Abstract
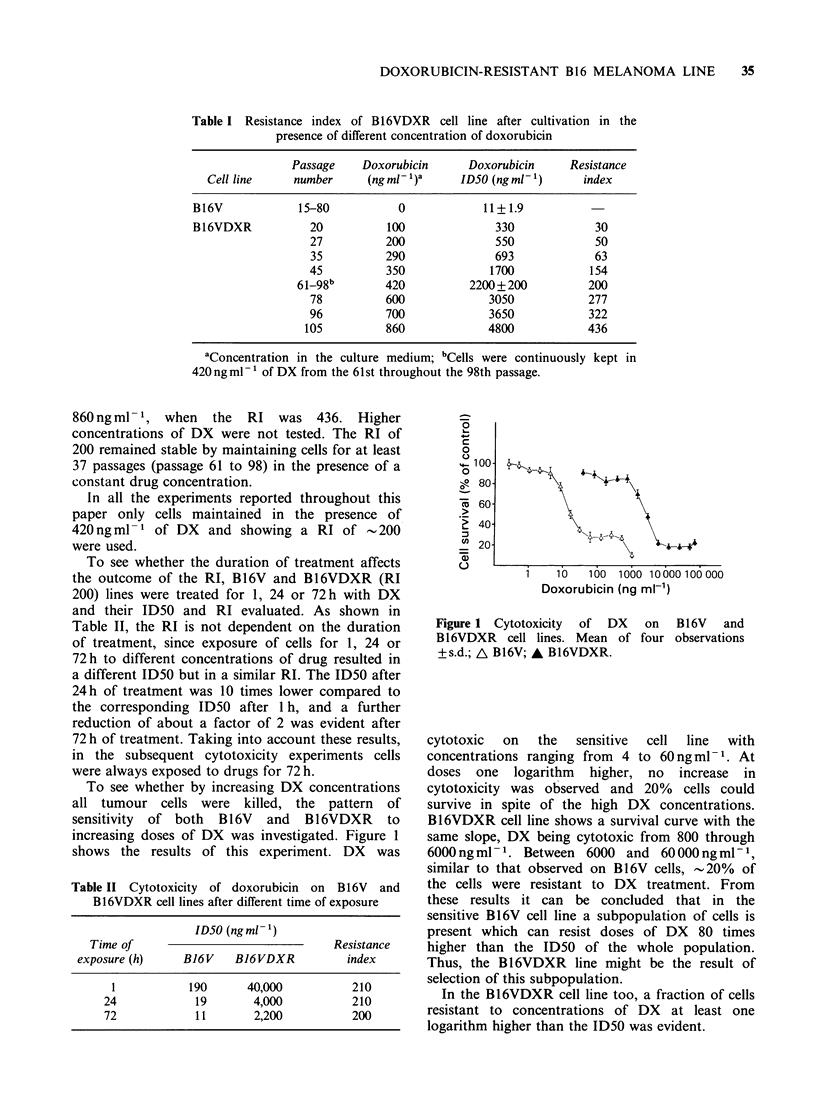
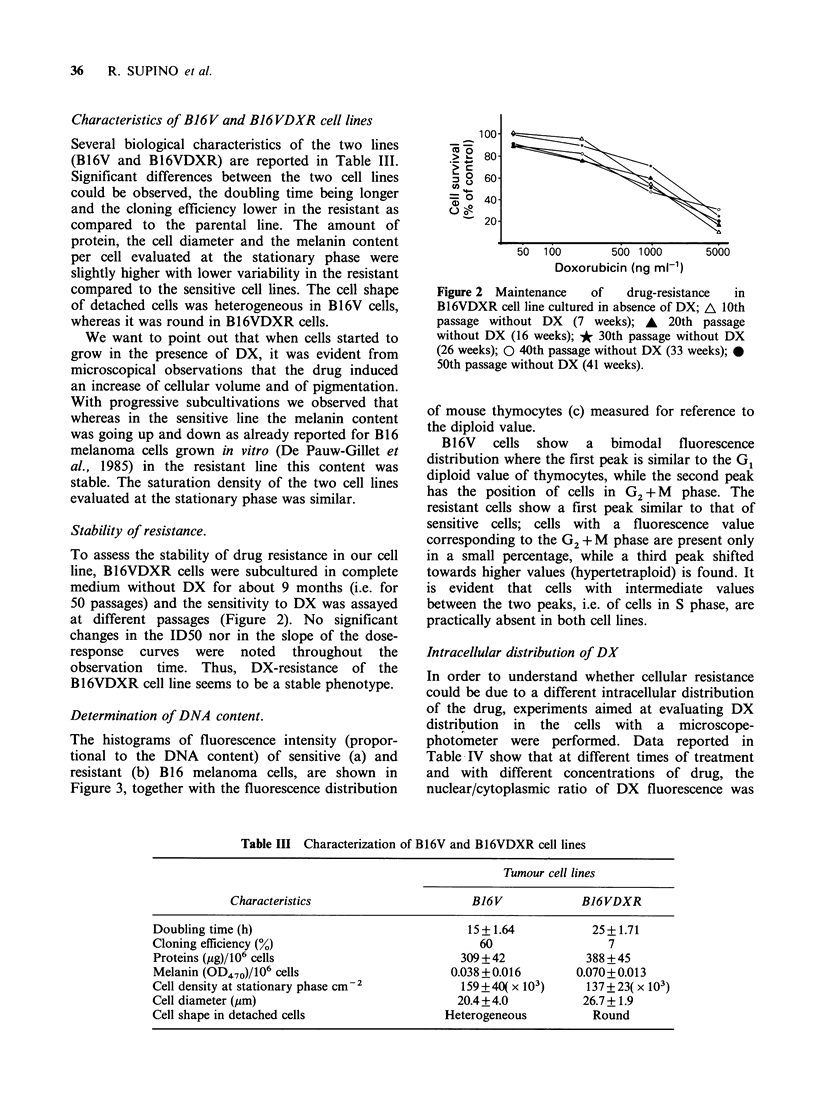
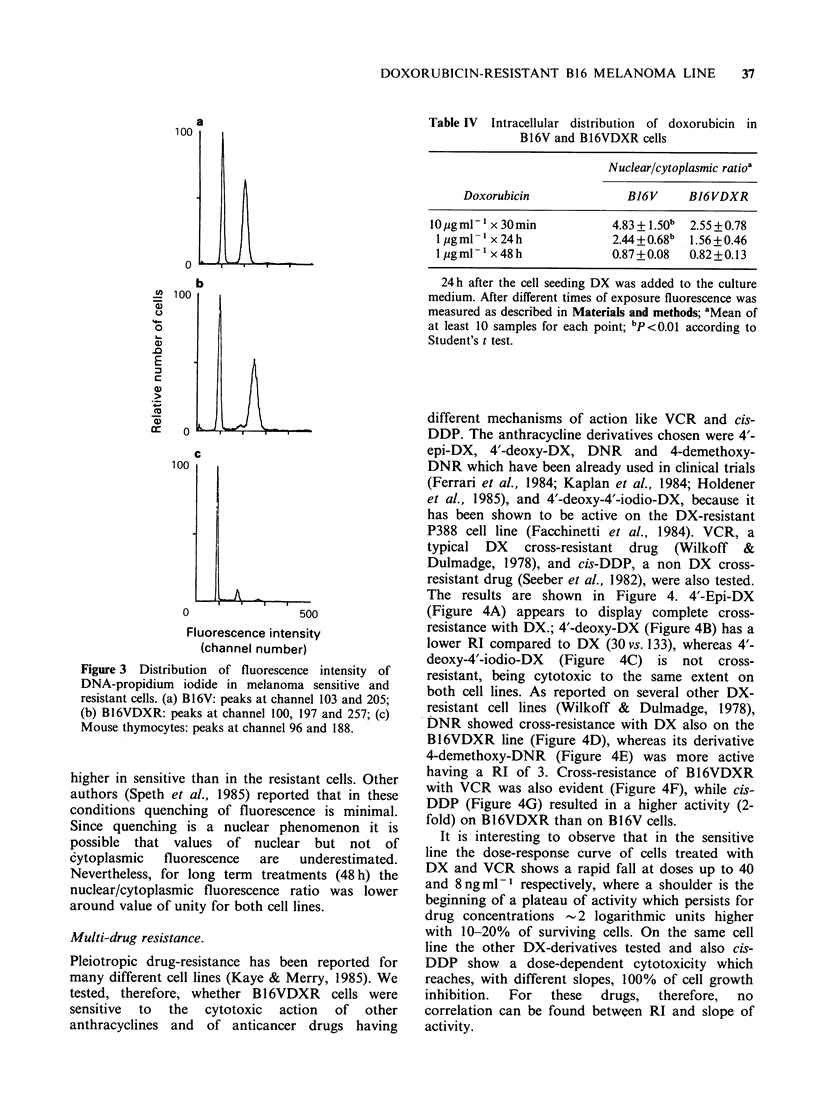
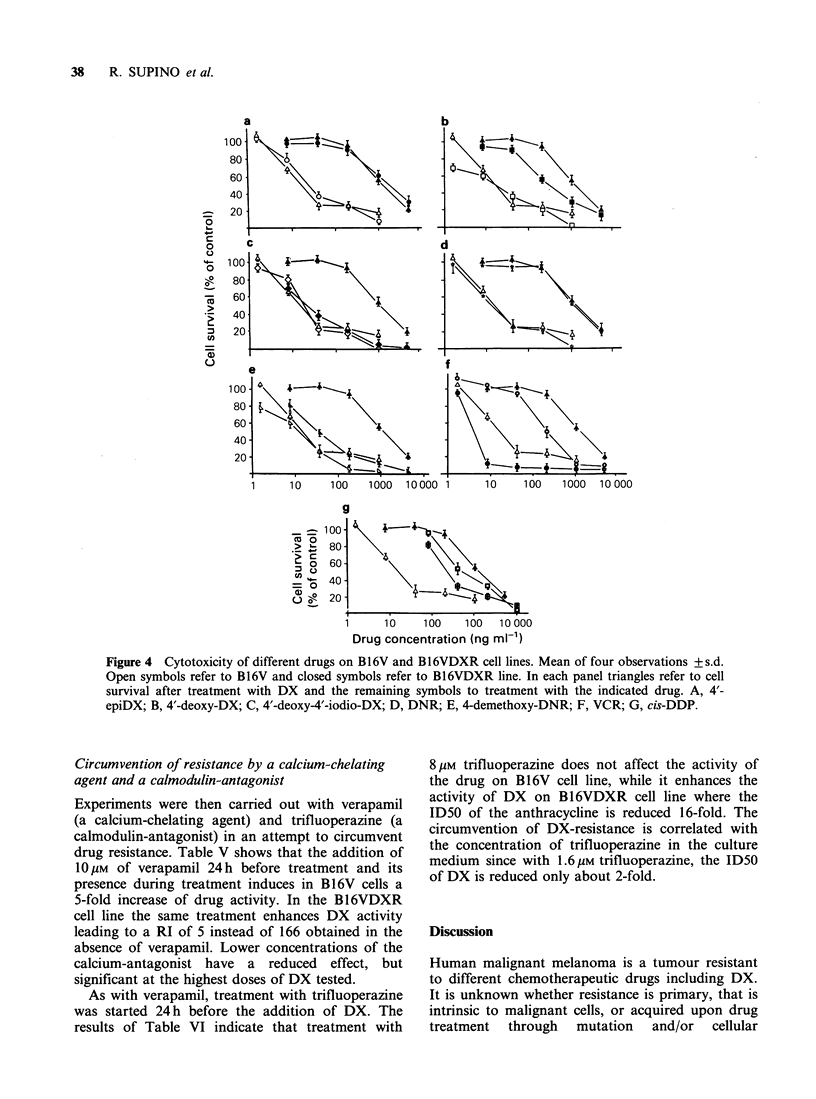
A B16 mouse melanoma cell line resistant to doxorubicin was obtained by continuous in vitro exposure to the drug. The ID50 for this line was 200 times higher than that for the parental cell line. The resistant cell line had some biological characteristics similar to those of the sensitive parental cell line, like saturation density and protein content. Differences were found in doubling time which was longer, cloning efficiency which was lower and DNA content which was higher in the resistant as compared to the parental line. Intracellular distribution of doxorubicin was also different having a nuclear-cytoplasmic ratio higher in sensitive than in resistant cells. Melanin content was an unstable feature in the sensitive cell line, whereas melanin was always present in resistant cells. Resistance to doxorubicin was maintained during 50 in vitro passages in the absence of the drug. Cross-resistance was found with vincristine and other anthracyclines, like daunorubicin and 4'-epi-doxorubicin but not with cis-platinum, and a new doxorubicin derivative, 4'-deoxy-4'-iodio-doxorubicin. The B16 line showed a lower resistance index to 4'-deoxy-doxorubicin and 4-demethoxy-daunorubicin (30 and 3 respectively), as compared to doxorubicin. Doxorubicin-resistance was partially circumvented by pretreatment of resistant cells with verapamil, a calcium chelating agent, and by trifluoperazine, a calmodulin-antagonist.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biedler J. L., Riehm H., Peterson R. H., Spengler B. A. Membrane-mediated drug resistance and phenotypic reversion to normal growth behavior of Chinese hamster cells. J Natl Cancer Inst. 1975 Sep;55(3):671–680. doi: 10.1093/jnci/55.3.671. [DOI] [PubMed] [Google Scholar]
- Chauffert B., Martin F., Caignard A., Jeannin J. F., Leclerc A. Cytofluorescence localization of adriamycin in resistant colon cancer cells. Cancer Chemother Pharmacol. 1984;13(1):14–18. doi: 10.1007/BF00401439. [DOI] [PubMed] [Google Scholar]
- Davis H. L., Davis T. E. Daunorubicin and adriamycin in cancer treatment: an analysis of their roles and limitations. Cancer Treat Rep. 1979 May;63(5):809–815. [PubMed] [Google Scholar]
- Ellem K. A., Kay G. F. Ferricyanide can replace pyruvate to stimulate growth and attachment of serum restricted human melanoma cells. Biochem Biophys Res Commun. 1983 Apr 15;112(1):183–190. doi: 10.1016/0006-291x(83)91814-4. [DOI] [PubMed] [Google Scholar]
- Ferrari L., Rossi A., Brambilla C., Bonfante V., Villani F., Crippa F., Bonadonna G. Phase I study with 4'-deoxydoxorubicin. Invest New Drugs. 1984;2(3):287–295. doi: 10.1007/BF00175379. [DOI] [PubMed] [Google Scholar]
- Freitas M. I., Giordano P. A., Bottiroli G. Improvement in microscope photometry by voltage to frequency conversion: analogue measurement and digital processing. J Microsc. 1981 Nov;124(Pt 2):211–218. doi: 10.1111/j.1365-2818.1981.tb00315.x. [DOI] [PubMed] [Google Scholar]
- Ganapathi R., Grabowski D. Enhancement of sensitivity to adriamycin in resistant P388 leukemia by the calmodulin inhibitor trifluoperazine. Cancer Res. 1983 Aug;43(8):3696–3699. [PubMed] [Google Scholar]
- Goldin A., Venditti J. M., Macdonald J. S., Muggia F. M., Henney J. E., Devita V. T., Jr Current results of the screening program at the Division of Cancer Treatment, National Cancer Institute. Eur J Cancer. 1981 Feb;17(2):129–142. doi: 10.1016/0014-2964(81)90027-x. [DOI] [PubMed] [Google Scholar]
- Hill B. T., Dennis L. Y., Li X. T., Whelan R. D. Identification of anthracycline analogues with enhanced cytotoxicity and lack of cross-resistance to adriamycin using a series of mammalian cell lines in vitro. Cancer Chemother Pharmacol. 1985;14(3):194–201. doi: 10.1007/BF00258115. [DOI] [PubMed] [Google Scholar]
- Kaplan S., Sessa C., Willems Y., Pacciarini M. A., Tamassia V., Cavalli F. Phase I trial of 4-demethoxydaunorubicin (idarubicin) with single oral doses. Invest New Drugs. 1984;2(3):281–286. doi: 10.1007/BF00175378. [DOI] [PubMed] [Google Scholar]
- Kaye S., Merry S. Tumour cell resistance to anthracyclines--a review. Cancer Chemother Pharmacol. 1985;14(2):96–103. doi: 10.1007/BF00434344. [DOI] [PubMed] [Google Scholar]
- Kessel D., Wilberding C. Anthracycline resistance in P388 murine leukemia and its circumvention by calcium antagonists. Cancer Res. 1985 Apr;45(4):1687–1691. [PubMed] [Google Scholar]
- Kessel D., Wilberding C. Mode of action of calcium antagonists which alter anthracycline resistance. Biochem Pharmacol. 1984 Apr 1;33(7):1157–1160. doi: 10.1016/0006-2952(84)90533-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lotan R., Lotan D. Stimulation of melanogenesis in a human melanoma cell line by retinoids. Cancer Res. 1980 Sep;40(9):3345–3350. [PubMed] [Google Scholar]
- McMillan T. J., Stephens T. C., Steel G. G. Development of drug resistance in a murine mammary tumour. Br J Cancer. 1985 Dec;52(6):823–832. doi: 10.1038/bjc.1985.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyskens F. L., Jr, Fuller B. B. Characterization of the effects of different retinoids on the growth and differentiation of a human melanoma cell line and selected subclones. Cancer Res. 1980 Jul;40(7):2194–2196. [PubMed] [Google Scholar]
- Peterson R. H., Meyers M. B., Spengler B. A., Biedler J. L. Alteration of plasma membrane glycopeptides and gangliosides of Chinese hamster cells accompanying development of resistance to daunorubicin and vincristine. Cancer Res. 1983 Jan;43(1):222–228. [PubMed] [Google Scholar]
- Seeber S., Osieka R., Schmidt C. G., Achterrath W., Crooke S. T. In vivo resistance towards anthracyclines, etoposide, and cis-diamminedichloroplatinum(II). Cancer Res. 1982 Nov;42(11):4719–4725. [PubMed] [Google Scholar]
- Skipper H. E., Schabel F. M., Jr, Lloyd H. H. Experimental therapeutics and kinetics: selection and overgrowth of specifically and permanently drug-resistant tumor cells. Semin Hematol. 1978 Jul;15(3):207–219. [PubMed] [Google Scholar]
- Skovsgaard T. Mechanism of cross-resistance between vincristine and daunorubicin in Ehrlich ascites tumor cells. Cancer Res. 1978 Dec;38(12):4722–4727. [PubMed] [Google Scholar]
- Speth P. A., Linssen P. C., Boezeman J. B., Wessels H. M., Haanen C. Quantitation of anthracyclines in human hematopoietic cell subpopulations by flow cytometry correlated with high pressure liquid chromatography. Cytometry. 1985 Mar;6(2):143–150. doi: 10.1002/cyto.990060210. [DOI] [PubMed] [Google Scholar]
- Supino R., Necco A., Dasdia T., Casazza A. M., Di Marco A. Relationship between effects on nucleic acid synthesis in cell cultures and cytotoxicity of 4-demethoxy derivatives of daunorubicin and adriamycin. Cancer Res. 1977 Dec;37(12):4523–4528. [PubMed] [Google Scholar]
- Tanigawa N., Mizuno Y., Hashimura T., Honda K., Satomura K., Hikasa Y., Niwa O., Sugahara T., Yoshida O., Kern D. H. Comparison of drug sensitivity among tumor cells within a tumor, between primary tumor and metastases, and between different metastases in the human tumor colony-forming assay. Cancer Res. 1984 Jun;44(6):2309–2312. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Nojiri M., Tsukagoshi S., Sakurai Y. Circumvention of vincristine and Adriamycin resistance in vitro and in vivo by calcium influx blockers. Cancer Res. 1983 Jun;43(6):2905–2910. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982 Nov;42(11):4730–4733. [PubMed] [Google Scholar]
- Wilkoff L. J., Dulmadge E. A. Resistance and cross-resistance of cultured leukemia P388 cells to vincristine, adriamycin, adriamycin analogs, and actinomycin D. J Natl Cancer Inst. 1978 Dec;61(6):1521–1524. [PubMed] [Google Scholar]
- Yanovich S., Preston L. Effects of verapamil on daunomycin cellular retention and cytotoxicity in P388 leukemic cells. Cancer Res. 1984 May;44(5):1743–1747. [PubMed] [Google Scholar]
- de Pauw-Gillet M. C., Hennet J. J., Bassleer R. J. Quantitative cytochemical analysis by microdensitometry of spontaneous or alpha-MSH-stimulated melanogenesis in B16 melanoma cells cultivated in vitro. Eur J Cancer Clin Oncol. 1985 Aug;21(8):951–956. doi: 10.1016/0277-5379(85)90114-2. [DOI] [PubMed] [Google Scholar]


