Abstract
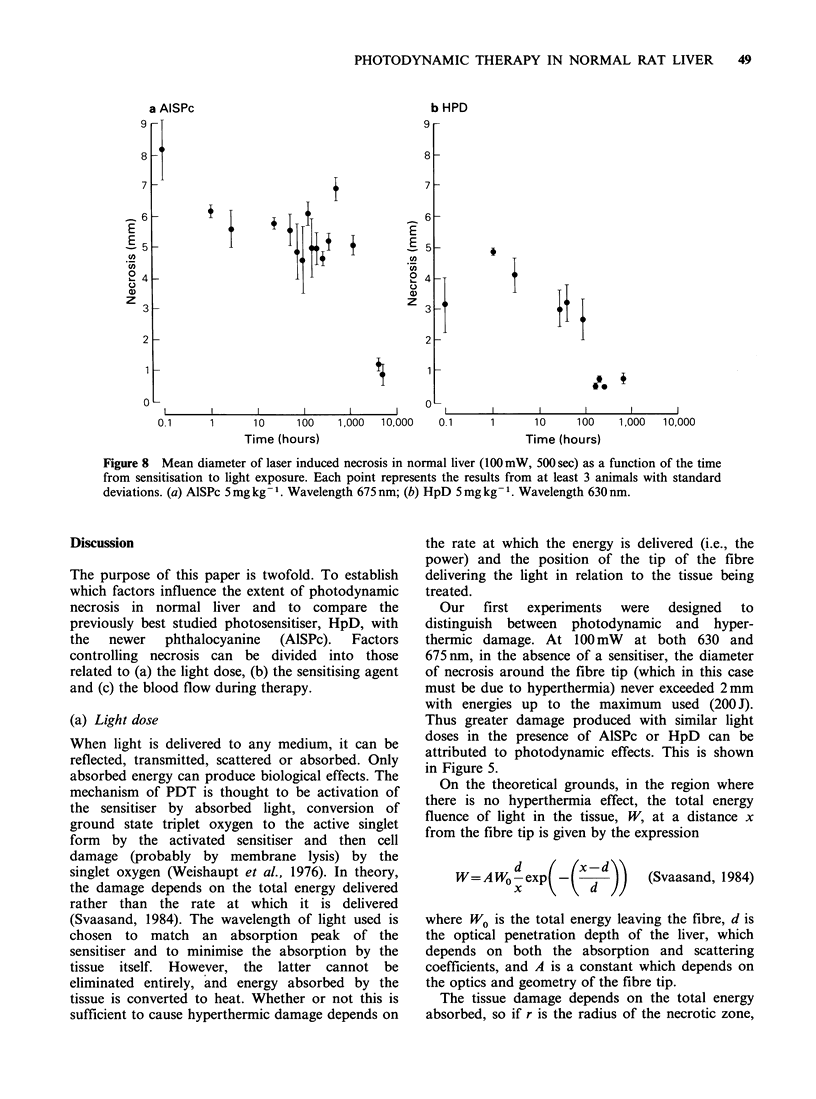
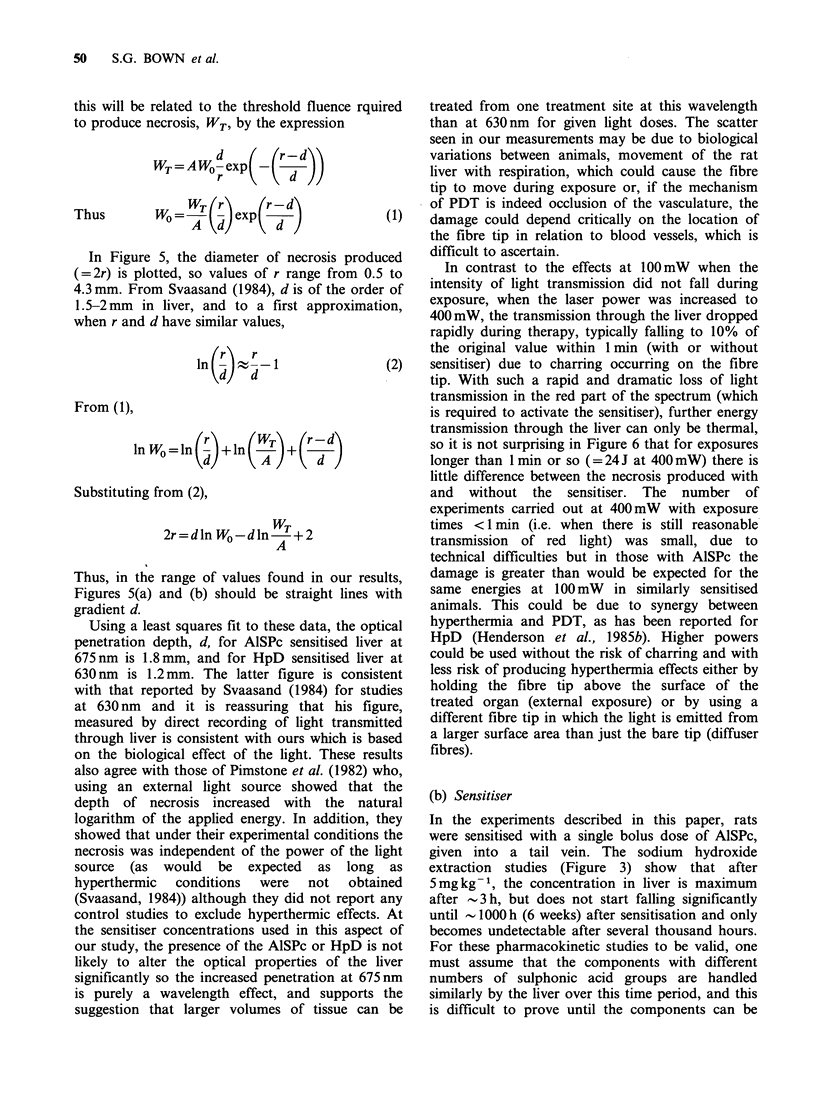
Selective sensitisation of malignant tumours to monochromatic light (photodynamic therapy, PDT) is a promising approach to cancer treatment, but current sensitisers are unsatisfactory and the parameters controlling effects produced in normal and neoplastic tissue are poorly understood. To quantify the effects in a relatively homogeneous organ, we carried out experiments in the livers of normal rats following systemic sensitisation with haematoporphyrin derivative (HpD) and a new sensitiser, a sulphonated aluminium phthalocyanine (AlSPc) using light from an Argon pumped tunable dye laser. Damage from PDT (dominant at 100 mW laser power) could be distinguished from that due to local hyperthermia (dominant at 400 mW). For both sensitisers, the extent of PDT necrosis increased with the applied light energy and was abolished by occluding the hepatic blood flow during therapy. With HpD, the extent of PDT necrosis was maximum with only a few hours between sensitisation and therapy, and was not detectable when this interval was increased to a week. With AlSPc, the extent of necrosis in liver changed little with sensitisation times from 1 h to 1000 h (6 weeks), and declined slowly thereafter, matching the amount of AlSPc measurable by alkali extraction, although prolonged photosensitisation was not seen with AlSPc in muscle. Less cutaneous photosensitivity was seen with AlSPc than with HpD. AlSPc is easier to produce and handle than HpD, has a more appropriate strong absorption peak (at 675 nm) and from these results, warrants further study as a photosensitiser for PDT.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Hur E., Rosenthal I. The phthalocyanines: a new class of mammalian cells photosensitizers with a potential for cancer phototherapy. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Feb;47(2):145–147. doi: 10.1080/09553008514550211. [DOI] [PubMed] [Google Scholar]
- Berenbaum M. C., Bonnett R., Scourides P. A. In vivo biological activity of the components of haematoporphyrin derivative. Br J Cancer. 1982 Apr;45(4):571–581. doi: 10.1038/bjc.1982.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugelski P. J., Porter C. W., Dougherty T. J. Autoradiographic distribution of hematoporphyrin derivative in normal and tumor tissue of the mouse. Cancer Res. 1981 Nov;41(11 Pt 1):4606–4612. [PubMed] [Google Scholar]
- Christensen T., Feren K., Moan J., Pettersen E. Photodynamic effects of haematoporphyrin derivative on synchronized and asynchronous cells of different origin. Br J Cancer. 1981 Nov;44(5):717–724. doi: 10.1038/bjc.1981.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J., Grindey G. B., Fiel R., Weishaupt K. R., Boyle D. G. Photoradiation therapy. II. Cure of animal tumors with hematoporphyrin and light. J Natl Cancer Inst. 1975 Jul;55(1):115–121. doi: 10.1093/jnci/55.1.115. [DOI] [PubMed] [Google Scholar]
- Gomer C. J., Dougherty T. J. Determination of [3H]- and [14C]hematoporphyrin derivative distribution in malignant and normal tissue. Cancer Res. 1979 Jan;39(1):146–151. [PubMed] [Google Scholar]
- Gregorie H. B., Jr, Horger E. O., Ward J. L., Green J. F., Richards T., Robertson H. C., Jr, Stevenson T. B. Hematoporphyrin-derivative fluorescence in malignant neoplasms. Ann Surg. 1968 Jun;167(6):820–828. doi: 10.1097/00000658-196806000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B. W., Waldow S. M., Mang T. S., Potter W. R., Malone P. B., Dougherty T. J. Tumor destruction and kinetics of tumor cell death in two experimental mouse tumors following photodynamic therapy. Cancer Res. 1985 Feb;45(2):572–576. [PubMed] [Google Scholar]
- LIPSON R. L., BALDES E. J., OLSEN A. M. The use of a derivative of hematoporhyrin in tumor detection. J Natl Cancer Inst. 1961 Jan;26:1–11. [PubMed] [Google Scholar]
- Star W. M., Marijnissen J. P., van den Berg-Blok A. E., Reinhold H. S. Destructive effect of photoradiation on the microcirculation of a rat mammary tumor growing in "sandwich" observation chambers. Prog Clin Biol Res. 1984;170:637–645. [PubMed] [Google Scholar]
- Weishaupt K. R., Gomer C. J., Dougherty T. J. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976 Jul;36(7 Pt 1):2326–2329. [PubMed] [Google Scholar]