Abstract
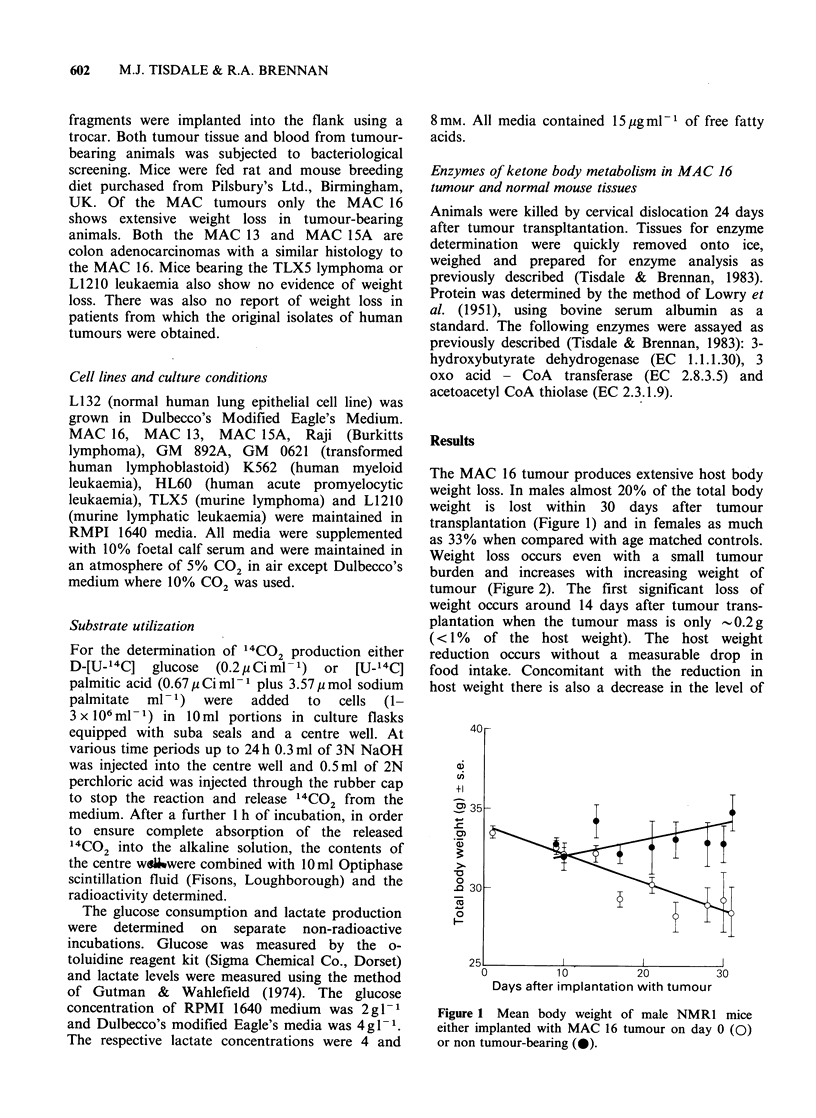
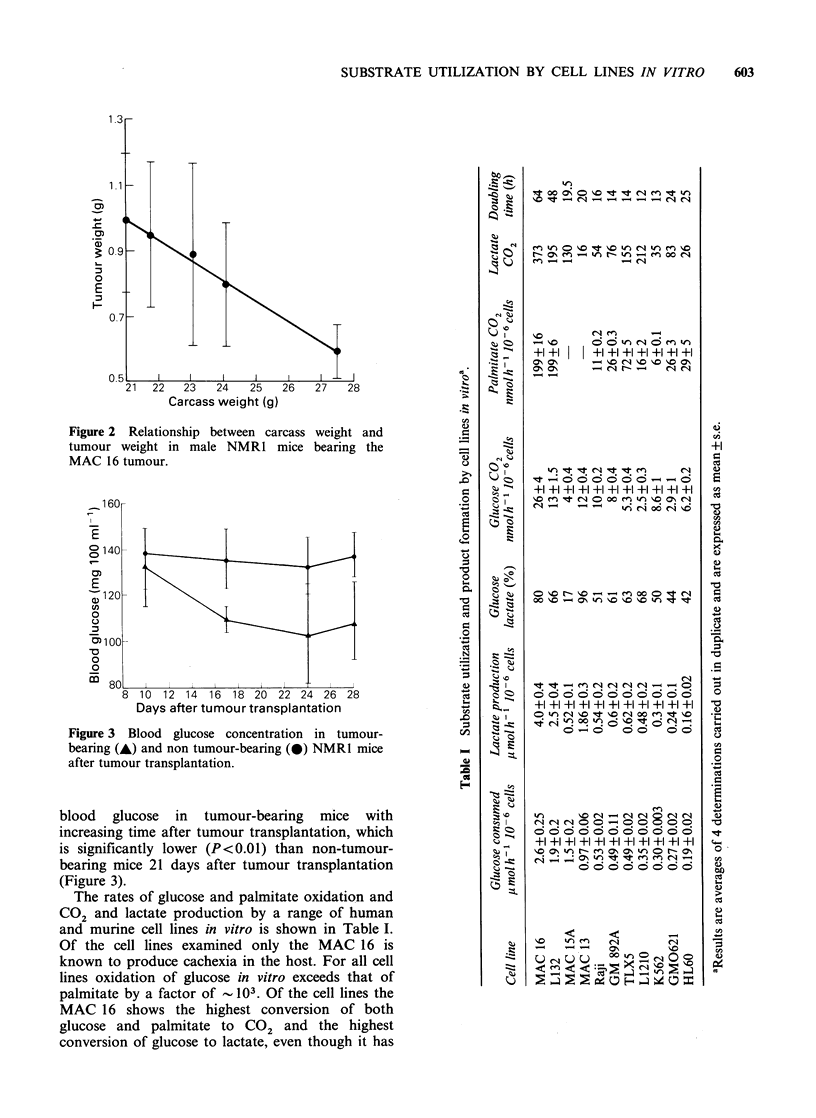
The MAC 16 is a transplantable murine carcinoma of the colon producing extensive weight loss in tumour-bearing animals. The weight loss is proportional to the size of the tumour and occurs without a reduction in food intake when compared with non tumour-bearing control mice. Weight loss produced by the MAC 16 tumour is accompanied by hypoglycaemia which becomes more extensive as the tumour mass increases. In order to understand the mechanism of the cachexia produced by the MAC 16 tumour the rate of substrate utilization and CO2 formation from both glucose and palmitate has been compared in vitro, with other colon carcinoma cell lines known not to produce cachexia as well as a range of murine and human tumour cell lines. The rate of glucose consumption, lactate production and CO2 formation from both glucose and palmitate is much higher for the MAC 16 than for the other tumour cells. For all cell lines in vitro the consumption of glucose exceeds that of palmitate by a factor of 10(3). Excessive consumption of glucose by the MAC 16 tumour may account for the hypoglycaemic effect on the host. The level of 3 oxo acid CoA transferase, an initiator of ketone body utilization, was found to be much lower in the MAC 16 tumour than non-involved colon. This suggests that the tumour may not be able to metabolize ketone bodies effectively.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOCH-FRANKENTHAL L., LANGAN J., MORRIS H. P., WEINHOUSE S. FATTY ACID OXIDATION AND KETOGENESIS IN TRANSPLANTABLE LIVER TUMORS. Cancer Res. 1965 Jun;25:732–736. [PubMed] [Google Scholar]
- Double J. A., Ball C. R. Chemotherapy of transplanted adenocarcinomas of the colon in mice. Cancer Chemother Rep. 1975 Nov-Dec;59(6):1083–1089. [PubMed] [Google Scholar]
- Fenselau A., Wallis K. Comparative studies on 3-oxo acid coenzyme A transferase from various rat tissues. Biochem J. 1974 Sep;142(3):619–627. doi: 10.1042/bj1420619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields A. L., Wolman S. L., Cheema-Dhadli S., Morris H. P., Halperin M. L. Regulation of energy metabolism in Morris hepatoma 7777 and 7800. Cancer Res. 1981 Jul;41(7):2762–2766. [PubMed] [Google Scholar]
- Fredericks M., Ramsey R. B. 3-Oxo acid coenzyme A transferase activity in brain and tumors of the nervous system. J Neurochem. 1978 Dec;31(6):1529–1531. doi: 10.1111/j.1471-4159.1978.tb06581.x. [DOI] [PubMed] [Google Scholar]
- Fung K. P., Chan T. W., Choy Y. M. Suppression of Ehrlich ascites tumor growth in mice by starvation and streptozotocin-induced diabetes. Cancer Lett. 1985 Sep 30;28(3):273–280. doi: 10.1016/0304-3835(85)90035-7. [DOI] [PubMed] [Google Scholar]
- Gold J. Hydrazine sulfate in the treatment of cancer. Cancer Treat Rep. 1976 Jul;60(7):964–965. [PubMed] [Google Scholar]
- Holroyde C. P., Gabuzda T. G., Putnam R. C., Paul P., Reichard G. A. Altered glucose metabolism in metastatic carcinoma. Cancer Res. 1975 Dec;35(12):3710–3714. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Magee B. A., Potezny N., Rofe A. M., Conyers R. A. The inhibition of malignant cell growth by ketone bodies. Aust J Exp Biol Med Sci. 1979 Oct;57(5):529–539. doi: 10.1038/icb.1979.54. [DOI] [PubMed] [Google Scholar]
- Nakashima R. A., Paggi M. G., Pedersen P. L. Contributions of glycolysis and oxidative phosphorylation to adenosine 5'-triphosphate production in AS-30D hepatoma cells. Cancer Res. 1984 Dec;44(12 Pt 1):5702–5706. [PubMed] [Google Scholar]
- Tisdale M. J., Brennan R. A. Loss of acetoacetate coenzyme A transferase activity in tumours of peripheral tissues. Br J Cancer. 1983 Feb;47(2):293–297. doi: 10.1038/bjc.1983.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale M. J. Role of acetoacetyl-CoA synthetase in acetoacetate utilization by tumor cells. Cancer Biochem Biophys. 1984 Jun;7(2):101–107. [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Page M. A., Krebs H. A. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J. 1971 Jan;121(1):41–47. doi: 10.1042/bj1210041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke H. R., Zielke C. L., Ozand P. T. Glutamine: a major energy source for cultured mammalian cells. Fed Proc. 1984 Jan;43(1):121–125. [PubMed] [Google Scholar]


