Abstract
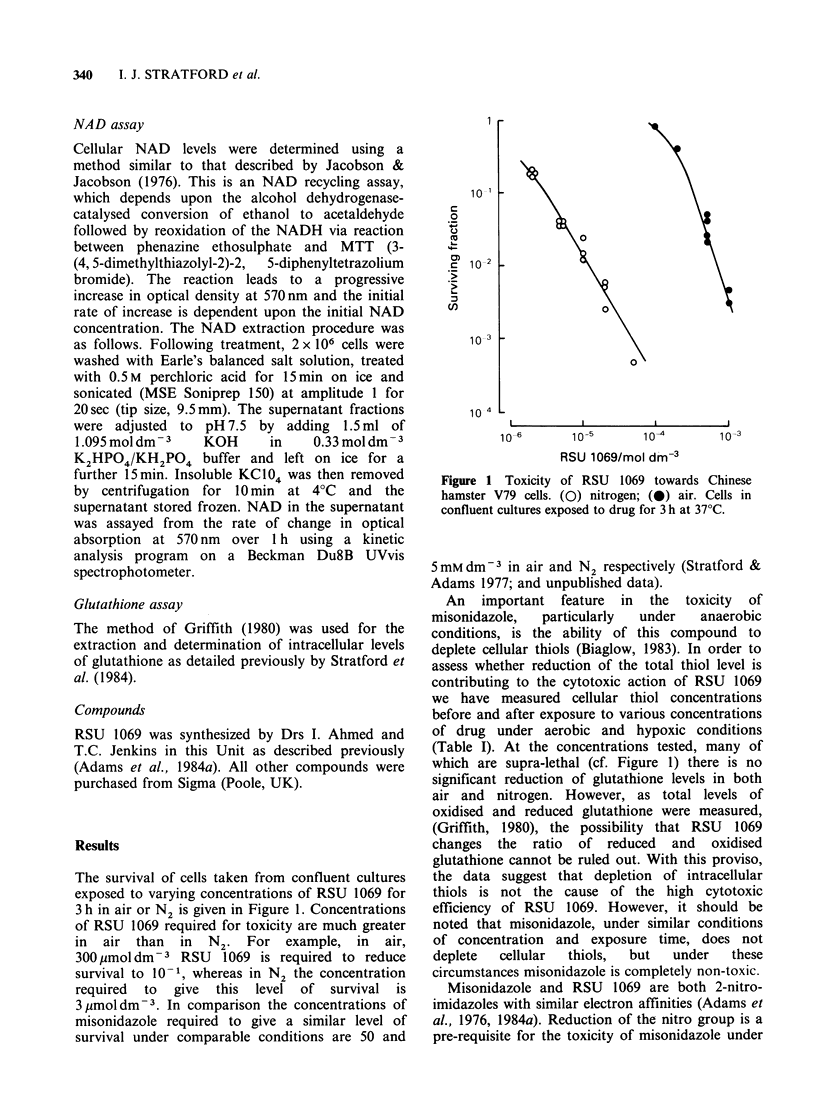
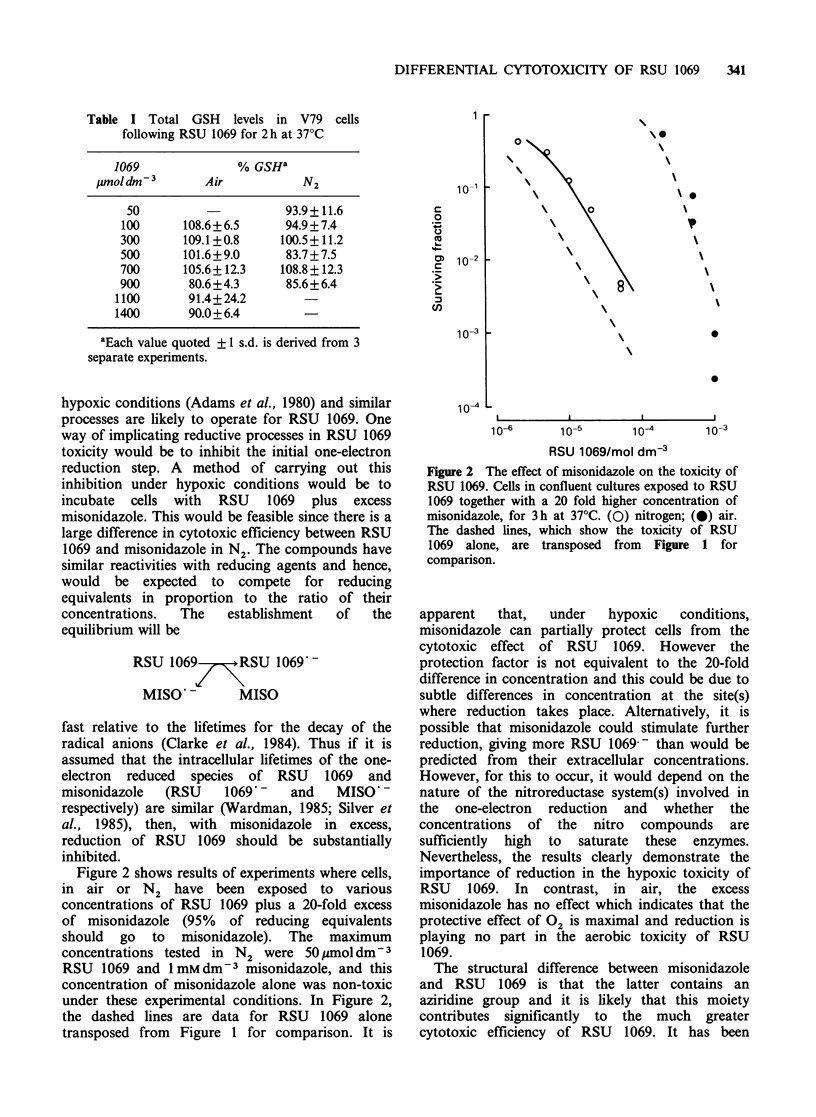
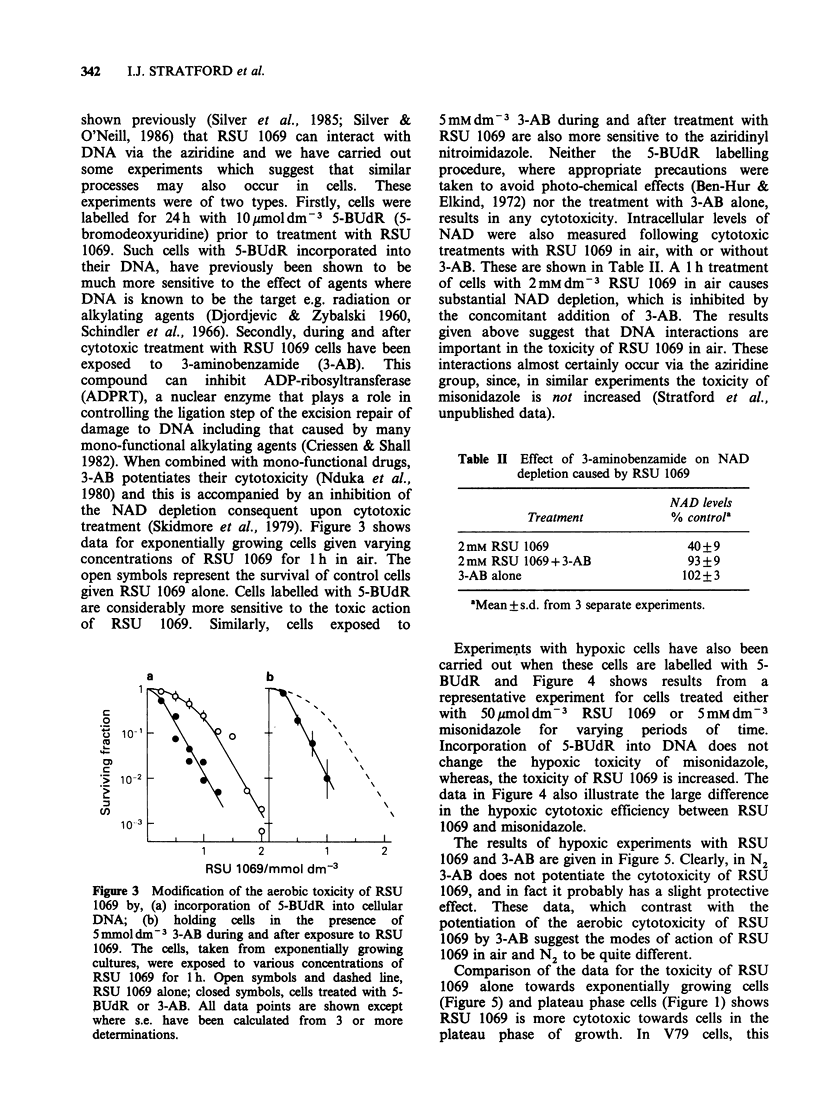
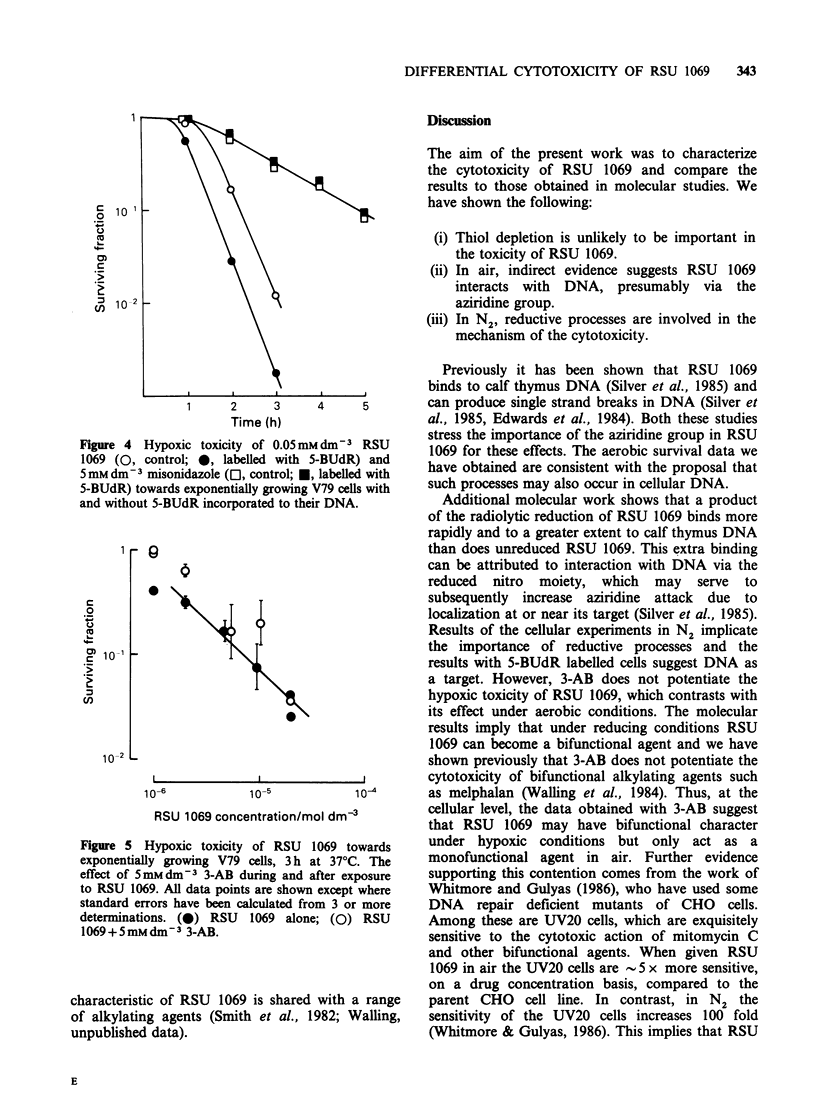
The hypoxic cell radiosensitizer RSU 1069 (1-(2-nitro-1-imidazolyl)-3-(1-aziridinyl)-2-propanol) shows, on a concentration basis, a 100-fold greater toxicity towards hypoxic relative to aerobic cells. This toxicity is substantially greater than that of misonidazole, a compound of similar electron affinity. Reductive processes are important for hypoxic toxicity; this is demonstrated by the fact that misonidazole, in excess, can protect against the hypoxic but not aerobic toxicity of RSU 1069. The importance of the interaction of RSU 1069 with DNA, suggested initially by molecular studies, is supported by the fact that cells containing 5-bromodeoxyuridine (5-BUdR) incorporated into their DNA show greater sensitivity towards the lethal effects of RSU 1069 both in air and nitrogen, compared to cells not treated with 5-BUdR. Experiments with RSU 1069 and 3-aminobenzamide (3-AB) show the latter compound to potentiate aerobic toxicity, consistent with monofunctional alkylation by RSU 1069. In contrast, 3-AB has no effect on the hypoxic cytotoxicity of RSU 1069, which would be predicted if RSU 1069 is functioning as a bifunctional agent under these conditions. It is our contention that in air, RSU 1069 functions as a typical monofunctional alkylating agent, presumably due to the presence of the aziridine group whereas, in hypoxia, reduction of the nitro group provides an additional alkylating species, converting the compound into a bifunctional agent.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. E., Ahmed I., Sheldon P. W., Stratford I. J. RSU 1069, a 2-nitroimidazole containing an alkylating group: high efficiency as a radio- and chemosensitizer in vitro and in vivo. Int J Radiat Oncol Biol Phys. 1984 Sep;10(9):1653–1656. doi: 10.1016/0360-3016(84)90521-2. [DOI] [PubMed] [Google Scholar]
- Adams G. E., Ahmed I., Sheldon P. W., Stratford I. J. Radiation sensitization and chemopotentiation: RSU 1069, a compound more efficient than misonidazole in vitro and in vivo. Br J Cancer. 1984 May;49(5):571–577. doi: 10.1038/bjc.1984.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams G. E., Flockhart I. R., Smithen C. E., Stratford I. J., Wardman P., Watts M. E. Electron-affinic sensitization. VII. A correlation between structures, one-electron reduction potentials, and efficiencies of nitroimidazoles as hypoxic cell radiosensitizers. Radiat Res. 1976 Jul;67(1):9–20. [PubMed] [Google Scholar]
- Adams G. E., Stratford I. J., Wallace R. G., Wardman P., Watts M. E. Toxicity of nitro compounds toward hypoxic mammalian cells in vitro: dependence on reduction potential. J Natl Cancer Inst. 1980 Mar;64(3):555–560. [PubMed] [Google Scholar]
- Ben-Hur E., Elkind M. M. Survival response of asynchronous and synchronous Chinese hamster cells exposed to fluorescent light following 5-bromodeoxyuridine incorporation. Mutat Res. 1972 Feb;14(2):237–245. doi: 10.1016/0027-5107(72)90050-4. [DOI] [PubMed] [Google Scholar]
- Biaglow J. E., Varnes M. E., Clark E. P., Epp E. R. The role of thiols in cellular response to radiation and drugs. Radiat Res. 1983 Sep;95(3):437–455. [PubMed] [Google Scholar]
- Creissen D., Shall S. Regulation of DNA ligase activity by poly(ADP-ribose). Nature. 1982 Mar 18;296(5854):271–272. doi: 10.1038/296271a0. [DOI] [PubMed] [Google Scholar]
- DJORDJEVIC B., SZYBALSKI W. Genetics of human cell lines. III. Incorporation of 5-bromo- and 5-iododeoxyuridine into the deoxyribonucleic acid of human cells and its effect on radiation sensitivity. J Exp Med. 1960 Sep 1;112:509–531. doi: 10.1084/jem.112.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. I., Knox R. J., Skolimowski I. M., Zahoor A., Knight R. C. Photosensitive interaction of RSU 1069 with DNA. Int J Radiat Oncol Biol Phys. 1984 Aug;10(8):1319–1322. doi: 10.1016/0360-3016(84)90340-7. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Jacobson E. L., Jacobson M. K. Pyridine nucleotide levels as a function of growth in normal and transformed 3T3 cells. Arch Biochem Biophys. 1976 Aug;175(2):627–634. doi: 10.1016/0003-9861(76)90553-1. [DOI] [PubMed] [Google Scholar]
- Nduka N., Skidmore C. J., Shall S. The enhancement of cytotoxicity of N-methyl-N-nitrosourea and of gamma-radiation by inhibitors of poly(ADP-ribose) polymerase. Eur J Biochem. 1980 Apr;105(3):525–530. doi: 10.1111/j.1432-1033.1980.tb04528.x. [DOI] [PubMed] [Google Scholar]
- Schindler R., Ramseier L., Grieder A. Increased sensitivity of mammalian cell cultures to radiomimetic alkylating agents following incorporation of 5-bromodeoxyuridine into cellular DNA. Biochem Pharmacol. 1966 Dec;15(12):2013–2023. doi: 10.1016/0006-2952(66)90229-2. [DOI] [PubMed] [Google Scholar]
- Silver A. R., O'Neill P., Jenkins T. C. Induction of DNA strand breaks by RSU-1069, a nitroimidazole-aziridine radiosensitizer. Role of binding of both unreduced and radiation-reduced forms to DNA, in vitro. Biochem Pharmacol. 1985 Oct 1;34(19):3537–3542. doi: 10.1016/0006-2952(85)90730-0. [DOI] [PubMed] [Google Scholar]
- Skidmore C. J., Davies M. I., Goodwin P. M., Halldorsson H., Lewis P. J., Shall S., Zia'ee A. A. The involvement of poly(ADP-ribose) polymerase in the degradation of NAD caused by gamma-radiation and N-methyl-N-nitrosourea. Eur J Biochem. 1979 Nov 1;101(1):135–142. doi: 10.1111/j.1432-1033.1979.tb04225.x. [DOI] [PubMed] [Google Scholar]
- Smith E., Stratford I. J., Adams G. E. Enhancing effect of pre-treatment of cells with misonidazole in hypoxia on their response to melphalan in air. Br J Cancer. 1982 Jul;46(1):117–126. doi: 10.1038/bjc.1982.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford I. J., Adams G. E. Effect of hyperthermia on differential cytotoxicity of a hypoxic cell radiosensitizer, Ro-07-0582, on mammalian cells in vitro. Br J Cancer. 1977 Mar;35(3):307–313. doi: 10.1038/bjc.1977.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford I. J., Adams G. E., Hardy C., Hoe S., O'Neill P., Sheldon P. W. Thiol reactive nitroimidazoles: radiosensitization studies in vitro and in vivo. Int J Radiat Biol Relat Stud Phys Chem Med. 1984 Dec;46(6):731–745. doi: 10.1080/09553008414551971. [DOI] [PubMed] [Google Scholar]
- Walling J. M., Stratford I. J., Stephens M. A. Chemopotentiation by CB 1954: the importance of postincubations and the possible involvement of poly(ADP-ribosylation). Int J Radiat Oncol Biol Phys. 1984 Sep;10(9):1661–1664. doi: 10.1016/0360-3016(84)90523-6. [DOI] [PubMed] [Google Scholar]


