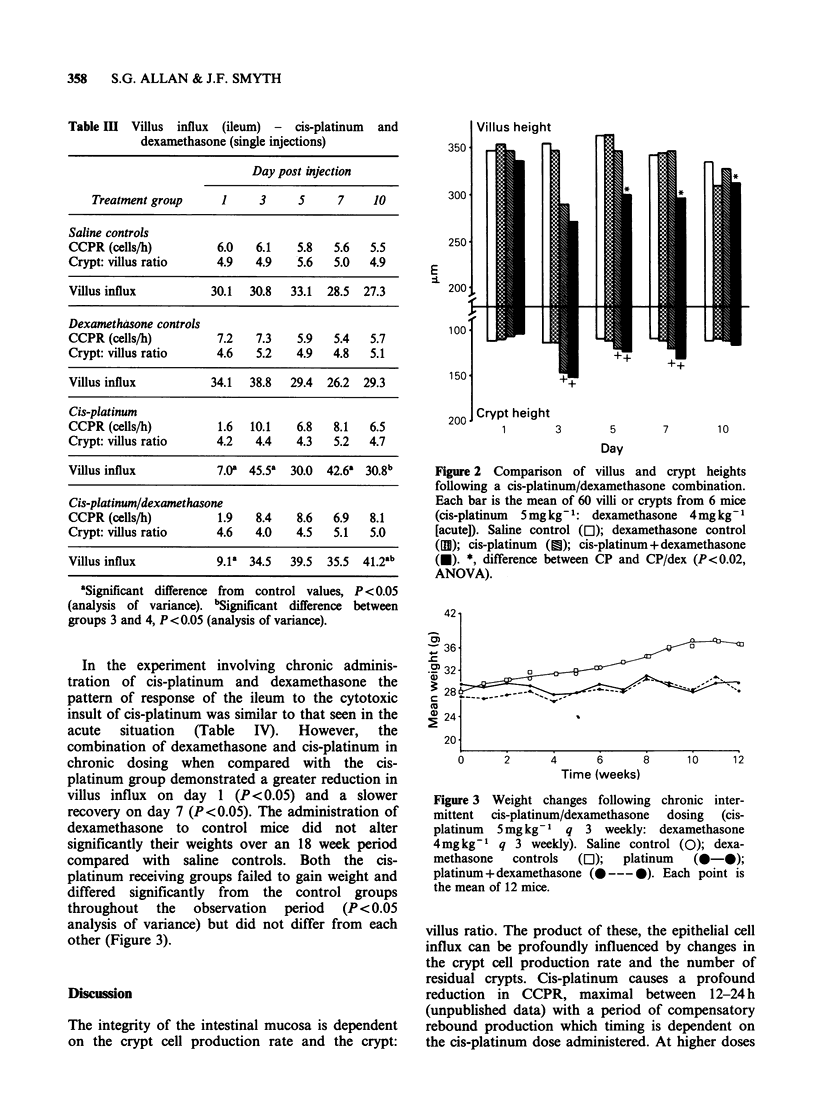
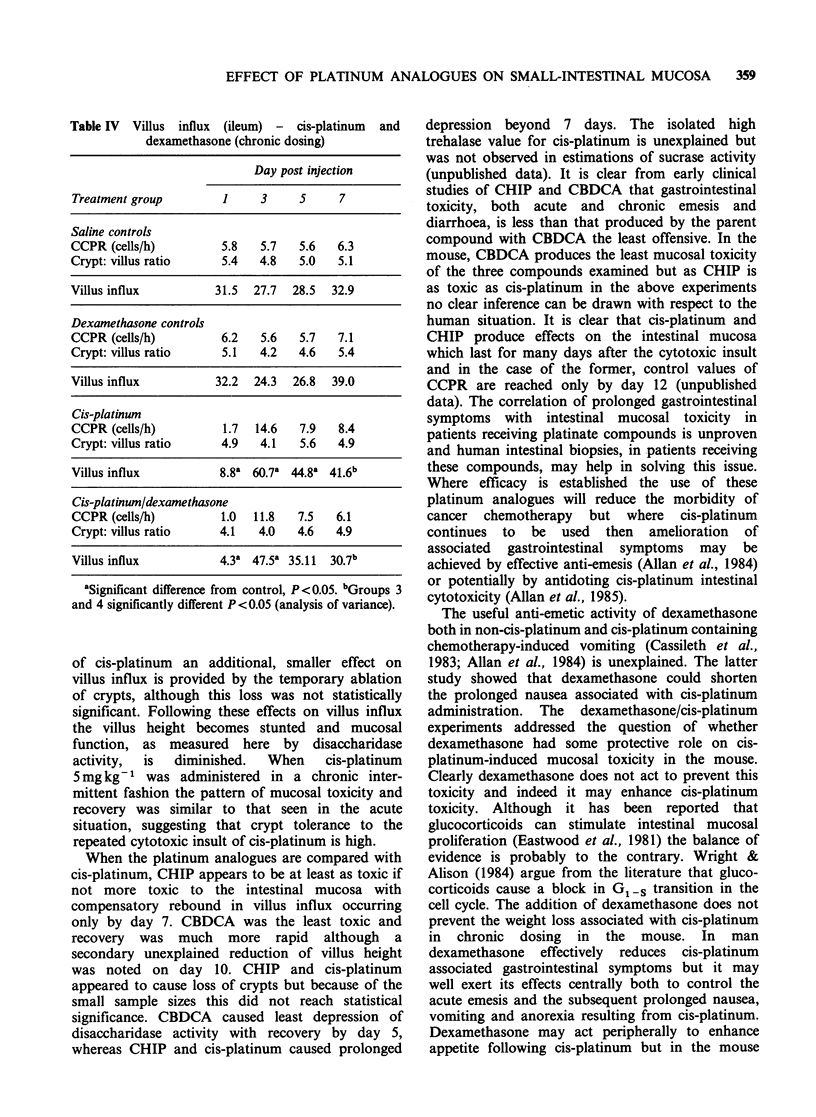
Cis-platinum causes profound gastrointestinal symptoms in patients and these may persist for many days after drug administration. Gut mucosal toxicity may be a factor in the pathogenesis of such prolonged nausea, vomiting and anorexia. The effects of cis-platinum on mouse ileal mucosal architecture, villus epithelial cell influx and disaccharidase activity are described in comparison with dhe effects of two platinum analogues, CBDCA and CHIP. In addition the effect of dexamethasone, a useful drug in the palliation of cis-platinum-induced emesis, in combination with cis-platinum is described. Cis-platinum, CBDCA and CHIP cause profound reduction in crypt cell production rate (CCPR) and thus villus epithelial cell influx within hours of administration leading to villus stunting and diminished function. CBDCA showed the least profound effect with early rebound in CCPR by day 3. Cis-platinum and CHIP were roughly equitoxic to ileal crypts with rebound in CCPR being delayed until day 7. Similarly, CBDCA caused least reduction in disaccharidase activity with cis-platinum and CHIP being equitoxic. The addition of dexamethasone had no protective effect on the effects of cis-platinum on murine ileal mucosa and mice given the combination chronically had no weight gain over 18 weeks, their weight paralleling those receiving cis-platinum alone. The platinate compounds have differing degrees of intestinal mucosal toxicity but no direct inference can be drawn in respect to the clinical situation where CBDCA causes less gastrointestinal symptomatology than CHIP but where both cause less than cis-platinum. Dexamethasone does not act by mucosal protection to provide its useful effects in prolonged cis-platinum-induced gastrointestinal symptoms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akwari O. E. The gastrointestinal tract in chemotherapy-induced emesis. A final common pathway. Drugs. 1983 Feb;25 (Suppl 1):18–34. doi: 10.2165/00003495-198300251-00004. [DOI] [PubMed] [Google Scholar]
- Allan S. G., Cornbleet M. A., Warrington P. S., Golland I. M., Leonard R. C., Smyth J. N. Dexamethasone and high dose metoclopramide: efficacy in controlling cisplatin induced nausea and vomiting. Br Med J (Clin Res Ed) 1984 Oct 6;289(6449):878–879. doi: 10.1136/bmj.289.6449.878-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borison H. L., McCarthy L. E. Neuropharmacology of chemotherapy-induced emesis. Drugs. 1983 Feb;25 (Suppl 1):8–17. doi: 10.2165/00003495-198300251-00003. [DOI] [PubMed] [Google Scholar]
- Calvert A. H., Harland S. J., Newell D. R., Siddik Z. H., Jones A. C., McElwain T. J., Raju S., Wiltshaw E., Smith I. E., Baker J. M. Early clinical studies with cis-diammine-1,1-cyclobutane dicarboxylate platinum II. Cancer Chemother Pharmacol. 1982;9(3):140–147. doi: 10.1007/BF00257742. [DOI] [PubMed] [Google Scholar]
- Cassileth P. A., Lusk E. J., Torri S., DiNubile N., Gerson S. L. Antiemetic efficacy of dexamethasone therapy in patients receiving cancer chemotherapy. Arch Intern Med. 1983 Jul;143(7):1347–1349. [PubMed] [Google Scholar]
- Creaven P. J., Madajewicz S., Pendyala L., Mittelman A., Pontes E., Spaulding M., Arbuck S., Solomon J. Phase I clinical trial of cis-dichloro-trans-dihydroxy-bis-isopropylamine platinum(IV) (CHIP). Cancer Treat Rep. 1983 Sep;67(9):795–800. [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Eastwood G. L., Quimby G. F., Laferriere J. R. Effects of chronic steroid ingestion on gastroduodenal epithelial renewal in the rat. Cell Tissue Kinet. 1981 Jul;14(4):405–411. doi: 10.1111/j.1365-2184.1981.tb00547.x. [DOI] [PubMed] [Google Scholar]
- Ferguson A., Sutherland A., MacDonald T. T., Allan F. Technique for microdissection and measurement in biopsies of human small intestine. J Clin Pathol. 1977 Nov;30(11):1068–1073. doi: 10.1136/jcp.30.11.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freireich E. J., Gehan E. A., Rall D. P., Schmidt L. H., Skipper H. E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966 May;50(4):219–244. [PubMed] [Google Scholar]
- Gralla R. J., Itri L. M., Pisko S. E., Squillante A. E., Kelsen D. P., Braun D. W., Jr, Bordin L. A., Braun T. J., Young C. W. Antiemetic efficacy of high-dose metoclopramide: randomized trials with placebo and prochlorperazine in patients with chemotherapy-induced nausea and vomiting. N Engl J Med. 1981 Oct 15;305(16):905–909. doi: 10.1056/NEJM198110153051601. [DOI] [PubMed] [Google Scholar]


