Abstract
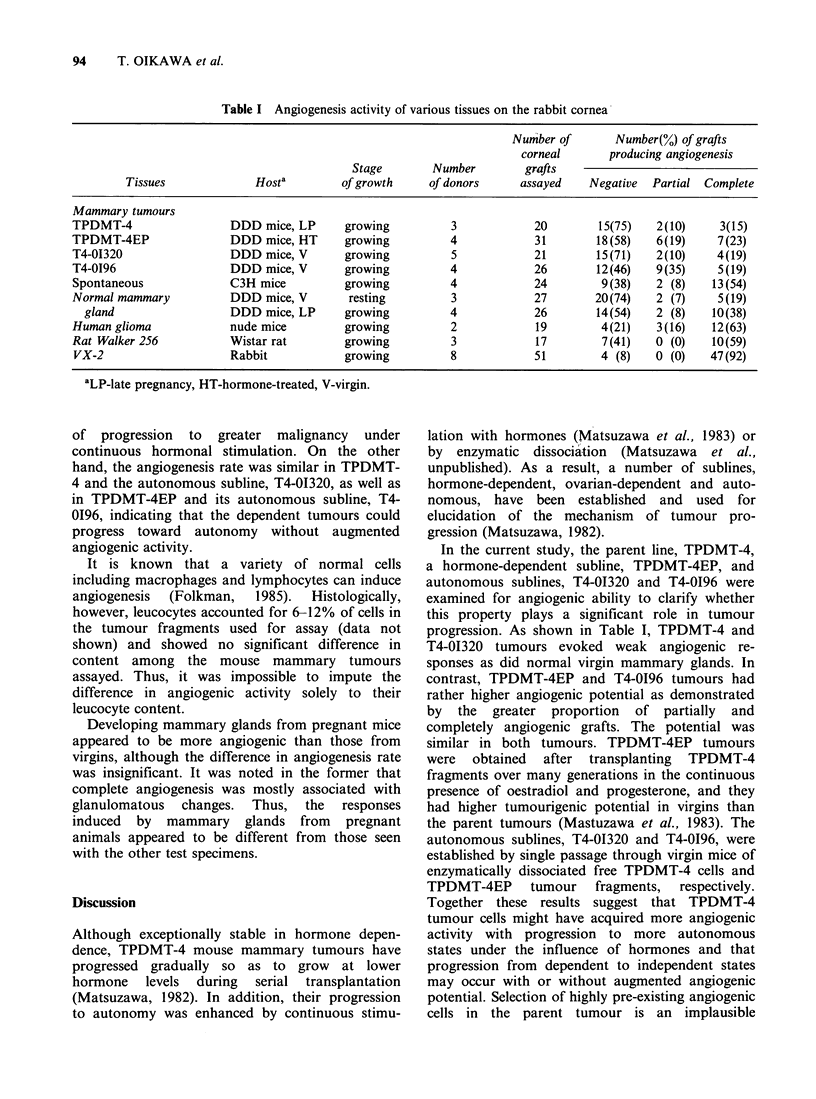
The transplantable pregnancy-dependent mammary tumour (TPDMT-4), the related hormone-dependent (TPDMT-4EP) and autonomous (T4-0I320 and T4-0I96) subline tumours, and the mammary glands from DDD mice were compared for angiogenic activity on the rabbit cornea by tissue implantation. The TPDMT-4EP tumour was established by serially transplanting TPDMT-4 tumour fragments in oestradiol plus progesterone treated mice. The T4-0I320 and T4-0I96 tumours directly derived from the TPDMT-4 and TPDMT-4EP tumours, respectively. Angiogenic activity was graded by macroscopic and microscopic examinations into 3 classes; negative, partial and complete angiogenesis. These tumours were comparable to mammary glands in activity and induced complete angiogenesis in only 15-23% of the implants. However, when partial and complete responses were combined as positive angiogenesis, TPDMT-4, T4-0I320, TPDMT-4EP and T4-0I96 tumour implants were angiogenic in 25, 29, 42 and 54%, respectively. The T4-0I96 tumour was significantly more angiogenic than the parent tumour but this was not so for the TPDMT-4EP tumour. Spontaneous C3H mouse mammary tumours, human gliomas from nude mice, rat Walker 256 carcinomas and rabbit VX-2 tumours induced complete angiogenesis in 54, 63, 59 and 92% of the implants, respectively. The results suggest that the TPDMT-4 tumour is unique in being weakly angiogenic and able to progress toward greater autonomy with or without augmented angiogenic activity in different conditions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessandri G., Raju K., Gullino P. M. Mobilization of capillary endothelium in vitro induced by effectors of angiogenesis in vivo. Cancer Res. 1983 Apr;43(4):1790–1797. [PubMed] [Google Scholar]
- Brem S. S., Jensen H. M., Gullino P. M. Angiogenesis as a marker of preneoplastic lesions of the human breast. Cancer. 1978 Jan;41(1):239–244. doi: 10.1002/1097-0142(197801)41:1<239::aid-cncr2820410133>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- DeOme K. B., Miyamoto M. J., Osborn R. C., Guzman R. C., Lum K. Detection of inapparent nodule-transformed cells in the mammary gland tissues of virgin female BALB/cfC3H mice. Cancer Res. 1978 Jul;38(7):2103–2111. [PubMed] [Google Scholar]
- Fenselau A., Watt S., Mello R. J. Tumor angiogenic factor. Purification from the Walker 256 rat tumor. J Biol Chem. 1981 Sep 25;256(18):9605–9611. [PubMed] [Google Scholar]
- Fett J. W., Strydom D. J., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985 Sep 24;24(20):5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Gullino P. M. Angiogenic capacity of preneoplastic lesions of the murine mammary gland as a marker of neoplastic transformation. Cancer Res. 1976 Jul;36(7 Pt 2):2611–2620. [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Gullino P. M. Neovascularization induced by intraocular xenografts of normal, preneoplastic, and neoplastic mouse mammary tissues. J Natl Cancer Inst. 1976 Feb;56(2):305–318. doi: 10.1093/jnci/56.2.305. [DOI] [PubMed] [Google Scholar]
- Gullino P. M. Natural history of breast cancer. Progression from hyperplasia to neoplasia as predicted by angiogenesis. Cancer. 1977 Jun;39(6 Suppl):2697–2703. doi: 10.1002/1097-0142(197706)39:6<2697::aid-cncr2820390656>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Kaminski M. J., Kieler J., Christensen B. Angiogenesis-inducing ability of human bladder epithelium cell lines and "spontaneously" transformed murine fibroblasts. Proc Soc Exp Biol Med. 1983 Dec;174(3):383–391. doi: 10.3181/00379727-174-41752. [DOI] [PubMed] [Google Scholar]
- Matsuno H. Tumor angiogenesis factor (TAF) in cultured cells derived from central nervous system tumors in humans. Neurol Med Chir (Tokyo) 1981 Jul;21(7):765–773. doi: 10.2176/nmc.21.765. [DOI] [PubMed] [Google Scholar]
- Matsuzawa A., Kaneko T., Ikeda Y. Accelerated progression to autonomy of a pregnancy-dependent mouse mammary tumor (TPDMT-4) by hormones. Cancer Res. 1983 May;43(5):2283–2289. [PubMed] [Google Scholar]
- Matsuzawa A., Kaneko T., Ikeda Y., Yamamoto T. Formation of duct-alveolar structures and new types of tumors by a pregnancy-dependent mouse mammary tumor (TPDMT-4) in virgin mice. Gan. 1982 Jun;73(3):372–376. [PubMed] [Google Scholar]
- Raju K. S., Alessandri G., Gullino P. M. Characterization of a chemoattractant for endothelium induced by angiogenesis effectors. Cancer Res. 1984 Apr;44(4):1579–1584. [PubMed] [Google Scholar]
- Shahabuddin S., Kumar S., West D., Arnold F. A study of angiogenesis factors from five different sources using a radioimmunoassay. Int J Cancer. 1985 Jan 15;35(1):87–91. doi: 10.1002/ijc.2910350114. [DOI] [PubMed] [Google Scholar]
- Stenzinger W., Brüggen J., Macher E., Sorg C. Tumor angiogenic activity (TAA) production in vitro and growth in the nude mouse by human malignant melanoma. Eur J Cancer Clin Oncol. 1983 May;19(5):649–656. doi: 10.1016/0277-5379(83)90181-5. [DOI] [PubMed] [Google Scholar]
- Strum J. M. Angiogenic responses elicited from chorioallantoic membrane vessels by neoplastic, preneoplastic, and normal mammary tissues from GR mice. Am J Pathol. 1983 Jun;111(3):282–287. [PMC free article] [PubMed] [Google Scholar]
- Tanaka N., Nagao S., Tohgo A., Sekiguchi F., Kohno M., Ogawa H., Matsui T., Matsutani M. Effects of human fibroblast interferon on human gliomas transplanted into nude mice. Gan. 1983 Apr;74(2):308–316. [PubMed] [Google Scholar]
- Tapper D., Langer R., Bellows A. R., Folkman J. Angiogenesis capacity as a diagnostic marker for human eye tumors. Surgery. 1979 Jul;86(1):36–40. [PubMed] [Google Scholar]



