Abstract
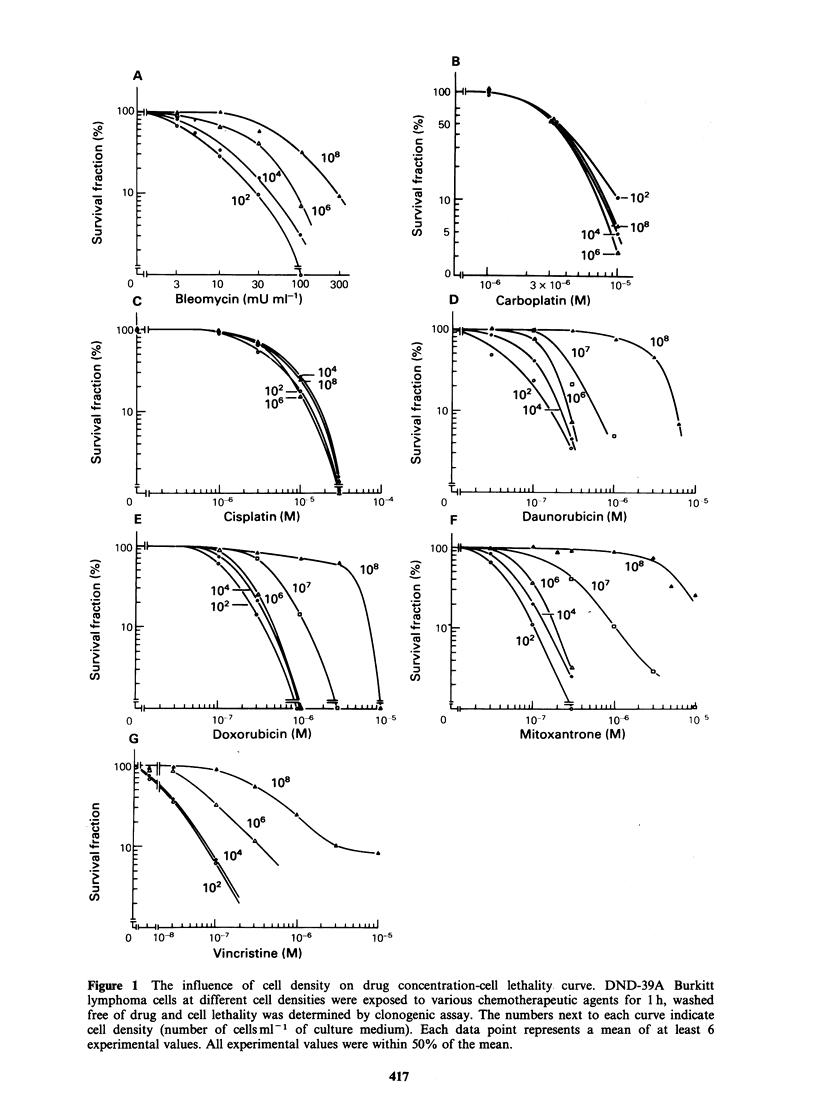
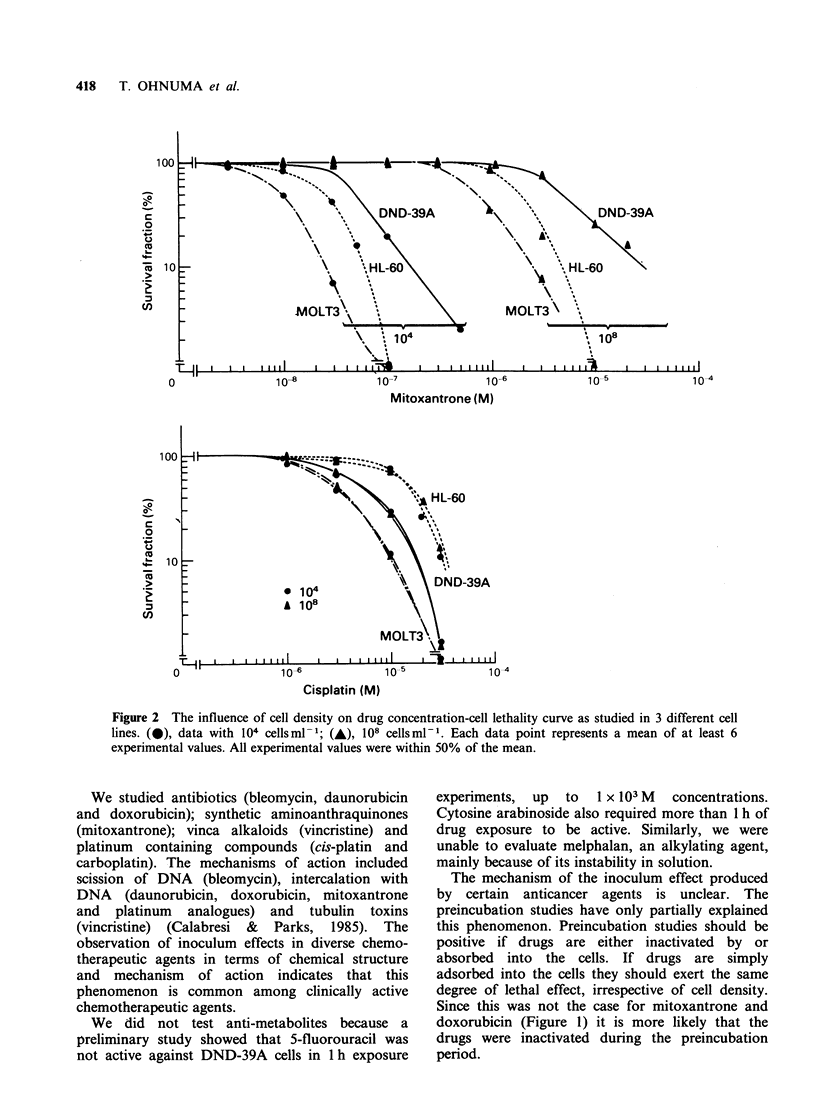
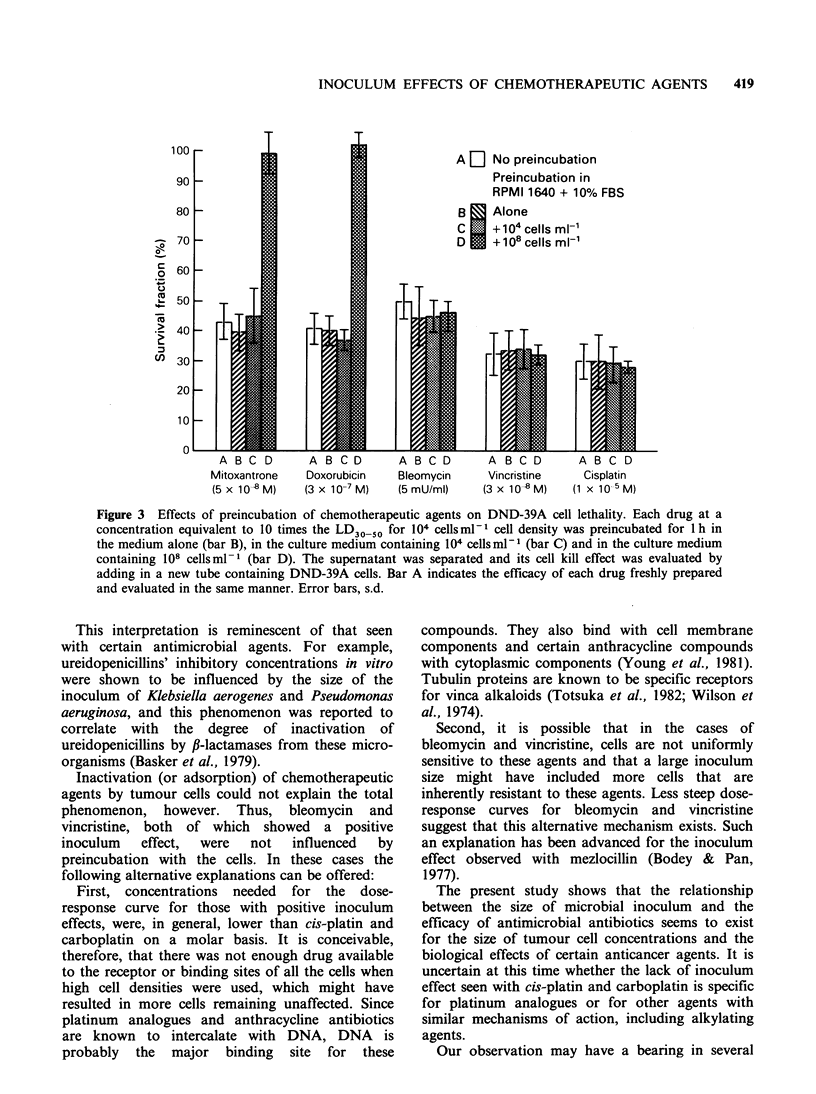
The effects of cell density on drug-induced cell kill kinetics were studied by means of clonogenic assay using 3 human leukaemia-lymphoma cell lines. Mitoxantrone, daunorubicin, doxorubicin, vincristine and bleomycin were progressively less efficacious when cell density increased (positive inoculum effects), whereas the effects of cis-platin and carboplatin were not influenced by cell density. Inoculum effects were related to the kind of chemotherapeutic agents tested, irrespective of the type of cell lines used. Preincubation of mitoxantrone or doxorubicin in the presence of cells in high density resulted in decreases in the cytocidal activity, whereas the effects of bleomycin, vincristine and cis-platin were unaffected. These results show that cell density affects the biological effect of certain chemotherapeutic agents. Inactivation of drugs by high densities of cells partially explains this phenomenon.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basker M. J., Edmondson R. A., Sutherland R. Comparative antibacterial activity of azlocillin, mezlocillin, carbenicillin and ticarcillin and relative stability to beta-lactamases of pseudomonas aeruginosa and klebsiella aerogenes. Infection. 1979;7(2):67–73. doi: 10.1007/BF01641616. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Pan T. Mezlocillin: in vitro studies of a new broad-spectrum penicillin. Antimicrob Agents Chemother. 1977 Jan;11(1):74–79. doi: 10.1128/aac.11.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther D., Beard M. E., Bateman C. J., Sewell R. L. Factors influencing prognosis in adults with acute myelogenous leukaemia. Br J Cancer. 1975 Oct;32(4):456–464. doi: 10.1038/bjc.1975.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREIREICH E. J., GEHAN E. A., SULMAN D., BOGGS D. R., FREI E., 3rd The effect of chemotherapy on acute leukemia in the human. J Chronic Dis. 1961 Dec;14:593–608. doi: 10.1016/0021-9681(61)90118-7. [DOI] [PubMed] [Google Scholar]
- Gallagher R., Collins S., Trujillo J., McCredie K., Ahearn M., Tsai S., Metzgar R., Aulakh G., Ting R., Ruscetti F. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979 Sep;54(3):713–733. [PubMed] [Google Scholar]
- George S. L., Fernbach D. J., Vietti T. J., Sullivan M. P., Lane D. M., Haggard M. E., Berry D. H., Lonsdale D., Komp D. Factors influencing survival in pediatric acute leukemia. The SWCCSG experience, 1958-1970. Cancer. 1973 Dec;32(6):1542–1553. doi: 10.1002/1097-0142(197312)32:6<1542::aid-cncr2820320634>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Hug V., Keating M., McCredie K., Hester J., Bodey G. P., Freireich E. J. Clinical course and response to treatment of patients with acute myelogenous leukemia presenting with a high leukocyte count. Cancer. 1983 Sep 1;52(5):773–779. doi: 10.1002/1097-0142(19830901)52:5<773::aid-cncr2820520503>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Kuroki T. Colony formation of mammalian cells on agar plates and its application to Lederberg's replica plating. Exp Cell Res. 1973 Jul;80(1):55–62. doi: 10.1016/0014-4827(73)90274-7. [DOI] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Ohnuma T., Arkin H., Holland J. F. Differences in chemotherapeutic susceptibility of human T-, B-, and non-T-/non-B-lymphocytes in culture. Recent Results Cancer Res. 1980;75:61–67. doi: 10.1007/978-3-642-81491-4_10. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Hamburger A. W., Soehnlen B., Durie B. G., Alberts D. S., Moon T. E. Quantitation of differential sensitivity of human-tumor stem cells to anticancer drugs. N Engl J Med. 1978 Jun 15;298(24):1321–1327. doi: 10.1056/NEJM197806152982401. [DOI] [PubMed] [Google Scholar]
- Schauer P., Arlin Z. A., Mertelsmann R., Cirrincione C., Friedman A., Gee T. S., Dowling M., Kempin S., Straus D. J., Koziner B. Treatment of acute lymphoblastic leukemia in adults: results of the L-10 and L-10M protocols. J Clin Oncol. 1983 Aug;1(8):462–470. doi: 10.1200/JCO.1983.1.8.462. [DOI] [PubMed] [Google Scholar]
- Simone J. V., Verzosa M. S., Rudy J. A. Initial features and prognosis in 363 children with acute lymphocytic leukemia. Cancer. 1975 Dec;36(6):2099–2108. doi: 10.1002/cncr.2820360926. [DOI] [PubMed] [Google Scholar]
- Totsuka K., Oshimi K., Mizoguchi H. Vindesine receptors in cells of a human leukaemia cell line. Br J Cancer. 1982 Sep;46(3):392–396. doi: 10.1038/bjc.1982.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hoff D. D., Casper J., Bradley E., Sandbach J., Jones D., Makuch R. Association between human tumor colony-forming assay results and response of an individual patient's tumor to chemotherapy. Am J Med. 1981 May;70(5):1027–1041. doi: 10.1016/0002-9343(81)90859-7. [DOI] [PubMed] [Google Scholar]
- Wilson L., Bamburg J. R., Mizel S. B., Grisham L. M., Creswell K. M. Interaction of drugs with microtubule proteins. Fed Proc. 1974 Feb;33(2):158–166. [PubMed] [Google Scholar]
- Young R. C., Ozols R. F., Myers C. E. The anthracycline antineoplastic drugs. N Engl J Med. 1981 Jul 16;305(3):139–153. doi: 10.1056/NEJM198107163050305. [DOI] [PubMed] [Google Scholar]


