Abstract
We isolated hematopoietic stem cells (HSC) from mice treated with cyclophosphamide (CY) and granulocyte colony-stimulating factor (G-CSF). All mobilized multipotent progenitor activity was contained in two populations: Thy-1loSca-1+Lin−Mac-1−CD4−c-kit+ long-term reconstituting progenitors and Thy-1loSca-1+Lin−Mac-1loCD4− transiently reconstituting progenitors. CY/G-CSF treatment drove both long-term and transient multipotent progenitors into cycle, leading to a more than 12-fold expansion in the number of long-term self-renewing HSC prior to mobilization. After CY and 2 days of G-CSF treatment the number of bone marrow HSC began to decline and the number of blood and splenic HSC increased. HSC continued to proliferate in the bone marrow and spleen through 8 days of G-CSF treatment, but HSC released into the blood tended to be in G0/G1 phase. Mobilized multipotent progenitors isolated from the spleen were less efficient than normal bone marrow multipotent progenitors in engrafting irradiated mice but did not differ in colony forming unit-spleen (CFU-S) activity or single cell in vitro assays of primitive progenitor activity. The data suggest that mobilized HSC isolated from the spleen are less efficient at homing to and engrafting the bone marrow of irradiated recipient mice.
Treatment with any of a wide variety of chemotherapeutics or cytokines leads to an increase in the frequency of hematopoietic progenitor cells in the peripheral blood and, in mice, the spleen (1–6). The increase in peripheral progenitors has been coupled to a decrease in bone marrow progenitors, suggesting that peripheral progenitors are mobilized from bone marrow (7, 8). In some cases, mobilization appeared to consist only of a redistribution of primitive progenitor cells (9), whereas in other cases this redistribution was coupled to an increase in the absolute number of progenitor cells (7, 10, 11). Hematopoietic stem cells (HSC) are included in mobilized progenitors as shown by the great increase in the long-term multilineage reconstituting (LTMR) potential of the peripheral blood and spleen (for example see ref. 12); however, highly enriched populations of mobilized multipotent progenitors have rarely been studied (10, 13, 14). Many properties of mobilized HSC have not been examined directly and their developmental potentials have not been compared with those of normal bone marrow HSC at the single cell level. The mechanisms that regulate the expansion of HSC into the periphery are poorly understood. These mechanisms may be a fundamental aspect of HSC biology as progenitor mobilization occurs in all species examined so far, including humans (3), primates (15), dogs (1), and mice (6).
Despite the lack of a basic understanding of the mechanism of progenitor mobilization, the phenomenon is widely and increasingly exploited clinically. Cyclophosphamide (CY), followed by multiple granulocyte colony-stimulating factor (G-CSF) doses, is commonly used to peripheralize hematopoietic progenitors in humans for transplantation. We isolated mobilized HSC and studied the effects of CY/G-CSF on the HSC pool.
MATERIALS AND METHODS
Mouse Strains.
C57BL/J (Ly5.1) and C57BL/Ka-Thy1.1 (Ly 5.2) mouse strains were bred and maintained on acidified water (pH 2.5). The mice used in this study were generally 6–12 weeks old.
Mobilization Protocol.
Mice were injected i.p. with 4 mg of CY (≈200 mg/kg) (Bristol–Myers Squibb) and then on successive days with 5 μg of human G-CSF (≈250 μg/kg per day) (Amgen Biologicals, supplied by the Stanford University Hospital pharmacy) administered as a single daily s.c. injection. The day of CY treatment was considered day −1 and the first day of G-CSF treatment was counted as day 0. For example, mice sacrificed on day 3 of the mobilization protocol were sacrificed on the day after the third G-CSF injection.
Tissue Preparation and Staining.
Marrow was flushed from the femurs and tibias of donor mice. Single cell suspensions were prepared by drawing the bone marrow cells through a 25-gauge needle, then expelling them back through the needle and through a nylon mesh screen. Spleens were cut into pieces and then gently pressed through a nylon screen to obtain a single cell suspension. Blood cells were collected by cardiac incision and diluted into two tubes, each containing 0.5 ml of 10 mM EDTA in PBS. One milliliter of 2% dextran T500 was then added to each tube and the red blood cells were depleted by sedimentation for 45 min at 37°C. Red blood cells were not lysed during stem cell purification from bone marrow and spleen, but were lysed using ammonium chloride as described (16) during stem cell isolation from blood.
Antibodies.
The antibodies used in immunofluorescence staining included 19XE5 (anti-Thy1.1), AL1–4A2 (anti-Ly5.2), A20.1 (anti-Ly5.1), 2B8 (anti-c-kit), E13 (anti-Sca-1, Ly6A/E). Lineage marker antibodies included KT31.1 (anti-CD3), 53–7.3 (anti-CD5), 53–6.7 (anti-CD8), Ter119 (anti-erythrocyte specific antigen), 6B2 (anti-B220), and 8C5 (anti-Gr-1). M1/70 (anti-Mac-1) and GK1.5 (anti-CD4) staining of hematopoietic populations were characterized separately from the lineage cocktail to distinguish long-term and transient progenitor populations.
Progenitor Isolation.
Progenitors were isolated as described (17). Briefly, cells were incubated with a cocktail of unlabeled lineage marker antibodies including those specific for CD3, CD4, CD5, CD8, Gr-1, Ter119, B220, and Mac-1, followed by incubation with phycoerythrin-conjugated anti-rat antibody (to visualize the lineage markers), and 19XE5FITC, E13bio, and 2B8APC or M1/70APC, prior to FACS (Beckman Center shared FACS facility) analysis. Sca-1+ cells were sometimes enriched by positive selection using MACS (Miltenyi Biotec) streptavidin-conjugated magnetic beads according to the manufacturer’s instructions.
Cell cycle analyses were performed as described (16).
Functional Analyses.
Recipient mice were lethally irradiated (920 rads) using an x-ray machine operated at 200 kV, delivering 85 rad/min. The radiation was delivered in two doses, with ≈3 hr between doses. After irradiation, mice were maintained on antibiotic water containing 1.1 g/liter of neomycin sulfate and 106 units/liter of polymixin B sulfate. For reconstitution assays, double-sorted progenitor populations were injected into the retro-orbital venous sinus of irradiated recipient mice along with 200,000 recipient-type whole bone marrow (WBM) cells to protect recipient mice from radiation-induced hematopoietic failure. Reconstituted mice were periodically bled via the tail vein to monitor reconstitution by donor marked progenitors as described (16).
CFU-S and radioprotection assays were performed as described (16).
Methylcellulose medium contained 40% Methocult (Stem Cell Technologies, Vancouver), 30% fetal bovine serum (FBS) (embryonic stem cell qualified: lot 32P5431) (GIBCO), 30% alpha MEM (ICN). The medium was supplemented with penicillin/streptomycin, 1% BSA, 2 mM l-glutamine, 50 μM 2-mercaptoethanol, 30 ng/ml interleukin (IL)-3, 10 ng/ml IL-6, and 20 ng/ml steel factor (SlF) (Genzyme). Medium (0.1 ml) was added to each well of a 96-well plate, and single cells were cloned into each well by FACS. Colony formation in methylcellulose was scored using an inverted microscope after 6, 8, and 10 days of culture. AC-6 and S17 cultures were established in 24-well plates prior to being seeded by single candidate progenitor cells by FACS. The medium [containing RPMI 1640 medium (Irvine Scientific)/10% FBS (Gemini Biological Products, Calabasas, CA: lot A1056K)/pyruvate/penicillin/streptomycin/2-mercaptoethanol) was changed weekly. Colony formation was monitored from 1 to 4 weeks after cloning HSC into the cultures.
RESULTS AND DISCUSSION
Thy-1.1+Sca-1+Lin−Mac-1−CD4−c-kit+ and Thy-1.1+Sca-1+Lin−Mac-1lo CD4−c-kit+ Cells Are the Only Mobilized HSC Populations.
In an effort to purify mobilized HSC from the spleens of CY/G-CSF-treated mice, we tested which markers are associated with LTMR activity in whole mobilized spleen cells (Table 1). We injected mice i.p. with 4 mg of CY followed by 7 days of 5 μg per day s.c. rhG-CSF. Then, splenocytes were separated into positive and negative fractions for Thy-1.1, Sca-1, Mac-1, lineage markers (including CD4), and c-kit. Each fraction was equivalent to the number of cells of that population contained in approximately 300,000 whole spleen cells, the minimum dose of mobilized splenocytes that LTMR all recipients. As has been previously shown with normal mouse bone marrow (18), all of the observed HSC activity in the spleens of these mice was Sca-1+, lineage−, and c-kit+. Most progenitor activity was Thy-1.1+. HSC activity was found in both the Mac-1− and Mac-1+ fractions. HSC mobilized by CY/G-CSF treatment are Thy-1.1+Sca-1+Lin−Mac-1−CD4−c-kit+ and Thy-1.1+Sca-1+Lin−Mac-1+CD4−c-kit+.
Table 1.
The surface markers associated with HSC activity in the spleens of CY/G-CSF-treated mice
| Antigen used to separate donor cells | Antigen negative
|
Antigen positive
|
||
|---|---|---|---|---|
| LTMR mice | Level of donor WBC* | LTMR mice | Level of donor WBC* | |
| Thy-1.1 | 1/5 | 11.1 ± 7.2 | 5/5 | 52.5 ± 38.0 |
| Sca-1 | 0/5 | 0.9 ± 0.7 | 5/5 | 67.1 ± 11.5 |
| Mac-1 | 3/5 | 11.0 ± 16.6 | 4/4 | 51.2 ± 19.9 |
| Lineage + CD4 | 5/5 | 61.1 ± 24.3 | 0/4 | 2.6 ± 0.3 |
| c-kit | 0/5 | 3.3 ± 5.8 | 4/4 | 65.1 ± 28.5 |
Whole spleen cells from Ly5.2+ mice on the 7th day of CY/G-CSF treatment were separated by FACS into positive and negative fractions for each antigen, and tested for HSC activity in irradiated Ly5.1+ recipients. A dose of Ly5.2+ separated cells, equivalent to the number of cells of that population contained in approximately 300,000 whole spleen cells, was injected along with a radioprotective dose of Ly5.1+ WBM cells into irradiated recipients. LTMR mice had donor-derived monocytes, granulocytes, B, and T cells for more than 20 weeks after reconstitution. Donor type white blood cell levels are presented as a mean of all recipients at 20 weeks after reconstitution.
*Mean ± SD [% of white blood cells (WBC)].
The Reconstituting Activities of CY/G-CSF Mobilized Thy-1loSca-1+Lin−Mac-1−CD4−c-kit+ and Thy-1loSca-1+Lin−Mac-1loCD4− Cells.
Two populations were purified from the spleens of CY/G-CSF-treated mice with fluorescence profiles consistent with those associated with multipotent progenitor activity: Thy-1loSca-1+Lin−Mac-1−CD4−c-kit+ (referred to as Mac-1−CD4−c-kit+) and Thy-1loSca-1+Lin−Mac-1loCD4− (referred to as Mac-1loCD4−) cells. The same two populations exist in normal bone marrow, where they are nearly pure populations of long-term and transiently self-renewing multipotent progenitors, respectively (16). The fluorescence profiles of these populations in normal bone marrow and the spleens of CY/G-CSF-treated mice were indistinguishable except for a reduction of up to 10-fold in the binding of anti-c-kit antibody to Mac-1−CD4−c-kit+ cells from the spleens of CY/G-CSF-treated mice (data not shown). This is consistent with previous observations of reduced c-kit expression upon mobilization (19) or bone marrow ablation (20).
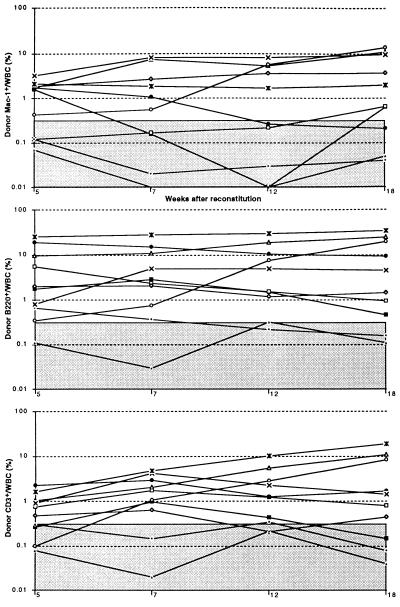
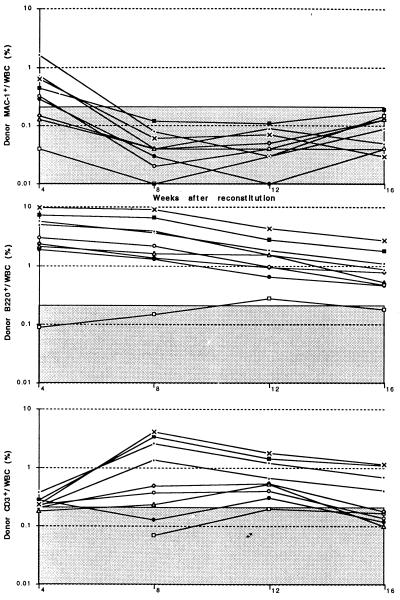
We assayed splenic Mac-1−CD4−c-kit+ cells and Mac-1loCD4− cells from CY/G-CSF-treated mice for functional activities to determine if these populations exhibited the same functional activities as in normal bone marrow. Ten cells of either population from normal bone marrow competitively reconstitutes a limit dilution frequency (60–70%) of irradiated recipients (16); however, when purified from CY/G-CSF-treated mice, similar cell doses infrequently yielded detectable reconstitution of any type (assayed from 4–12 weeks after reconstitution). Larger doses of splenic Mac-1−CD4−c-kit+ cells exhibited mostly LTMR activity, whereas larger doses of splenic Mac-1loCD4− cells exhibited mostly transient multilineage progenitor activity. Fig. 1 shows the donor myeloid, B, and T reconstitution of 10 lethally irradiated mice injected with 150 donor-type Mac-1−CD4−c-kit+ cells from the spleens of CY/G-CSF-treated mice and 200,000 recipient-type WBM cells. Six recipients were LTMR, one was transiently multilineage reconstituted, two were reconstituted only by B and T cells, and one recipient was unreconstituted by donor-type cells. In 2 experiments, only 4 of 8 irradiated mice injected with 50 Mac-1−CD4−c-kit+ cells isolated from the spleens of CY/G-CSF mice became reconstituted, of which two or three were LTMR (data not shown). Fig. 2 shows the donor myeloid, B, and T reconstitution of 9 lethally irradiated mice injected with 50 donor-type Mac-1loCD4− spleen cells and 200,000 recipient-type WBM cells. Six mice were transiently multilineage reconstituted, as evidenced by the loss-of-donor-type myeloid cells by 8 weeks after reconstitution, and the slowly declining levels of donor-type B and T cells. Two mice were reconstituted only by B and T cells, and one mouse was unreconstituted by donor-type cells.
Figure 1.
Donor myeloid, B, and T lineage reconstitution profiles of 10 mice injected with by 150 donor-type Thy-1.1loSca-1+Lin−Mac-1−CD4−c-kit+ cells (purified from the spleens of donor mice on day 7 of CY/G-CSF treatment) plus 200,000 recipient-type WBM cells. Each line represents a separate mouse, and the shaded area represents the range of background signals based on negative control mice reconstituted only with recipient-type WBM cells. Donor cells are presented as a percentage of all white blood cells.
Figure 2.
Donor myeloid, B, and T lineage reconstitution profiles of 9 mice injected with 50 donor-type Thy-1.1loSca-1+Lin−Mac-1loCD4− cells (purified from the spleens of donor mice on day 7 of CY/G-CSF treatment) plus 200,000 recipient-type WBM cells. Each line represents a separate mouse, and the shaded area represents the range of background signals based on negative control mice reconstituted only with recipient-type WBM cells. Donor cells are presented as a percentage of all white blood cells.
Mac-1−CD4−c-kit+ cells isolated from the blood of CY/G-CSF mice were also less efficient at engrafting irradiated mice than the same population isolated from normal bone marrow. For example, only 4 of 15 mice injected with 20 Mac-1−CD4−c-kit+ cells purified from the blood of day 7 CY/G-CSF-treated mice became reconstituted by donor-type cells. Preliminary experiments suggest inefficient engraftment by Mac-1−CD4−c-kit+ cells isolated from the bone marrow of CY/G-CSF-treated mice as well.
The functional activities of Mac-1−CD4−c-kit+ and Mac-1loCD4− cells from the spleens of CY/G-CSF-treated mice are summarized in Table 2. On average, injection of 100 splenic Mac-1−CD4−c-kit+ cells along with a radioprotective dose of recipient-type normal WBM cells yielded a limit dilution frequency of donor reconstituted mice. Of mice reconstituted by near-limit dilution doses of Mac-1−CD4−c-kit+ cells, 66% were LTMR, 13% were transiently multilineage reconstituted, and 20% were reconstituted in only 1 or 2 lineages. On average, 20 Mac-1loCD4− cells were required to competitively reconstitute a limit dilution frequency of irradiated recipients. Only 16% of mice reconstituted with near-limit dilution doses of Mac-1loCD4− cells were LTMR; most (57%) were transiently multilineage reconstituted and some (27%) were reconstituted in only 1 or 2 lineages. As in normal bone marrow, the Mac-1−CD4−c-kit+ population is enriched for long-term multipotent progenitors, whereas the Mac-1loCD4− population is enriched for transiently reconstituting multipotent progenitors. Although the Mac-1loCD4− population is depleted of LTMR progenitors, its more efficient engraftment explains why significant amounts of LTMR activity were observed in both the Mac-1− and Mac-1+ fractions of splenocytes (Table 1).
Table 2.
Functional properties of purified Thy-1.1lo Sca-1+ Lin−/lo subpopulations in the spleens of day 7 CY/G-CSF treated mice
| Splenic multipotent progenitor population
|
||
|---|---|---|
| Mac-1−CD4−c-kit+ | Mac-1loCD4− | |
| Limit dilution dose upon i.v. injection | 100 ± 39 | 20 ± 9 |
| Long-term progenitors (%) | 66 | 15 |
| Day 12 CFU-S (1/frequency) | 20 ± 8 | 13 ± 5 |
| Clonogenic on stroma (%) | 46 ± 16 | 47 ± 18 |
| Clonogenic in SlF, IL-3, IL-6 (%) | 68 ± 9 | 71 ± 12 |
| Clonogenic in IL-3 alone (%) | 12 | 9 |
| Clonogenic in GM-CSF alone (%) | 8 | 5 |
The limit dilution dose of a progenitor is the dose at which an average of 63% of recipients become reconstituted by donor type cells and Poisson statistics predict that the recipients are reconstituted by an average of a single progenitor. The long-term progenitor content was based on the percentage of reconstituted mice that became LTMR by near limit dilution doses of the given population. Hematopoietic stroma were AC-6 or S17 in 24-well plates. Clonogenicity in response to cytokines was assayed in methylcellulose. Colony formation in culture was quantified by sorting a single cell per well and counting the percentage of wells that formed colonies.
The LTMR ability of whole splenocytes from day 7 CY/G-CSF-treated mice is consistent with the Mac-1−CD4−c-kit+ and Mac-1loCD4− populations containing all of the HSC activity. The frequencies of Mac-1−CD4−c-kit+ and Mac-1loCD4− cells in day 7 CY/G-CSF mobilized spleens (0.06 and 0.05% of spleen cells, respectively), limit dilution engraftment efficiencies, and proportions of long-term multipotent progenitors within each population (Table 2) can be used to estimate the LTMR potential of those populations relative to whole mobilized spleen cells. When the number of Mac-1−CD4−c-kit+ and Mac-1loCD4− cells contained in 200,000 whole mobilized splenocytes is injected into irradiated recipients, the recipients should be engrafted on average by 1.6 long-term multipotent progenitors. Poisson statistics predict that when mice are reconstituted by an average of 1.6 engrafting progenitors that 8 of 10 mice should be reconstituted (21). This is consistent with our observations that 100,000, 200,000, or 400,000 whole mobilized splenocytes LTMR 5 of 9, 7 of 9, or 10 of 10 recipients, respectively (data not shown). The LTMR activity observed in the Mac-1−CD4−c-kit+ and Mac-1loCD4− populations is consistent with the amount of LTMR activity observed in whole mobilized spleen cells. This again is consistent with these two populations containing all of the HSC activity in the spleens of CY/G-CSF-treated mice.
The Mobilized Mac-1−CD4−c-kit+ and Mac-1loCD4− Populations May Be Nearly Pure Populations of Multipotent Progenitors.
Relative to normal bone marrow multipotent progenitors, 10-fold more Mac-1−CD4−c-kit+ cells from the spleens of CY/G-CSF-treated mice or 2-fold more splenic Mac-1loCD4− cells were required to reconstitute a limit dilution frequency of recipients. The reduced engraftment efficiency of mobilized multipotent progenitors may have resulted from changes in their homing properties relative to normal bone marrow HSC; however, another possible explanation is that the splenic Mac-1−CD4−c-kit+ and Mac-1loCD4− populations in CY/G-CSF-treated mice are not pure populations of multipotent progenitors, but contain mostly nonprogenitor cells that dilute the activities of these populations. To distinguish between these possibilities, we tested the activities of the splenic Mac-1−CD4−c-kit+ and Mac-1loCD4− populations in multipotent progenitor assays that function independently of the ability of cells to home to and/or engraft the bone marrow (Table 2). Day 12 CFU-S activity derives from long-term and transient multipotent progenitors (16, 22, 23). The CFU-S activities of the splenic Mac-1−CD4−c-kit+ and Mac-1loCD4− populations are equal to or greater than the same populations from normal marrow (16). This is not consistent with the mobilized Mac-1−CD4−c-kit+ and Mac-1loCD4− populations containing mostly nonprogenitor cells, or mostly progenitors more differentiated than multipotent progenitors.
We next tested the ability of the mobilized populations to form colonies in methylcellulose supplemented with IL-3, IL-6, and SlF. These cytokines optimize colony formation by multipotent progenitors (24, 25). Eighty-three percent of single Mac-1−CD4−c-kit+ cells or 62% of Mac-1loCD4− cells from normal bone marrow form colonies under such conditions (17); data not shown). Sixty-eight percent of mobilized Mac-1−CD4−c-kit+ cells and 71% of mobilized Mac-1loCD4− cells formed colonies in methylcellulose with IL-3,6 and SlF (Table 2). Most of the colonies were primitive in appearance, including macrophages, granulocytes, and/or megakaryocytes. Methylcellulose culture results were also inconsistent with either population containing a large non-progenitor population that could reduce the read-out rate in vivo by 2 to 10 fold.
The low efficiency of mobilized HSC engraftment in bone marrow could result from an impaired ability to interact with bone marrow stroma. In an effort to test this possibility, we assayed the ability of single cells to form colonies on the stromal cell lines AC-6.2.1 or S17. When single Mac-1−CD4−c-kit+ or Mac-1loCD4− cells from normal bone marrow are deposited into stromal cell cultures in 24-well plates, around 50% of the cells form colonies of hundreds of thousands of cells that persist for more than 6 weeks (17). Formation of such colonies on stroma has been shown to be equivalent to LTC-IC (26, 27). Almost 50% of Mac-1−CD4−c-kit+ and Mac-1loCD4− cells from the spleens of CY/G-CSF-treated mice also formed colonies on stroma and these colonies were indistinguishable from those formed by normal bone marrow multipotent progenitors (Table 2). The ability of mobilized progenitor populations to proliferate on stroma was indistinguishable from that of nearly pure multipotent progenitor populations from normal bone marrow.
It remains formally possible that some as yet unidentified early myeloerythroid progenitor could give the observed activities in culture, without having detectable levels of progenitor activity in vivo. We tested the ability of mobilized splenic Mac-1−CD4−c-kit+ and Mac-1loCD4− cells to proliferate in methylcellulose in response to IL-3 or GM-CSF alone. Each of these factors is known to widely stimulate colony formation by progenitors committed to myeloid or myeloerythroid lineages (24, 28, 29). Only 8–12% of mobilized Mac-1−CD4−c-kit+ cells and 5–9% of mobilized Mac-1loCD4− cells formed colonies in response to these factors (Table 2). Even if none of the colonies that formed in response to these factors were derived from multipotent progenitors, this level of contaminating committed progenitors could not account for a 2- to 10-fold reduction in the ability of these populations to engraft irradiated mice.
No progenitor population other than lymphomyeloid multipotent progenitors has ever been identified that could give rise to the activities observed in the CFU-S and in vitro clonogenic assays (30). Since all such multipotent progenitors can be detected in the long-term competitive reconstitution assay we used, the functional data strongly suggest that the Mac-1−CD4−c-kit+ and Mac-1loCD4− populations from the spleens of CY/G-CSF-treated mice are highly enriched populations of long-term and transiently reconstituting multipotent progenitors despite their inefficient engraftment of irradiated mice. Perhaps changes in adhesion molecule expression that permit multipotent progenitors to be mobilized from the bone marrow and home to the spleen also reduce the efficiency of bone marrow homing in irradiated recipient mice. Although, to the best of our knowledge, this is the first study to present evidence that cytokine mobilized HSC engraft bone marrow less efficiently, the phenomenon of impaired bone marrow engraftment is not novel. Hematopoietic progenitors were impaired in their ability to home to bone marrow upon transplantation into irradiated recipients after treatment with antibodies against VLA-4 (31, 32). HSC in very old mice (17) and reconstituted mice (S.J.M., A. M. Wandycz, H. Hemmati, and I.L.W., unpublished work) exhibited properties similar to mobilized Mac-1−CD4−c-kit+ cells, including evidence of impaired marrow engraftment. Proof of the variability of HSC engraftment in bone marrow after intravenous injection will depend on the development of an in vivo homing assay sensitive enough to detect the trafficking of small numbers of cells.
Radioprotection.
Despite the reduced engraftment efficiency of mobilized Mac-1−CD4−c-kit+ cells, the radioprotective capacity of the population was similar to that of long-term reconstituting HSC from normal bone marrow (16) and fetal liver (33). Forty, 80, or 120 mobilized Mac-1−CD4−c-kit+ cells radioprotected 40%, 70%, or 90% of recipient mice, respectively (data not shown). The radioprotective capacity of HSC populations would appear to depend more on their CFU-S activity than on their ability to reconstitute bone marrow.
CY/G-CSF Treatment Induces a Massive Expansion in Bone Marrow HSC First, and in Blood and Spleen Later.
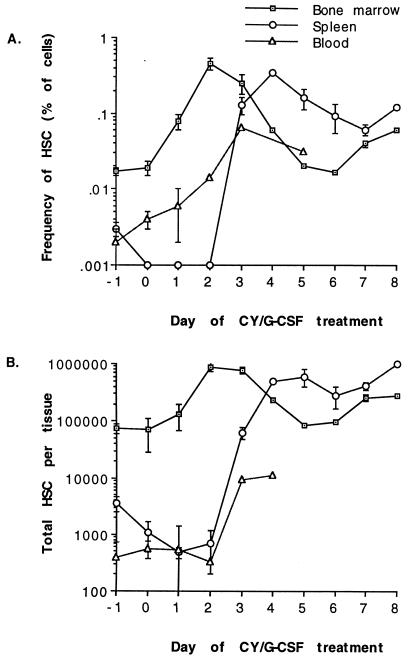
Both Mac-1−CD4−c-kit+ and Mac-1loCD4− cells increased in absolute number and were mobilized to the spleen by CY/G-CSF treatment. We studied the kinetics of long-term reconstituting Mac-1−CD4−c-kit+ cells in detail. Fig. 3 shows the frequency and absolute numbers of Mac-1−CD4−c-kit+ cells in the bone marrow, blood, and spleen after CY and sequential days of G-CSF administration. CY administration did not detectably reduce the numbers of bone marrow Mac-1−CD4−c-kit+ cells, but splenic Mac-1−CD4−c-kit+ cell numbers may have initially declined after CY treatment. After 2 days of G-CSF administration the Mac-1−CD4−c-kit+ population had increased in number more than 12-fold in the bone marrow, but no expansion was yet detectable in the spleen. If all of the Mac-1−CD4−c-kit+ cells noted on day 2 were derived from self-renewing divisions of bone marrow Mac-1−CD4−c-kit+ cells, then these cells would have to have undergone an average of 3.6 rounds of division over the 3 days of CY and G-CSF treatment. This would correspond to an average cell cycle time of around 20 hr if all Mac-1−CD4−c-kit+ cells were induced to self-renew. During the first day after CY treatment, Mac-1−CD4−c-kit+ cells may have been recruited into cycle, but no increase was observed in the number of Mac-1−CD4−c-kit+ cells. Most of the increase in Mac-1−CD4−c-kit+ cell numbers occurred between days 1 and 2 of CY/G-CSF treatment, when a 6.7-fold increase was observed, corresponding to an average cell cycle time during this interval of 9 hr.
Figure 3.
The frequency (A) and total number (B) of Thy-1.1loSca-1+Lin−Mac-1−CD4−c-kit+ HSC in the bone marrow, spleen, and blood of mice on successive days of CY/G-CSF treatment. CY was administered on day −1 and G-CSF was administered on each successive day. Mice were sacrificed to determine HSC levels, so measurements were performed in lieu of drug administration on that day. Thus, HSC measurements presented for day −1 correspond to normal mice (0.017% of WBM or 74,000 HSC/bone marrow whole body) and mice sacrificed on day 1 had received CY plus one dose of G-CSF. Variation is presented as the standard error of the mean. Total bone marrow HSC were calculated by assuming that the femurs and tibias (less the epiphyses) contained 15% of all bone marrow in the mouse (34). Total HSC in the blood was calculated by assuming that the total blood volume was 1.8 ml (35). Frequencies of HSC in the blood are expressed as a proportion of nucleated cells; frequencies in bone marrow and spleen are expressed as a proportion of all cells.
Increased numbers of HSC were first observed in the spleen after 3 days of G-CSF treatment; their appearance was abrupt and corresponded precisely with the first appearance of increased numbers of HSC in the blood and a decline in the frequency and number of HSC in the bone marrow. The frequency of HSC peaked in the spleen on day 4. After day 4 there was a rapid increase in spleen size as G-CSF drove an expansion of peripheral myeloid cells. The absolute number of Mac-1−CD4−c-kit+ cells in the spleen was considerably increased through day 8. Levels of blood Mac-1−CD4−c-kit+ cells remained elevated from day 3 onward, but the total number of Mac-1−CD4−c-kit+ cells in the blood was small relative to the spleen. It is conceivable that the rapid rise of Mac-1−CD4−c-kit+ cell levels in the bone marrow by day 2 could result partly from an influx of Mac-1−CD4−c-kit+ cells from the liver or other sites; however, the number of HSC in the liver has been observed to be insignificant relative to the bone marrow (36).
CY/G-CSF treatment caused a dramatic expansion in the number of Mac-1−CD4−c-kit+ cells. Interestingly, most of the observed expansion occurred prior to the appearance of HSC in the periphery. The changes in Mac-1−CD4−c-kit+ cell levels were consistent with migration from the bone marrow to the spleen beginning after the second day of G-CSF treatment and continuing for the duration of G-CSF treatment. These kinetics contrast markedly with those observed for IL-8, which induces detectable mobilization within 30 min (37). The initial 90-fold expansion in HSC numbers in the spleen between days 2 and 3 was too fast and too large to be explained only by the proliferation of HSC endogenous to the spleen: large numbers of Mac-1−CD4−c-kit+ cells must have migrated from the bone marrow through the blood. The relatively small numbers of Mac-1−CD4−c-kit+ cells in the blood between days 2 and 3 suggest that the transit time of HSC in the blood is short. Later increases in Mac-1−CD4−c-kit+ cell numbers in the spleen between days 6 and 8 could be caused by proliferation of cells within the spleen or by immigration from the bone marrow, or both. The variation in Mac-1−CD4−c-kit+ cell frequency and number between mice was high, even among littermates that were treated concurrently with CY/G-CSF in the same cage. High variability between individuals may be inherent in the response to CY/G-CSF, even in an inbred population.
Cell Cycle Status of HSC in CY/G-CSF-Treated Mice.
Only 4% and 7% of normal bone marrow Mac-1−CD4−c-kit+ and Mac-1loCD4− cells are in the S/G2/M phases of the cell cycle (16, 17). At all times analyzed from day 2 through day 8, bone marrow and splenic Mac-1−CD4−c-kit+ and Mac-1loCD4− cells in CY/G-CSF-treated mice had increased proportions of S/G2/M phase cells (Table 3). After 1–3 days of G-CSF treatment, Mac-1−CD4−c-kit+ cells appeared to be cycling rapidly, with 21–33% of the cells in S/G2/M phases of the cell cycle. CY/G-CSF treatment drove multipotent progenitors into cycle, consistent with the observed rapid expansion of those populations. In contrast, HSC isolated from the blood were not in cycle. Only 6.6% of blood Thy-1.1loSca-1+Lin−/loc-kit+ cells (including both Mac-1−CD4−c-kit+ and Mac-1loCD4− cells) were in S/G2/M phases. This is consistent with the results of others (13, 38–40).
Table 3.
Cell cycle status of mutipotent progenitor populations after CY/G-CSF treatment
| Thy-1.1loSca-1+Lin−/lo subpopulation | S/G2/M cells after successive days of G-CSF, %
|
|||||||
|---|---|---|---|---|---|---|---|---|
| d1 | d2 | d3 | d4 | d5 | d6 | d7 | d8 | |
| Bone marrow Mac-1−CD4−c-kit+ | 33 | 21 | 27 | ND | 11 | ND | 15 | 23 |
| Splenic Mac-1−CD4−c-kit+ | ∗ | ∗ | 8 | ND | 10 | 16 | 12 | 13 |
| Splenic Mac-1loCD4− | ∗ | ∗ | ND | ND | 17 | 21 | 14 | 14 |
The percentage of cells in each population with greater than 2n DNA was determined by Hoechst 33342 staining of FACS purified populations. ∗, Number of multipotent progenitors present in the spleen on these days was insufficient for analysis. d, day; ND, not determined.
While HSC mobilization is evolutionarily conserved, the selective advantage of mobilization is unclear. One possibility is that mobilization in adults is the vestige of a mechanism evolved to regulate transitions between hematopoietic tissues during fetal development. The fetal liver is the major site of hematopoiesis during mid-gestation, but on day 15 the spleen becomes hematopoietic. Prior to day 15, the number of HSC in the fetal liver doubles daily, but on day 15 the number of fetal liver HSC declines dramatically (33). The spleen may be seeded by a large scale mobilization of fetal liver HSC. This would require precisely regulated changes in HSC adhesion properties, like those that may mediate cytokine mobilization of adult HSC. Another possibility is that consumption of myeloablative compounds is a sufficiently frequent event in the natural history of most species that mechanisms evolved to quickly expand hematopoiesis (Christopher Goodnow, personal communication). Understanding the mechanism of cytokine mobilization may shed light on its evolutionary value.
Acknowledgments
We thank Libuse Jerabek for laboratory management, Tim Knaak for FACS machine operation, Veronica Braunstein and Andreea Salinas for antibody preparation, Lucino Hidalgo and Ricardo Salazar for animal care. We thank Koichi Akashi for help in conducting methylcellulose assays. We also thank Henry Chiu, Sherman Fong, Robert Negrin, Ian McNiece, Nobuko Uchida, and Ann Tsukamoto for helpful discussions. D.E.W. is a Medical Student Fellow of the Howard Hughes Medical Institute and S.J.M. was a Howard Hughes Medical Institute Predoctoral Fellow. This work was supported by a grant from SyStemix/Sandoz.
ABBREVIATIONS
- HSC
hematopoietic stem cells
- CY
cyclophosphamide
- G-CSF
granulocyte colony-stimulating factor
- IL
interleukin
- LTMR
long-term multilineage reconstituting(ed)
- WBM
whole bone marrow
- CFU-S
colony forming unit-spleen
- SlF
steel factor
References
- 1.Appelbaum F R, Deeg H J, Storb R, Graham R C, Charrier K, Bensinger W. Transplantation. 1986;42:19–22. doi: 10.1097/00007890-198607000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Abrams R A, McCormack K, Bowles C, Deisseroth A B. J Clin Invest. 1981;67:1392–1399. doi: 10.1172/JCI110167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juttner C A, To L B, Haylock D N, Branford A, Kimber R J. Br J Haematol. 1985;61:739–745. doi: 10.1111/j.1365-2141.1985.tb02888.x. [DOI] [PubMed] [Google Scholar]
- 4.Richman C, Weiner R, Yankee R. Blood. 1976;47:1031–1039. [PubMed] [Google Scholar]
- 5.Siena S, Bregni M, Brando B, Ravagnani F, Bonadonna G, Gianni A M. Blood. 1989;74:1905–1914. [PubMed] [Google Scholar]
- 6.Molineux G, Pojda Z, Hampson I N, Lord B I, Dexter T M. Blood. 1990;76:2153–2158. [PubMed] [Google Scholar]
- 7.Haan G d, Dontje B, Engel C, Loeffler M, Nijhof W. Blood. 1995;86:2986–2992. [PubMed] [Google Scholar]
- 8.Molineux G, Pojda Z, Dexter T M. Blood. 1990;75:563–569. [PubMed] [Google Scholar]
- 9.Fleming W H, Alpern E J, Uchida N, Ikuta K, Weissman I L. Proc Natl Acad Sci USA. 1993;90:3760–3764. doi: 10.1073/pnas.90.8.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodine D M, Seidel N E, Zsebo K M, Orlic D. Blood. 1993;82:445–455. [PubMed] [Google Scholar]
- 11.Bodine D, Seidel N E, Orlic D. Blood. 1996;88:89–97. [PubMed] [Google Scholar]
- 12.Yan X-Q, Briddell R, Hartley C, Stoney G, Samal B, McNiece I. Blood. 1994;84:795–799. [PubMed] [Google Scholar]
- 13.Uchida N, He D, Friera A, Reitsma M, Sasaki D, Chen B, Tsukamoto A. Blood. 1997;89:465–472. [PubMed] [Google Scholar]
- 14.Murray L, Chen B, Galy A, Chen S, Tushinski R, Uchida N, Negrin R, Tricot G, Jagannath S, Vesole D, Barlogie E, Hoffman R, Tsukamoto A. Blood. 1995;85:368–378. [PubMed] [Google Scholar]
- 15.Andrews R G, Briddell R A, Knitter G H, Opie T, Bronsden M, Myerson D, Applebaum F R, McNiece I K. Blood. 1994;84:800–810. [PubMed] [Google Scholar]
- 16.Morrison S J, Weissman I L. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 17.Morrison S J, Wandycz A M, Akashi K, Globerson A, Weissman I L. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 18.Uchida N, Weissman I L. J Exp Med. 1992;175:175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.To L B, Haylock D N, Dowse T, Simmons P J, Trimboli S, Ashman L K, Juttner C A. Blood. 1994;84:2930–2939. [PubMed] [Google Scholar]
- 20.Katayama N, Shih J-P, Nishikawa S-I, Kina T, Clark S C, Ogawa M. Blood. 1993;82:2353–2360. [PubMed] [Google Scholar]
- 21.Henry C, Marbrook J, Vann D C, Kodlin D, Wofsy C. In: Limiting Dilution Analysis. Mishell B B, Shiigi S M, editors. San Francisco: Freeman; 1980. pp. 138–144. [Google Scholar]
- 22.Loo J C M V d, Bos C V d, Baert M R M, Wagemaker G, Ploemacher R E. Blood. 1994;83:1769–1777. [PubMed] [Google Scholar]
- 23.Spangrude G J, Heimfeld S, Weissman I L. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 24.Heimfeld S, Hudak S, Weissman I, Rennick D. Proc Natl Acad Sci USA. 1991;88:9902–9906. doi: 10.1073/pnas.88.21.9902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuji K, Lyman S D, Sudo T, Clark S C, Ogawa M. Blood. 1992;79:2855–2860. [PubMed] [Google Scholar]
- 26.Muller-Sieburg C E, Whitlock C A, Weissman I L. Cell. 1986;44:653–662. doi: 10.1016/0092-8674(86)90274-6. [DOI] [PubMed] [Google Scholar]
- 27.Muller-Sieburg C E, Riblet R. J Exp Med. 1996;183:1141–1150. doi: 10.1084/jem.183.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogawa M. Blood. 1993;81:2844–2853. [PubMed] [Google Scholar]
- 29.Inaba K, Inaba M, Deguchi M, Hagi K, Yasumizu R, Ikehara S, Muramatsu S, Steinman R M. Proc Natl Acad Sci USA. 1993;90:3038–3042. doi: 10.1073/pnas.90.7.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weilbaecher K, Weissman I, Blume K, Heimfeld S. Blood. 1991;78:945–952. [PubMed] [Google Scholar]
- 31.Williams D A, Rios M, Stephens C, Patel V P. Nature (London) 1991;352:438–441. doi: 10.1038/352438a0. [DOI] [PubMed] [Google Scholar]
- 32.Papayannopoulou T, Craddock C, Nakamoto B, Priestley G V, Wolf N S. Proc Natl Acad Sci USA. 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison S J, Hemmati H D, Wandycz A M, Weissman I L. Proc Natl Acad Sci USA. 1995;92:10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith L H, Clayton M L. Exp Hematol. 1970;20:82–86. [Google Scholar]
- 35.Russell E S, Bernstein S E. In: The Biology of the Laboratory Mouse. Green E L, editor. New York: Dover; 1966. p. 340. [Google Scholar]
- 36.Taniguchi H, Toyoshima T, Fukao K, Nakauchi H. Nat Med. 1996;2:198–203. doi: 10.1038/nm0296-198. [DOI] [PubMed] [Google Scholar]
- 37.Laterveer L, Lindley I J, Hamilton M S, Willemze R, Fibbe W E. Blood. 1995;85:2269–2275. [PubMed] [Google Scholar]
- 38.Donahue R, Kirby M, Metzger M, Agricola B, Sellers S, Cullis H. Blood. 1996;87:1644–1653. [PubMed] [Google Scholar]
- 39.Roberts A W, Metcalf D. Blood. 1995;86:1600–1605. [PubMed] [Google Scholar]
- 40.Siegert W, Serke S. Bone Marrow Transplantation. 1996;17:467–470. [PubMed] [Google Scholar]