Abstract
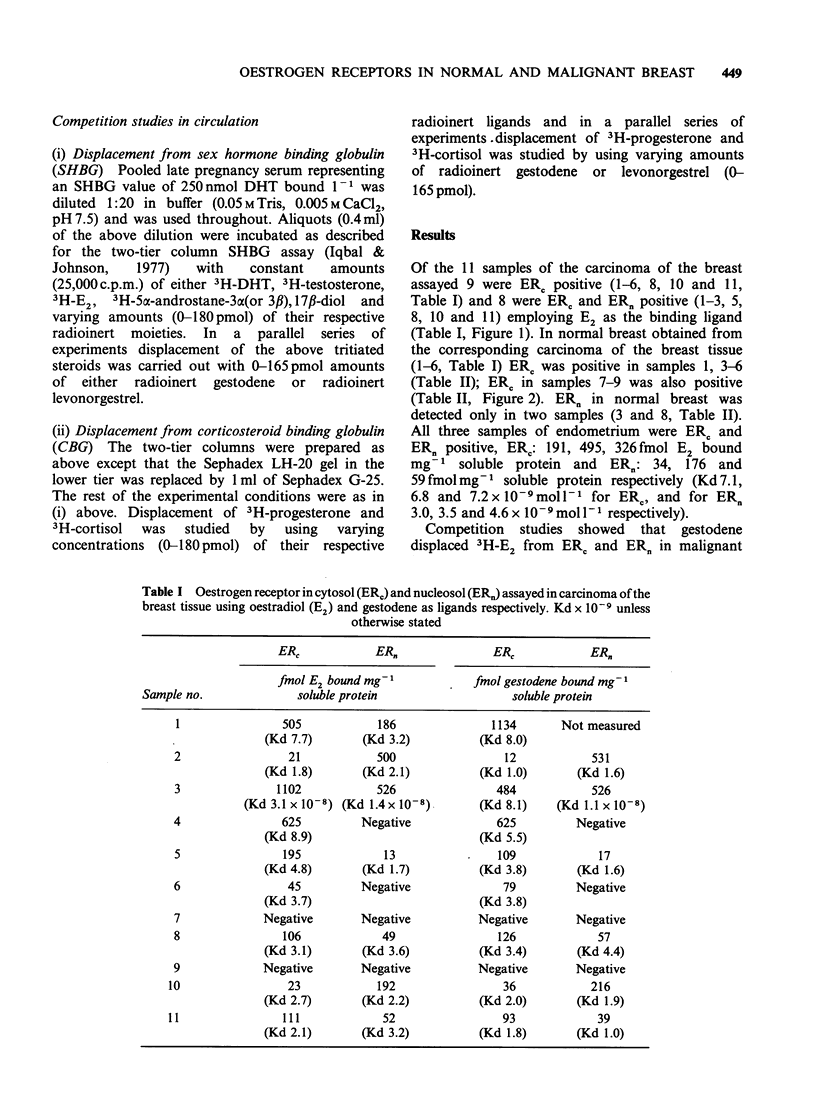
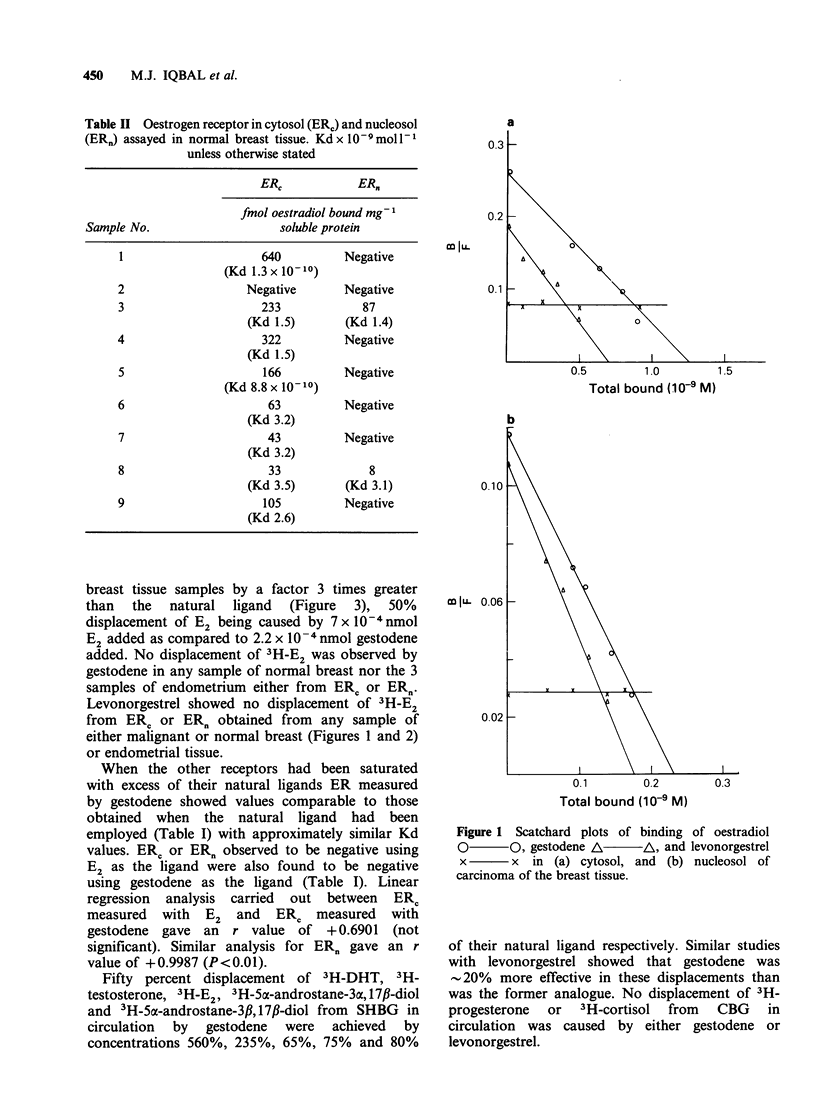
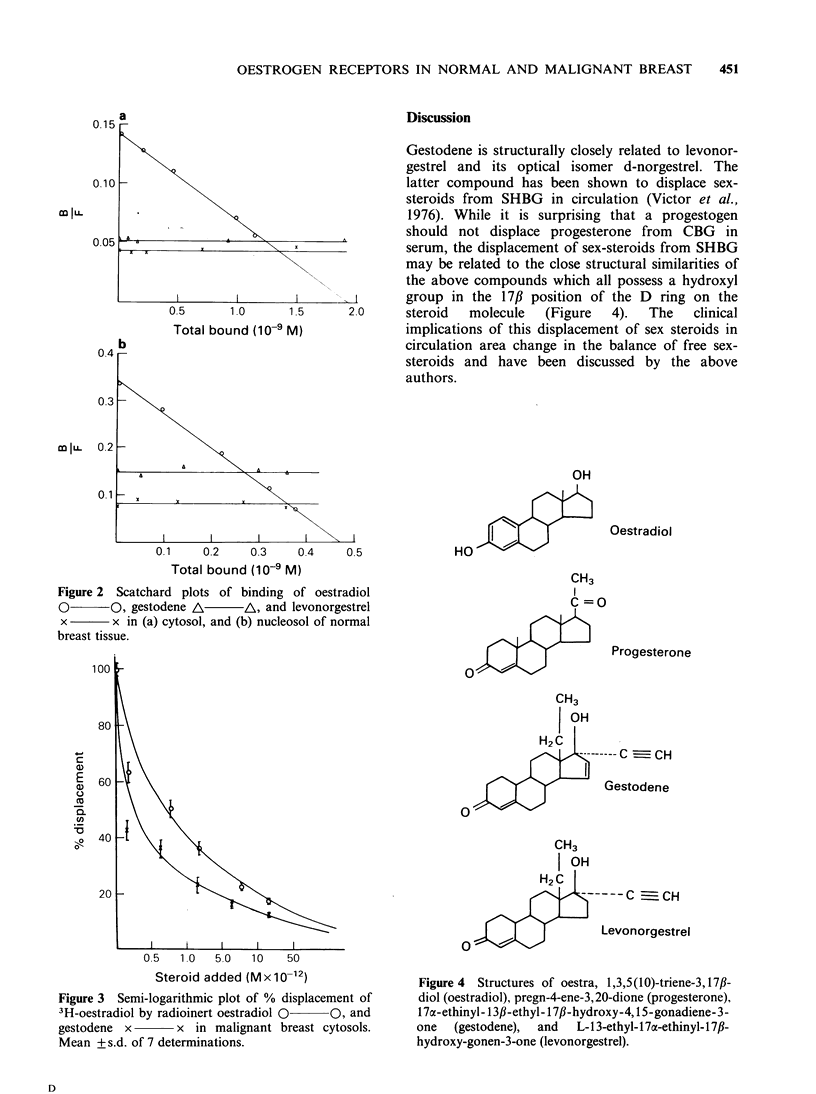
Oestrogen receptor protein (ER) was detected in 9 of 11 samples of malignant breast tissue and 8 of 9 samples of normal breast tissue. Levels of cytosolic ER (ERc) in malignant breast were 21-1102 fmol mg-1 soluble protein (Kd 1.8 X 10(-9)-3.1 X 10(-8) mol l-1) and those of nucleosolic ER (ERn), 13-526 fmol mg-1 soluble protein (Kd 2.1 X 10(-9)-1.4 X 10(-8) mol l-1). In normal breast tissue ERc levels were 33-640 fmol mg-1 soluble protein (Kd 1.3 X 10(-10)-3.2 X 10(-9) mol l-1), ERn was detected in only 2 samples, 8 and 87 fmol mg-1 soluble protein with Kd 3.2 X 10(-9) and 1.4 X 10(-9) l mol-1 respectively. 17 alpha-ethinyl-13 beta-ethyl-17 beta-hydroxy-4,15-gonadiene-3-one (gestodene), a new synthetic progestogen displaced 3H-oestradiol (3H-E2) from both ERc and ERn in malignant tissue but not in normal breast, or these receptors from endometrial tissue. In competition studies gestodene was approximately 3 times more effective in displacing 3H-E2 from ERc and ERn in malignant breast tissue than the natural ligand. Quantitation of ER by gestodene were ERc, 12-1134 fmol gestodene bound mg-1 soluble protein (Kd 1 X 10(-9)-8.1 X 10(-9) mol l-1); ERn, 17-531 fmol gestodene bound mg-1 soluble protein (Kd 1.6 X 10(-9)-1.1 X 10(-8) mol l-1). L-13-ethyl-17 alpha-ethinyl, 17 beta-hydroxy-gonen-3-one (levonorgestrel) showed no binding to ER in malignant breast, normal breast or endometrial tissue. In circulation both gestodene and levonorgestrel displaced E2 from sex hormone binding globulin more than any of the androgens tested. These results suggest that gestodene is a progestogen with oestrogenic and/or antioestrogenic properties and provide strong evidence for differences in ER from malignant and normal breast tissue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. M., Jeltsch J. M., Roberts M., Chambon P. Activation of pS2 gene transcription is a primary response to estrogen in the human breast cancer cell line MCF-7. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6344–6348. doi: 10.1073/pnas.81.20.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamness G. C., McGuire W. L. Scatchard plots: common errors in correction and interpretation. Steroids. 1975 Oct;26(4):538–542. doi: 10.1016/0039-128x(75)90073-2. [DOI] [PubMed] [Google Scholar]
- Greenway B., Iqbal M. J., Johnson P. J., Williams R. Oestrogen receptor proteins in malignant and fetal pancreas. Br Med J (Clin Res Ed) 1981 Sep 19;283(6294):751–753. doi: 10.1136/bmj.283.6294.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grody W. W., Schrader W. T., O'Malley B. W. Activation, transformation, and subunit structure of steroid hormone receptors. Endocr Rev. 1982 Spring;3(2):141–163. doi: 10.1210/edrv-3-2-141. [DOI] [PubMed] [Google Scholar]
- Iqbal M. J., Corbishley T. P., Wilkinson M. L., Williams R. A microassay for the determination of binding parameters of estrogen and androgen receptors employing affinity immobilization on Cibacron blue 3GA-Sepharose 6B. Anal Biochem. 1985 Jan;144(1):79–85. doi: 10.1016/0003-2697(85)90086-7. [DOI] [PubMed] [Google Scholar]
- Iqbal M. J., Johnson M. W. Study of steroid-protein binding by a novel "two-tier" column employing Cibacron Blue F3G-A-Sepharose 4B. I-Sex hormone binding globulin. J Steroid Biochem. 1977 Sep;8(9):977–983. doi: 10.1016/0022-4731(77)90196-0. [DOI] [PubMed] [Google Scholar]
- Iqbal M. J., Wilkinson M. L., Johnson P. J., Williams R. Sex steroid receptor proteins in foetal, adult and malignant human liver tissue. Br J Cancer. 1983 Dec;48(6):791–796. doi: 10.1038/bjc.1983.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper T. W., Ruh M. F., Ruh T. S. Estrogen and antiestrogen binding to rat uterine and pituitary estrogen receptor: evidence for at least two physicochemical forms of the estrogen receptor. J Steroid Biochem. 1985 Nov;23(5A):537–545. doi: 10.1016/0022-4731(85)90001-9. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S. Dynamics of steroid hormone receptor action. Annu Rev Physiol. 1980;42:17–35. doi: 10.1146/annurev.ph.42.030180.000313. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Katzenellenbogen J. A., Ferguson E. R., Krauthammer N. Anti-estrogen interaction with uterine estrogen receptors. Studies with a radiolabeled anti-estrogen (CI-628). J Biol Chem. 1978 Feb 10;253(3):697–707. [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Pavlik E. J., Robertson D. W., Katzenellenbogen J. A. Interaction of a high affinity anti-estrogen (alpha-[4-pyrrolidinoethoxy]phenyl-4-hydroxy-alpha'-nitrostilbene, CI628M) with uterine estrogen receptors. J Biol Chem. 1981 Mar 25;256(6):2908–2915. [PubMed] [Google Scholar]
- Keene J. L., Sweet F., Ruh M. F., Ruh T. S. Interaction of the radiolabelled high-affinity anti-oestrogen [3H]H1285 with the cytoplasmic oestrogen receptor. Biochem J. 1984 Feb 1;217(3):819–826. doi: 10.1042/bj2170819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W. J., Greene G. L. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984 Feb 23;307(5953):745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- McGuire W. L., Zava D. T., Horwitz K. B., Garola R. E., Chamness G. C. Receptors and breast cancer: do we know it all? J Steroid Biochem. 1978 May;9(5):461–466. doi: 10.1016/0022-4731(78)90616-7. [DOI] [PubMed] [Google Scholar]
- Szego C. M., Pietras R. J. Subcellular distribution of oestrogen receptors. Nature. 1985 Sep 5;317(6032):88–89. doi: 10.1038/317088a0. [DOI] [PubMed] [Google Scholar]
- Victor A., Weiner E., Johansson E. D. Sex hormone binding globulin: the carrier protein for d-norgestrel. J Clin Endocrinol Metab. 1976 Jul;43(1):244–247. doi: 10.1210/jcem-43-1-244. [DOI] [PubMed] [Google Scholar]
- Welshons W. V., Lieberman M. E., Gorski J. Nuclear localization of unoccupied oestrogen receptors. Nature. 1984 Feb 23;307(5953):747–749. doi: 10.1038/307747a0. [DOI] [PubMed] [Google Scholar]


