Abstract
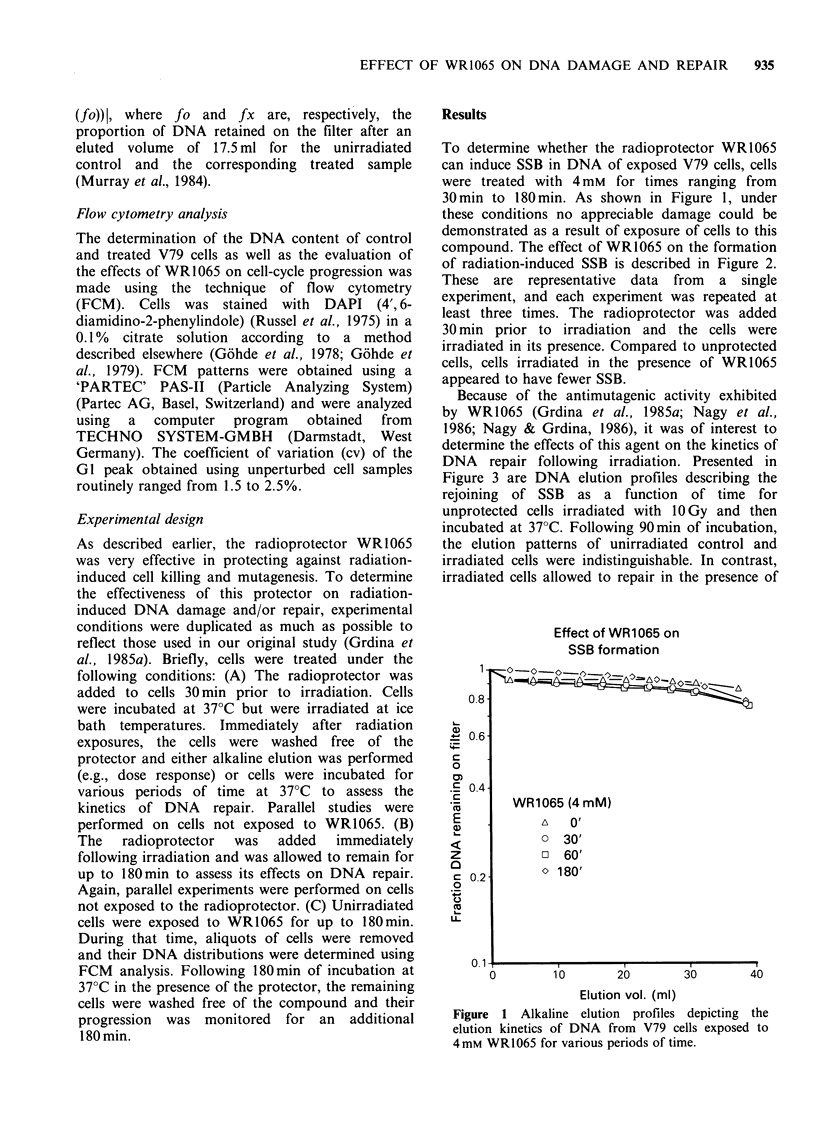
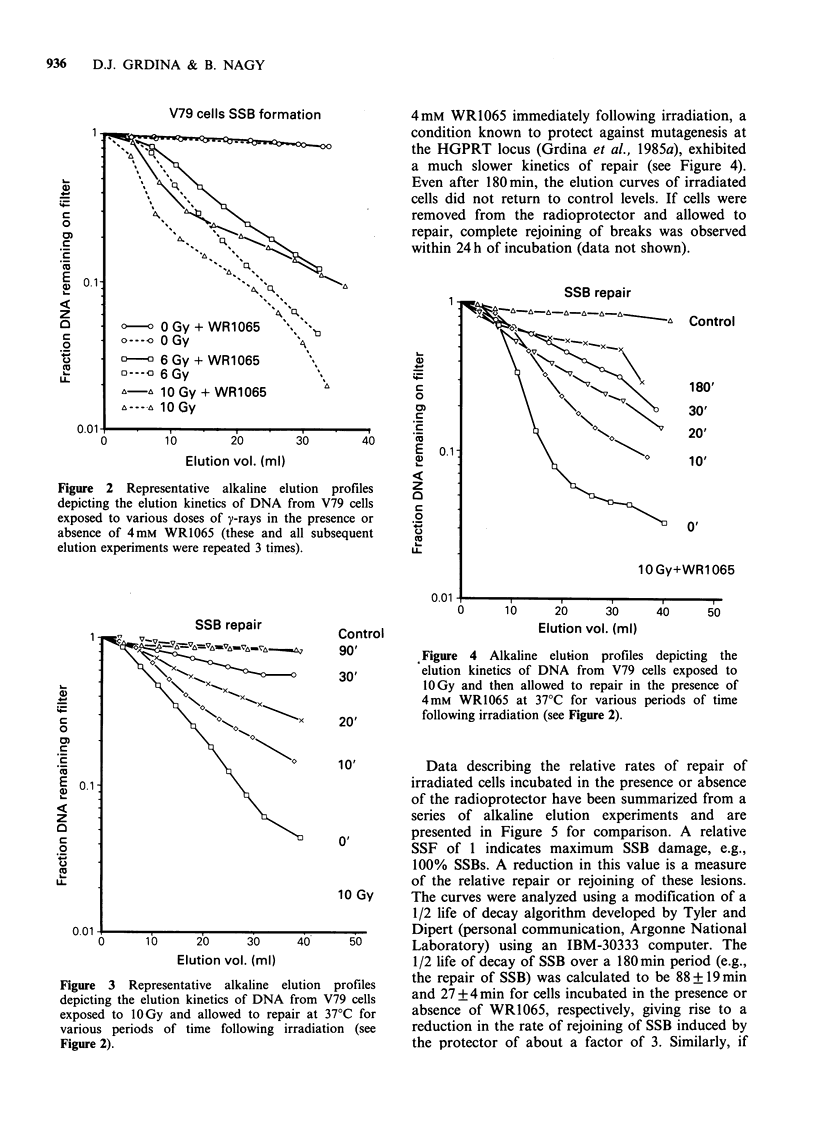
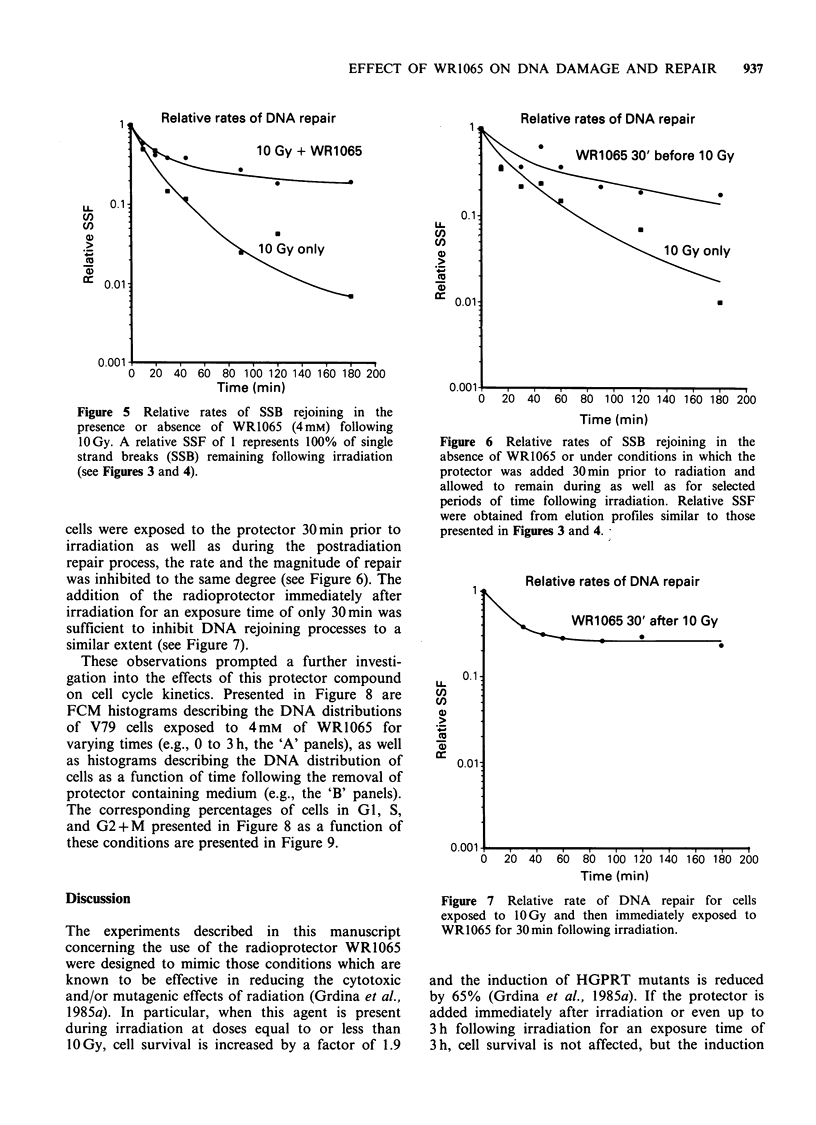
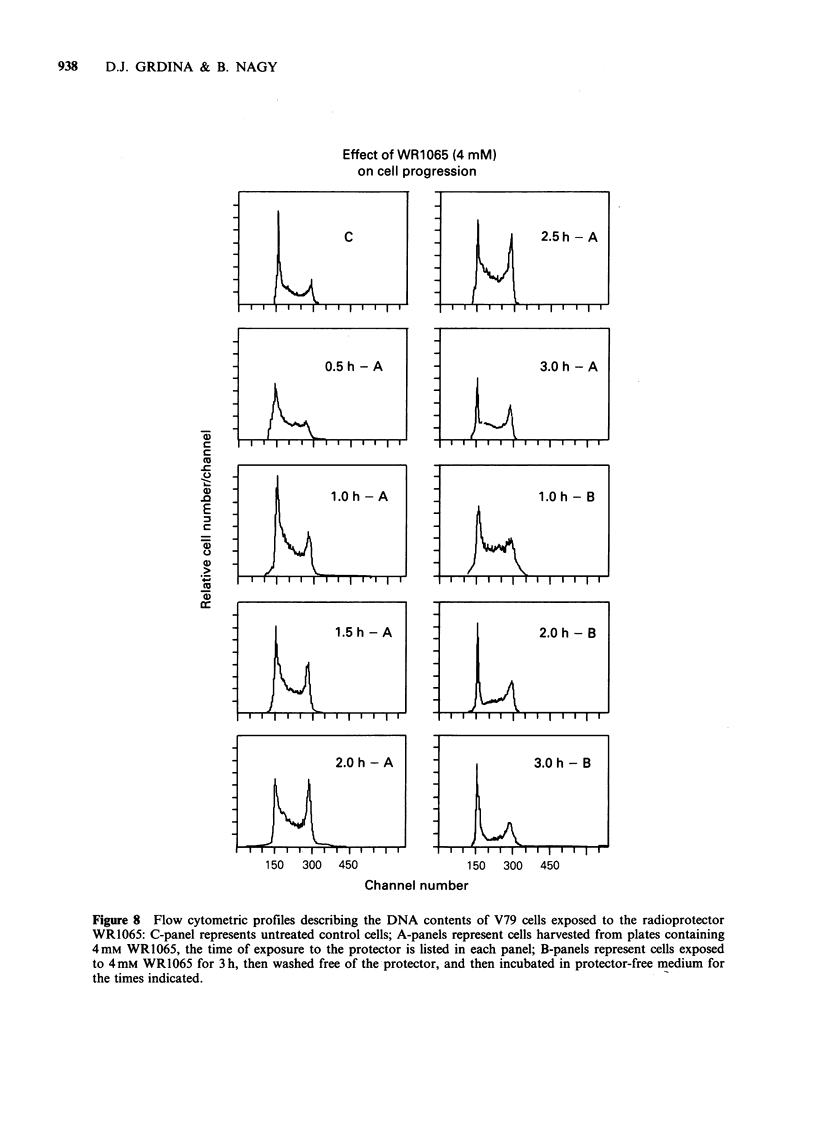
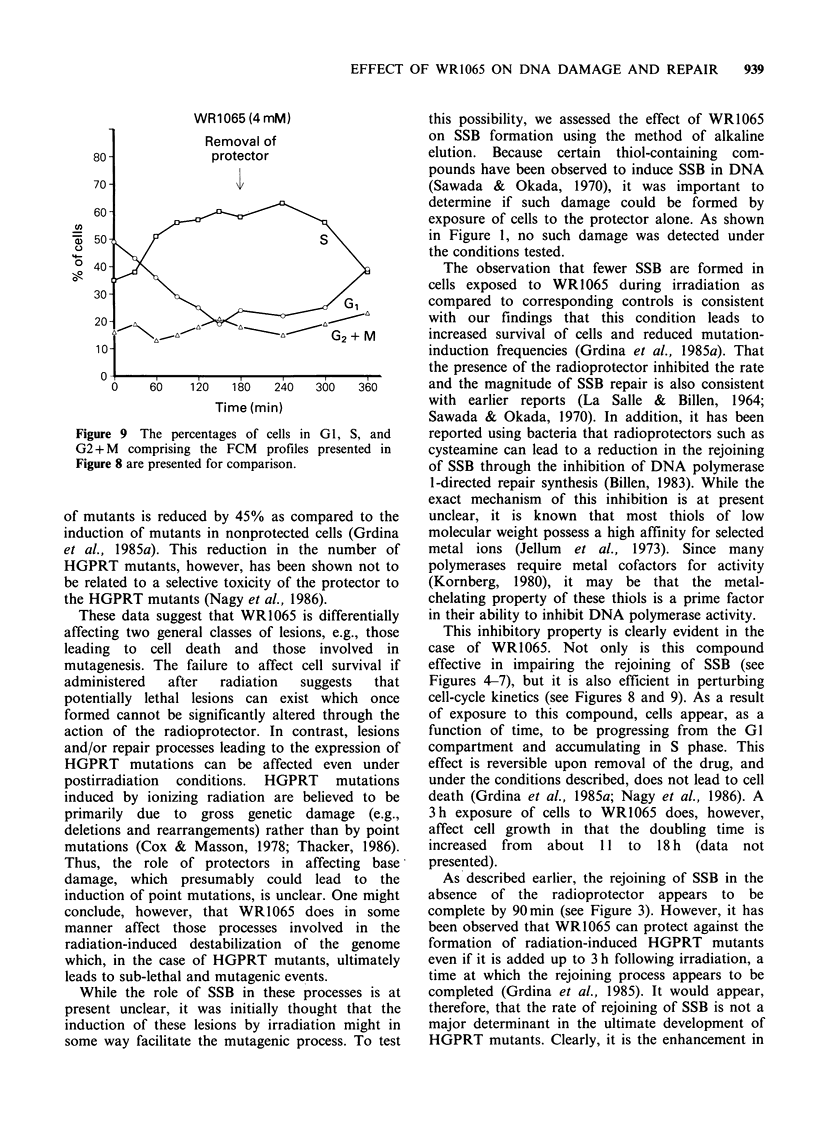
The radioprotector 2-[(aminopropyl)amino] ethanethiol (WR1065) was investigated with respect to its ability to affect radiation-induced DNA damage and repair in V79 cells. Studies were performed to evaluate the protector under conditions in which it is known to be effective in reducing the cytotoxic and mutagenic effects of gamma-irradiation. At a concentration of 4 mM, WR1065 protected against the formation of single strand breaks (SSB), as determined by the method of alkaline elution, when it was present during irradiation. The protector appeared, however, to inhibit the subsequent postirradiation repair or rejoining of SSB. While repair was complete within 24 h, the protector reduced the rate of repair by a factor of 3. This inhibitory effect on the rate of repair did not correlate with either measured differences in cell survival or mutagenesis. The radioprotector was also investigated with respect to its ability to affect cell cycle progression. WR1065 present in the growth medium inhibited the progression of cells through S-phase, and cell-doubling time following a 3 h exposure to the protector was increased from 11 to 18 h. These data are consistent with the well characterized property of thiols to inhibit DNA polymerase activity. It was concluded that, while the presence of WR1065 during irradiation reduced SSB-DNA damage, its effect on the subsequent rejoining of these breaks could not be correlated with its observed effect on protecting against radiation-induced mutagenesis. It may be that the inhibition of cell-cycle progression by the protector allowed more time to enhance the fidelity of repair as measured by the protector's ability to protect against radiation-induced mutagenesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billen D. The effects of radioprotectors on DNA polymerase I-directed repair synthesis and DNA strand breaks in toluene-treated and X-irradiated Escherichia coli. Radiat Res. 1983 Jul;95(1):158–164. [PubMed] [Google Scholar]
- Borek C., Troll W. Modifiers of free radicals inhibit in vitro the oncogenic actions of x-rays, bleomycin, and the tumor promoter 12-O-tetradecanoylphorbol 13-acetate. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1304–1307. doi: 10.1073/pnas.80.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarone C. F., Bolognesi C., Santi L. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal Biochem. 1979 Nov 15;100(1):188–197. doi: 10.1016/0003-2697(79)90131-3. [DOI] [PubMed] [Google Scholar]
- Chu E. H., Malling H. V. Mammalian cell genetics. II. Chemical induction of specific locus mutations in Chinese hamster cells in vitro. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1306–1312. doi: 10.1073/pnas.61.4.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R., Masson W. K. Do radiation-induced thioguanine-resistant mutants of cultured mammalian cells arise by HGPRT gene mutation or X-chromosome rearrangement? Nature. 1978 Dec 7;276(5688):629–630. doi: 10.1038/276629a0. [DOI] [PubMed] [Google Scholar]
- Farber E. The multistep nature of cancer development. Cancer Res. 1984 Oct;44(10):4217–4223. [PubMed] [Google Scholar]
- Grdina D. J., Nagy B., Hill C. K., Wells R. L., Peraino C. The radioprotector WR1065 reduces radiation-induced mutations at the hypoxanthine-guanine phosphoribosyl transferase locus in V79 cells. Carcinogenesis. 1985 Jun;6(6):929–931. doi: 10.1093/carcin/6.6.929. [DOI] [PubMed] [Google Scholar]
- Grdina D. J., Peraino C., Carnes B. A., Hill C. K. Protective effect of S-2-(3-aminopropylamino)ethylphosphorothioic acid against induction of altered hepatocyte foci in rats treated once with gamma-radiation within one day after birth. Cancer Res. 1985 Nov;45(11 Pt 1):5379–5381. [PubMed] [Google Scholar]
- Greenstock C. L. Redox processes in radiation biology and cancer. Radiat Res. 1981 May;86(2):196–211. [PubMed] [Google Scholar]
- Han A., Hill C. K., Elkind M. M. Repair of cell killing and neoplastic transformation at reduced dose rates of 60Co gamma-rays. Cancer Res. 1980 Sep;40(9):3328–3332. [PubMed] [Google Scholar]
- Hill C. K., Nagy B., Peraino C., Grdina D. J. 2-[(Aminopropyl)amino]ethanethiol (WR1065) is anti-neoplastic and anti-mutagenic when given during 60Co gamma-ray irradiation. Carcinogenesis. 1986 Apr;7(4):665–668. doi: 10.1093/carcin/7.4.665. [DOI] [PubMed] [Google Scholar]
- Jellum E., Aaseth J., Eldjarn L. Mercaptodextran, a metal-chelating and disulphide-reducing polythiol of high molecular weight. Biochem Pharmacol. 1973 May 15;22(10):1179–1188. doi: 10.1016/0006-2952(73)90235-9. [DOI] [PubMed] [Google Scholar]
- LASALLE M., BILLEN D. INHIBITION OF DNA SYNTHESIS IN MURINE BONE-MARROW CELLS BY AET AND CYSTEAMINE. Ann N Y Acad Sci. 1964 Mar 31;114:622–629. doi: 10.1111/j.1749-6632.1964.tb53616.x. [DOI] [PubMed] [Google Scholar]
- Meyn R. E., Jenkins W. T. Variation in normal and tumor tissue sensitivity of mice to ionizing radiation-induced DNA strand breaks in vivo. Cancer Res. 1983 Dec;43(12 Pt 1):5668–5673. [PubMed] [Google Scholar]
- Milas L., Hunter N., Stephens L. C., Peters L. J. Inhibition of radiation carcinogenesis in mice by S-2-(3-aminopropylamino)-ethylphosphorothioic acid. Cancer Res. 1984 Dec;44(12 Pt 1):5567–5569. [PubMed] [Google Scholar]
- Murray D., Jenkins W. T., Meyn R. E. The efficiency of DNA strand-break repair in two fibrosarcoma tumors and in normal tissues of mice irradiated in vivo with X rays. Radiat Res. 1984 Oct;100(1):171–181. [PubMed] [Google Scholar]
- Nagy B., Dale P. J., Grdina D. J. Protection against cis-diamminedichloroplatinum cytotoxicity and mutagenicity in V79 cells by 2-[(aminopropyl)amino]ethanethiol. Cancer Res. 1986 Mar;46(3):1132–1135. [PubMed] [Google Scholar]
- Nagy B., Grdina D. J. Protective effects of 2-[(aminopropyl)amino] ethanethiol against bleomycin and nitrogen mustard-induced mutagenicity in V79 cells. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1475–1478. doi: 10.1016/0360-3016(86)90197-5. [DOI] [PubMed] [Google Scholar]
- Peraino C., Staffeldt E. F., Carnes B. A., Ludeman V. A., Blomquist J. A., Vesselinovitch S. D. Characterization of histochemically detectable altered hepatocyte foci and their relationship to hepatic tumorigenesis in rats treated once with diethylnitrosamine or benzo(a)pyrene within one day after birth. Cancer Res. 1984 Aug;44(8):3340–3347. [PubMed] [Google Scholar]
- Phillips T. L. Rationale for initial clinical trials and future development of radioprotectors. Cancer Clin Trials. 1980 Summer;3(2):165–173. [PubMed] [Google Scholar]
- Purdie J. W. A comparative study of the radioprotective effects of cysteamine, WR-2721, and WR-1065 in cultured human cells. Radiat Res. 1979 Feb;77(2):303–311. [PubMed] [Google Scholar]
- Russell W. C., Newman C., Williamson D. H. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975 Feb 6;253(5491):461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- Sawada S., Okada S. Cysteamine, cystamine, and single-strand breaks of DNA in cultured mammalian cells. Radiat Res. 1970 Oct;44(1):116–132. [PubMed] [Google Scholar]
- Slaga T. J., Klein-Szanto A. J., Triplett L. L., Yotti L. P., Trosko K. E. Skin tumor-promoting activity of benzoyl peroxide, a widely used free radical-generating compound. Science. 1981 Aug 28;213(4511):1023–1025. doi: 10.1126/science.6791284. [DOI] [PubMed] [Google Scholar]
- Suzuki F., Han A., Lankas G. R., Utsumi H., Elkind M. M. Spectral dependencies of killing, mutation, and transformation in mammalian cells and their relevance to hazards caused by solar ultraviolet radiation. Cancer Res. 1981 Dec;41(12 Pt 1):4916–4924. [PubMed] [Google Scholar]
- Thacker J. The nature of mutants induced by ionising radiation in cultured hamster cells. III. Molecular characterization of HPRT-deficient mutants induced by gamma-rays or alpha-particles showing that the majority have deletions of all or part of the hprt gene. Mutat Res. 1986 May;160(3):267–275. doi: 10.1016/0027-5107(86)90137-5. [DOI] [PubMed] [Google Scholar]


