Abstract
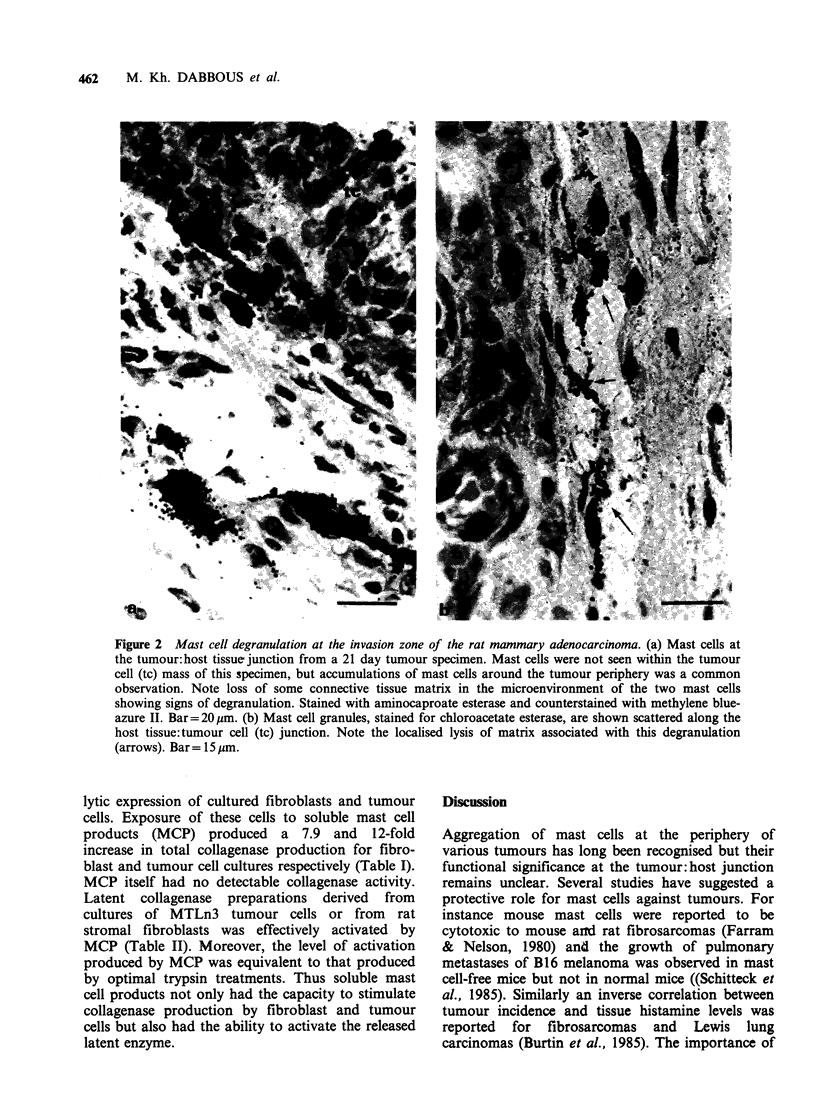
Significant numbers of mast cells have been demonstrated histologically around the periphery of the invasive rat mammary adenocarcinoma 13672NF. The number of mast cells at microfoci along the tumour:host tissue junction was significantly greater than that found in normal mammary tissues, and few mast cells were detected within the tumour itself. Mast cell degranulation, often associated with disruption and lysis of the connective tissue matrix, was a common feature in later stages of tumour proliferation. When soluble products derived from purified rat peritoneal mast cells were added to monolayer cultures of rat stromal fibroblasts or tumour cells they stimulated a significant increase in total collagenase production, and the mast cell products were also capable of activating the latent collagenases thus produced. Histological examination indicated that degradation of local collagenous matrix was a common feature of mast cell degranulation, an observation possibly explained by the release of mast cell enzymes and/or the potential of this cell to modulate the expression of collagenolytic activity by surrounding cells. These observations suggest that, at least in some tumours, mast cells contribute to the connective tissue breakdown commonly associated with tumour invasiveness and metastatic spread.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins F. M., Friedman M. M., Subba Rao P. V., Metcalfe D. D. Interactions between mast cells, fibroblasts and connective tissue components. Int Arch Allergy Appl Immunol. 1985;77(1-2):96–102. doi: 10.1159/000233760. [DOI] [PubMed] [Google Scholar]
- Barsky S. H., Togo S., Garbisa S., Liotta L. A. Type IV collagenase immunoreactivity in invasive breast carcinoma. Lancet. 1983 Feb 5;1(8319):296–297. doi: 10.1016/s0140-6736(83)91708-7. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Cobb C. M., Taylor R. E., Fullmer H. M. Activation of fibroblast procollagenase by mast cell proteases. Biochim Biophys Acta. 1976 Jun 7;438(1):273–286. doi: 10.1016/0005-2744(76)90243-6. [DOI] [PubMed] [Google Scholar]
- Biswas C. Tumor cell stimulation of collagenase production by fibroblasts. Biochem Biophys Res Commun. 1982 Dec 15;109(3):1026–1034. doi: 10.1016/0006-291x(82)92042-3. [DOI] [PubMed] [Google Scholar]
- Bromley M., Fisher W. D., Woolley D. E. Mast cells at sites of cartilage erosion in the rheumatoid joint. Ann Rheum Dis. 1984 Feb;43(1):76–79. doi: 10.1136/ard.43.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley M., Woolley D. E. Histopathology of the rheumatoid lesion. Identification of cell types at sites of cartilage erosion. Arthritis Rheum. 1984 Aug;27(8):857–863. doi: 10.1002/art.1780270804. [DOI] [PubMed] [Google Scholar]
- Burtin C., Ponvert C., Fray A., Scheinmann P., Lespinats G., Loridon B., Canu P., Paupe J. Inverse correlation between tumor incidence and tissue histamine levels in W/WV, WV/+, and +/+ mice. J Natl Cancer Inst. 1985 Mar;74(3):671–674. [PubMed] [Google Scholar]
- CSABA G., ACS T., HORVATH C., MOLD K. Genesis and function of mast cells. Mast cell and plasmacyte reaction to induced, homologous and heterologous tumours. Br J Cancer. 1961 Jun;15:327–335. doi: 10.1038/bjc.1961.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbous M. K., El-Torky M., Haney L., Brinkley S. B., Sobhy N. Collagenase activity in rabbit carcinoma: cell source and cell interactions. Int J Cancer. 1983 Mar 15;31(3):357–364. doi: 10.1002/ijc.2910310317. [DOI] [PubMed] [Google Scholar]
- Dabbous M. K., Roberts A. N., Brinkley B. Collagenase and neutral protease activities in cultures of rabbit VX-2 carcinoma. Cancer Res. 1977 Oct;37(10):3537–3544. [PubMed] [Google Scholar]
- Dabbous M. K., Woolley D. E., Haney L., Carter L. M., Nicolson G. L. Host-mediated effectors of tumor invasion: role of mast cells in matrix degradation. Clin Exp Metastasis. 1986 Apr-Jun;4(2):141–152. doi: 10.1007/BF00119080. [DOI] [PubMed] [Google Scholar]
- FISHER E. R., FISHER B. ROLE OF MAST CELLS IN TUMOR GROWTH. Arch Pathol. 1965 Feb;79:185–191. [PubMed] [Google Scholar]
- Farnoush A., Mackenzie I. C. Proliferation of mast cells in normal and DMBA-treated mouse skin. J Oral Pathol. 1984 Aug;13(4):359–365. doi: 10.1111/j.1600-0714.1984.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Farnoush A., Mackenzie I. C. Sequential histological changes and mast cell response in skin during chemically-induced carcinogenesis. J Oral Pathol. 1983 Aug;12(4):300–306. doi: 10.1111/j.1600-0714.1983.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Farram E., Nelson D. S. Mouse mast cells as anti-tumor effector cells. Cell Immunol. 1980 Oct;55(2):294–301. doi: 10.1016/0008-8749(80)90162-8. [DOI] [PubMed] [Google Scholar]
- Hartveit F. Mast cells and metachromasia in human breast cancer: their occurrence, significance and consequence: a preliminary report. J Pathol. 1981 May;134(1):7–11. doi: 10.1002/path.1711340103. [DOI] [PubMed] [Google Scholar]
- Hartveit F., Sandstad E. Stromal metachromasia: a marker for areas of infiltrating tumour growth? Histopathology. 1982 Jul;6(4):423–428. doi: 10.1111/j.1365-2559.1982.tb02739.x. [DOI] [PubMed] [Google Scholar]
- Hartveit F., Thoresen S., Tangen M., Maartmann-Moe H. Mast cell changes and tumour dissemination in human breast carcinoma. Invasion Metastasis. 1984;4(3):146–155. [PubMed] [Google Scholar]
- Henry N., van Lamsweerde A. L., Vaes G. Collagen degradation by metastatic variants of Lewis lung carcinoma: cooperation between tumor cells and macrophages. Cancer Res. 1983 Nov;43(11):5321–5327. [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. Mediation of local homeostasis and inflammation by leukotrienes and other mast cell-dependent compounds. Nature. 1981 Sep 10;293(5828):103–108. doi: 10.1038/293103a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Thorgeirsson U. P., Garbisa S. Role of collagenases in tumor cell invasion. Cancer Metastasis Rev. 1982;1(4):277–288. doi: 10.1007/BF00124213. [DOI] [PubMed] [Google Scholar]
- Meier H. L., Heck L. W., Schulman E. S., MacGlashan D. W., Jr Purified human mast cells and basophils release human elastase and cathepsin G by an IgE-mediated mechanism. Int Arch Allergy Appl Immunol. 1985;77(1-2):179–183. doi: 10.1159/000233779. [DOI] [PubMed] [Google Scholar]
- Neri A., Nicolson G. L. Phenotypic drift of metastatic and cell-surface properties of mammary adenocarcinoma cell clones during growth in vitro. Int J Cancer. 1981 Dec;28(6):731–738. doi: 10.1002/ijc.2910280612. [DOI] [PubMed] [Google Scholar]
- Neri A., Welch D., Kawaguchi T., Nicolson G. L. Development and biologic properties of malignant cell sublines and clones of a spontaneously metastasizing rat mammary adenocarcinoma. J Natl Cancer Inst. 1982 Mar;68(3):507–517. [PubMed] [Google Scholar]
- Norrby K., Eneström S. Cellular and extracellular changes following mast-cell secretion in avascular rat mesentery. An electron-microscopic study. Cell Tissue Res. 1984;235(2):339–345. doi: 10.1007/BF00217858. [DOI] [PubMed] [Google Scholar]
- Norrby K. Evidence of mast-cell histamine being mitogenic in intact tissue. Agents Actions. 1985 Apr;16(3-4):287–290. doi: 10.1007/BF01983162. [DOI] [PubMed] [Google Scholar]
- Parwaresch M. R., Horny H. P., Lennert K. Tissue mast cells in health and disease. Pathol Res Pract. 1985 Mar;179(4-5):439–461. doi: 10.1016/s0344-0338(85)80184-9. [DOI] [PubMed] [Google Scholar]
- Roche W. R. Mast cells and tumors. The specific enhancement of tumor proliferation in vitro. Am J Pathol. 1985 Apr;119(1):57–64. [PMC free article] [PubMed] [Google Scholar]
- SMYTH C. J., GUM O. B. Mast cells in connective tissue diseases. Arthritis Rheum. 1958 Apr;1(2):178–180. doi: 10.1002/art.1780010211. [DOI] [PubMed] [Google Scholar]
- Schittek A., Issa H. A., Stafford J. H., Young D., Zwilling B., James A. G. Growth of pulmonary metastases of B16 melanoma in mast cell-free mice. J Surg Res. 1985 Jan;38(1):24–28. doi: 10.1016/0022-4804(85)90006-x. [DOI] [PubMed] [Google Scholar]
- Schwartz L. B., Bradford T. R., Littman B. H., Wintroub B. U. The fibrinogenolytic activity of purified tryptase from human lung mast cells. J Immunol. 1985 Oct;135(4):2762–2767. [PubMed] [Google Scholar]
- Schwartz L. B. Enzyme mediators of mast cells and basophils. Clin Rev Allergy. 1983 Sep;1(3):397–416. [PubMed] [Google Scholar]
- Schwartz L. B., Lewis R. A., Austen K. F. Tryptase from human pulmonary mast cells. Purification and characterization. J Biol Chem. 1981 Nov 25;256(22):11939–11943. [PubMed] [Google Scholar]
- Subba Rao P. V., Friedman M. M., Atkins F. M., Metcalfe D. D. Phagocytosis of mast cell granules by cultured fibroblasts. J Immunol. 1983 Jan;130(1):341–349. [PubMed] [Google Scholar]
- Woolley D. E., Grafton C. A. Collagenase immunolocalization studies of cutaneous secondary melanomas. Br J Cancer. 1980 Aug;42(2):260–265. doi: 10.1038/bjc.1980.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoffe J. R., Taylor D. J., Wooley D. E. Mast cell products stimulate collagenase and prostaglandin E production by cultures of adherent rheumatoid synovial cells. Biochem Biophys Res Commun. 1984 Jul 18;122(1):270–276. doi: 10.1016/0006-291x(84)90470-4. [DOI] [PubMed] [Google Scholar]
- Yoffe J. R., Taylor D. J., Woolley D. E. Mast-cell products and heparin stimulate the production of mononuclear-cell factor by cultured human monocyte/macrophages. Biochem J. 1985 Aug 15;230(1):83–88. doi: 10.1042/bj2300083. [DOI] [PMC free article] [PubMed] [Google Scholar]