Abstract
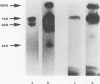
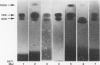
Permanent cell lines (UCT-Mel 1 through 7) were established from biopsies of metastatic tissue taken from seven patients with malignant melanoma. Cells from these lines were all aneuploid and all grew as non-contact-inhibited, adherent monolayers. All of the lines, with the remarkable exception of UCT-Mel 6, formed tumours in nude mice, expressed the melanoma M-18 antigen and synthesized plasminogen activators exclusively of the tissue-type. UCT-Mel 6 cells were non tumourigenic, they lacked the M-18 antigen and they synthesized plasminogen activators exclusively of the urokinase type. UCT-Mel 1 and UCT-Mel 2 formed pigment in vitro and both of these lines showed an increase in pigment content and tyrosinase synthesis with increasing cell density. The rate of plasminogen activator released by UCT-Mel 1 and UCT-Mel 3 declined strikingly as the cells became confluent. Assuming that proteolytic activity is required for cell migration in vivo; that tyrosinase synthesis reflects expression of the differentiated phenotype and that melanoma cells retain some of the characteristics of neural crest cells, we suggest that the effects of confluence and close cell-cell contact provide a useful experimental counterpart for the study of normal neural crest all behaviour that is characterized by an inverse relationship between migration and a protease secretion on the one hand and pigmentation on the other.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azar H. A., Hansen C. T., Costa J. N:NIH(S)-nu/nu mice with combined immunodeficiency: a new model for human tumor heterotransplantation. J Natl Cancer Inst. 1980 Aug;65(2):421–430. [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Chou I. N., O'Donnell S. P., Black P. H., Roblin R. O. Cell density-dependent secretion of plasminogen activator by 3T3 cells. J Cell Physiol. 1977 Apr;91(1):31–37. doi: 10.1002/jcp.1040910104. [DOI] [PubMed] [Google Scholar]
- Creasey A. A., Smith H. S., Hackett A. J., Fukuyama K., Epstein W. L., Madin S. H. Biological properties of human melanoma cells in culture. In Vitro. 1979 May;15(5):342–350. doi: 10.1007/BF02616140. [DOI] [PubMed] [Google Scholar]
- Dippold W. G., Lloyd K. O., Li L. T., Ikeda H., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: definition of six antigenic systems with mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6114–6118. doi: 10.1073/pnas.77.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner R. E., Kitamura H., Moore G. E. Studies of tumor cell lines derived from patients with malignant melanoma. Oncology. 1975;31(1):31–43. doi: 10.1159/000225003. [DOI] [PubMed] [Google Scholar]
- Glimelius B., Weston J. A. Analysis of developmentally homogeneous neural crest cell populations in vitro. III. Role of culture environment in cluster formation and differentiation. Cell Differ. 1981 Jan;10(1):57–67. doi: 10.1016/0045-6039(81)90048-8. [DOI] [PubMed] [Google Scholar]
- Glovanella B. C., Stehlin J. S., Santamaria C., Yim S. O., Morgan A. C., Williams L. J., Jr, Leibovitz A., Fialkow P. J., Mumford D. M. Human neoplastic and normal cells in tissue culture. I. Cell lines derived from malignant melanomas and normal melanocytes. J Natl Cancer Inst. 1976 Jun;56(6):1131–1142. doi: 10.1093/jnci/56.6.1131. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoal E. G., Wilson E. L., Dowdle E. B. The regulation of tissue plasminogen activator activity by human fibroblasts. Cell. 1983 Aug;34(1):273–279. doi: 10.1016/0092-8674(83)90158-7. [DOI] [PubMed] [Google Scholar]
- Hoal E., Wilson E. L., Dowdle E. B. Variable effects of retinoids on two pigmenting human melanoma cell lines. Cancer Res. 1982 Dec;42(12):5191–5195. [PubMed] [Google Scholar]
- Houghton A. N., Eisinger M., Albino A. P., Cairncross J. G., Old L. J. Surface antigens of melanocytes and melanomas. Markers of melanocyte differentiation and melanoma subsets. J Exp Med. 1982 Dec 1;156(6):1755–1766. doi: 10.1084/jem.156.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E. G. Latent tissue plasminogen activator produced by human endothelial cells in culture: evidence for an enzyme-inhibitor complex. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6804–6808. doi: 10.1073/pnas.80.22.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S. K., Dent P. B., McCulloch P. B. Characterization of human maligant melanoma cell lines. I. Morphology and growth characteristics in culture. J Natl Cancer Inst. 1975 May;54(5):1037–1044. [PubMed] [Google Scholar]
- Loskutoff D. J., van Mourik J. A., Erickson L. A., Lawrence D. Detection of an unusually stable fibrinolytic inhibitor produced by bovine endothelial cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2956–2960. doi: 10.1073/pnas.80.10.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus G., Kohga S., Camiolo S. M., Madeja J. M., Ambrus J. L., Karakousis C. Plasminogen activators in human malignant melanoma. J Natl Cancer Inst. 1984 Jun;72(6):1213–1222. [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Rijken D. C., Collen D. Purification and characterization of the plasminogen activator secreted by human melanoma cells in culture. J Biol Chem. 1981 Jul 10;256(13):7035–7041. [PubMed] [Google Scholar]
- Tucker W. S., Kirsch W. M., Martinez-Hernandez A., Fink L. M. In vitro plasminogen activator activity in human brain tumors. Cancer Res. 1978 Feb;38(2):297–302. [PubMed] [Google Scholar]
- Vetterlein D., Bell T. E., Young P. L., Roblin R. Immunological quantitation and immunoadsorption of urokinase-like plasminogen activators secreted by human cells. J Biol Chem. 1980 Apr 25;255(8):3665–3672. [PubMed] [Google Scholar]
- Vetterlein D., Young P. L., Bell T. E., Roblin R. Immunological characterization of multiple weight forms of human cell plasminogen activators. J Biol Chem. 1979 Feb 10;254(3):575–578. [PubMed] [Google Scholar]
- Wilson E. L., Becker M. L., Hoal E. G., Dowdle E. B. Molecular species of plasminogen activators secreted by normal and neoplastic human cells. Cancer Res. 1980 Mar;40(3):933–938. [PubMed] [Google Scholar]
- Wilson E. L., Dowdle E. Secretion of plasminogen activator by normal, reactive and neoplastic human tissues cultured in vitro. Int J Cancer. 1978 Oct 15;22(4):390–399. doi: 10.1002/ijc.2910220405. [DOI] [PubMed] [Google Scholar]
- Wilson E. L., Jacobs P., Dowdle E. B. The secretion of plasminogen activators by human myeloid leukemic cells in vitro. Blood. 1983 Mar;61(3):568–574. [PubMed] [Google Scholar]