Abstract
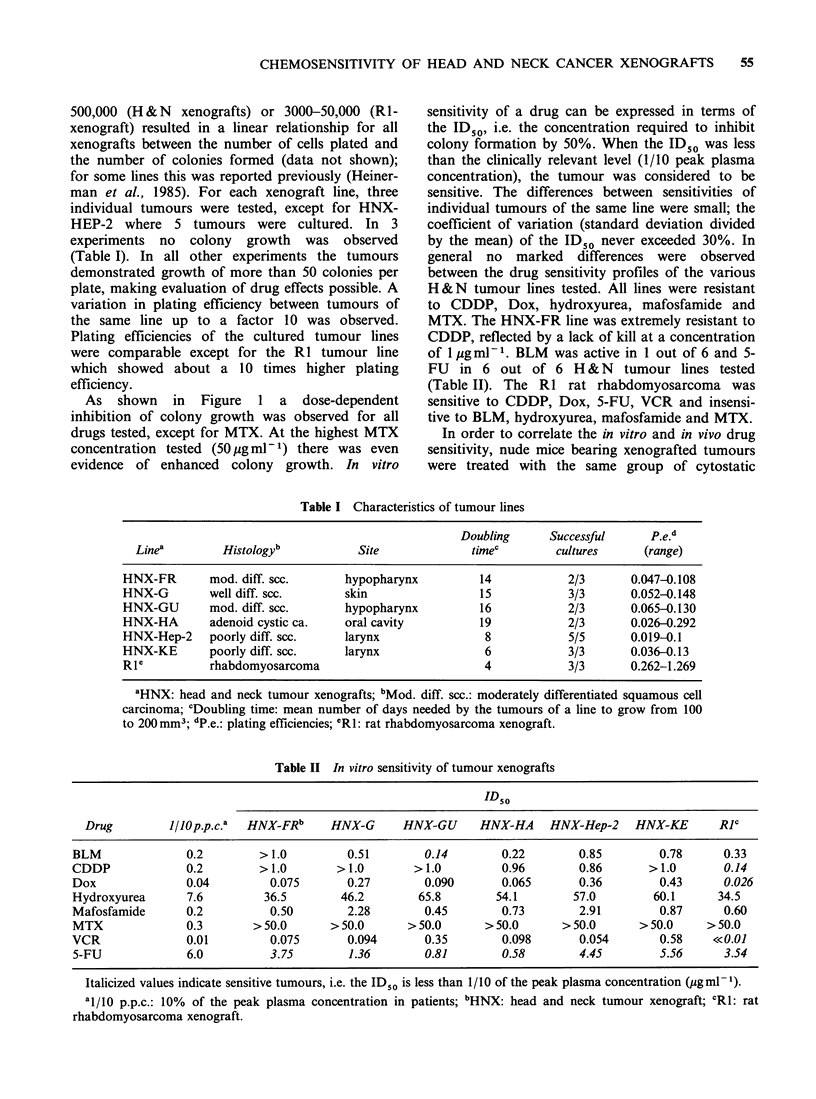
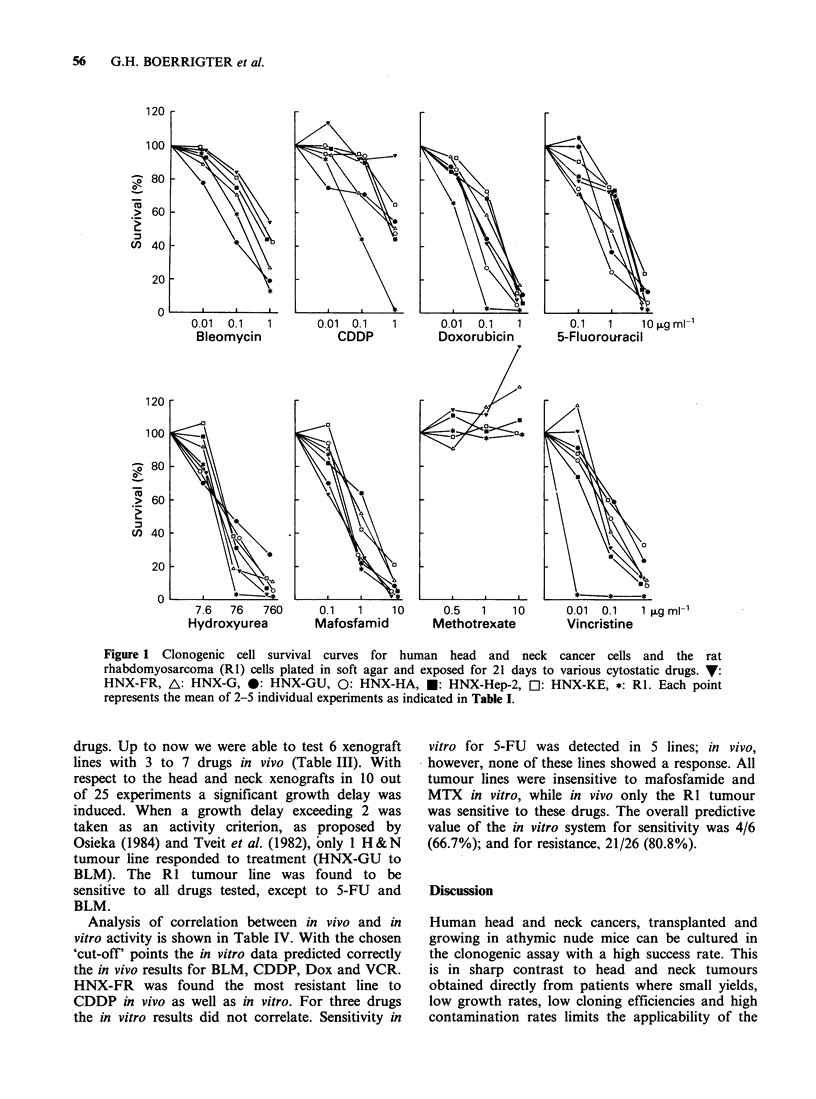
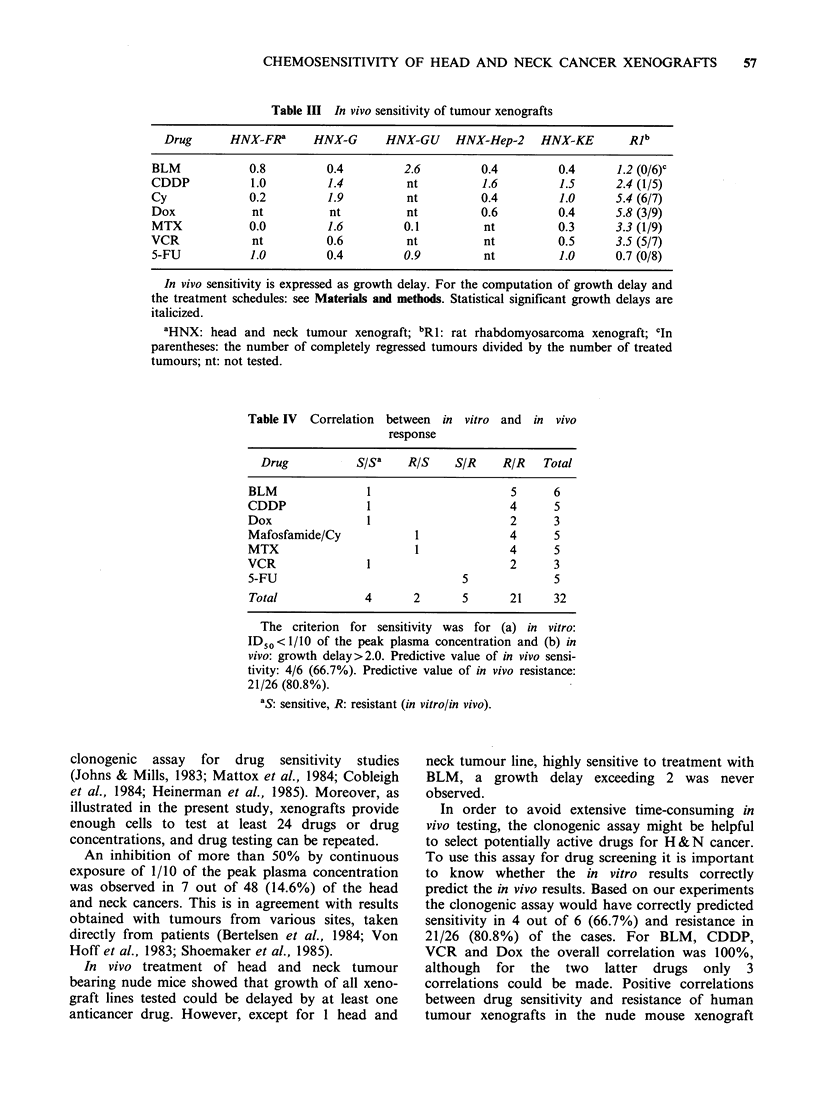
The potential use of human head and neck (H & N) tumours, growing in athymic nude mice, for preclinical assessment of cytostatic drug sensitivity in a soft agar cloning system was examined. Of 20 H & N tumour xenografts, obtained from 6 different xenograft lines, 17 demonstrated sufficient colony growth to evaluate in vitro drug sensitivity. Moreover, all xenografts provided enough cells to test 8 cytostatic drugs at 3 concentrations each. A dose-dependent inhibition of colony growth was obtained with all drugs tested, except methotrexate. Tumours were considered sensitive when the drug concentration required to inhibit colony formation by 50%, was less than 1/10 of the peak plasma concentration in patients. All H & N tumour lines were resistant to cisplatin, doxorubicin, hydroxyurea, mafosfamide (an in vitro active analogue of cyclophosphamide) and methotrexate. Bleomycin was active in 1/6 and 5-fluorouracil in 6/6 of the H & N tumour lines tested. In 32 cases the in vitro data of the H & N tumour lines and a chemosensitive rat rhabdomyosarcoma were compared directly with in vivo results obtained in nude mice. The clonogenic assay correctly predicted sensitivity in 4/6 (66.7%) and resistance in 21/26 (80.8%) of the cases. A lack of correlation was noted for methotrexate, 5-fluorouracil and cyclophosphamide. In vitro culture of human H & N xenografts may provide a means for a rapid and large scale screening to identify new drugs active against H & N malignancies. In addition the clonogenic assay may help to select drugs for subsequent testing in the nude mouse xenograft model. The lack of correlation for some drugs in the present study indicates that there are some limitations in the use of xenograft tumour material for in vitro testing of new drugs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts D. S., Chen H. S. Tabular summary of pharmacokinetic parameters relevant to in vitro drug assay. Prog Clin Biol Res. 1980;48:351–359. [PubMed] [Google Scholar]
- Bateman A. E., Selby P. J., Steel G. G., Towse G. D. In vitro chemosensitivity tests on xenografted human melanomas. Br J Cancer. 1980 Feb;41(2):189–198. doi: 10.1038/bjc.1980.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelsen C. A., Sondak V. K., Mann B. D., Korn E. L., Kern D. H. Chemosensitivity testing of human solid tumors. A review of 1582 assays with 258 clinical correlations. Cancer. 1984 Mar 15;53(6):1240–1245. doi: 10.1002/1097-0142(19840315)53:6<1240::aid-cncr2820530604>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Braakhuis B. J., Schoevers E. J., Heinerman E. C., Sneeuwloper G., Snow G. B. Chemotherapy of human head and neck cancer xenografts with three clinically active drugs: cis-platinum, bleomycin and methotrexate. Br J Cancer. 1983 Nov;48(5):711–716. doi: 10.1038/bjc.1983.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobleigh M. A., Gallagher P. A., Hill J. H., Applebaum E. L., McGuire W. P. Growth of human squamous head and neck cancer in vitro. Am J Pathol. 1984 Jun;115(3):397–402. [PMC free article] [PubMed] [Google Scholar]
- Fiebig H. H., Schuchhardt C., Henss H., Fiedler L., Löhr G. W. Comparison of tumor response in nude mice and in the patients. Behring Inst Mitt. 1984 May;(74):343–352. [PubMed] [Google Scholar]
- Friedman H. S., Schold S. C., Jr, Muhlbaier L. H., Bjornsson T. D., Bigner D. D. In vitro versus in vivo correlations of chemosensitivity of human medulloblastoma. Cancer Res. 1984 Nov;44(11):5145–5149. [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E. Primary bioassay of human tumor stem cells. Science. 1977 Jul 29;197(4302):461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- Heinerman E. C., Braakhuis B. J., Boerrigter G. H., Snow G. B. Successful in vitro growth of human head and neck cancer after transplantation in nude mice. Arch Otorhinolaryngol. 1985;241(3):225–232. doi: 10.1007/BF00453692. [DOI] [PubMed] [Google Scholar]
- Hilgard P., Brock N. The key role of hydroxylation for the cytostatic activity and selectivity of cyclophosphamide. Invest New Drugs. 1984;2(2):131–132. doi: 10.1007/BF00232341. [DOI] [PubMed] [Google Scholar]
- Johns M. E., Mills S. E. Cloning efficiency. A possible prognostic indicator in squamous cell carcinoma of the head and neck. Cancer. 1983 Oct 15;52(8):1401–1404. doi: 10.1002/1097-0142(19831015)52:8<1401::aid-cncr2820520811>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Looney W. B., Mayo A. A., Allen P. M., Morrow J. Y., Morris H. P. A mathematical evaluation of tumour growth curves in rapid, intermediate and slow growing rat hepatomata. Br J Cancer. 1973 Apr;27(4):341–344. doi: 10.1038/bjc.1973.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE A. E., SABACHEWSKY L., TOOLAN H. W. Culture characteristics of four permanent lines of human cancer cells. Cancer Res. 1955 Oct;15(9):598–602. [PubMed] [Google Scholar]
- Mattox D. E., Von Hoff D. D., Clark G. M., Aufdemorte T. B. Factors that influence growth of head and neck squamous carcinoma in the soft agar cloning assay. Cancer. 1984 Apr 15;53(8):1736–1740. doi: 10.1002/1097-0142(19840415)53:8<1736::aid-cncr2820530820>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Nowak K., Peckham M. J., Steel G. G. Variation in response of xenografts of colo-rectal carcinoma to chemotherapy. Br J Cancer. 1978 Apr;37(4):576–584. doi: 10.1038/bjc.1978.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osieka R. Studies on drug resistance in a human melanoma xenograft system. Cancer Treat Rev. 1984 Mar;11 (Suppl A):85–98. doi: 10.1016/0305-7372(84)90047-1. [DOI] [PubMed] [Google Scholar]
- Salmon S. E. Human tumor colony assay and chemosensitivity testing. Cancer Treat Rep. 1984 Jan;68(1):117–125. [PubMed] [Google Scholar]
- Selby P., Buick R. N., Tannock I. A critical appraisal of the "human tumor stem-cell assay". N Engl J Med. 1983 Jan 20;308(3):129–134. doi: 10.1056/NEJM198301203080304. [DOI] [PubMed] [Google Scholar]
- Shoemaker R. H., Wolpert-DeFilippes M. K., Kern D. H., Lieber M. M., Makuch R. W., Melnick N. R., Miller W. T., Salmon S. E., Simon R. M., Venditti J. M. Application of a human tumor colony-forming assay to new drug screening. Cancer Res. 1985 May;45(5):2145–2153. [PubMed] [Google Scholar]
- Shorthouse A. J., Jones J. M., Steel G. G., Peckham M. J. Experimental combination and single-agent chemotherapy in human lung-tumour xenografts. Br J Cancer. 1982 Jul;46(1):35–44. doi: 10.1038/bjc.1982.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singletary S. E., Umbach G. E., Spitzer G., Drewinko B., Tomasovic B., Ajani J., Hug V., Blumenschein G. The human tumor stem cell assay revisited. Int J Cell Cloning. 1985 Mar;3(2):116–128. doi: 10.1002/stem.5530030205. [DOI] [PubMed] [Google Scholar]
- Steel G. G., Courtenay V. D., Peckham M. J. The response to chemotherapy of a variety of human tumour xenografts. Br J Cancer. 1983 Jan;47(1):1–13. doi: 10.1038/bjc.1983.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taetle R., Howell S. B., Giuliani F. C., Koziol J., Koessler A. Comparison of the activity of doxorubicin analogues using colony-forming assays and human xenografts. Cancer. 1982 Oct 15;50(8):1455–1461. doi: 10.1002/1097-0142(19821015)50:8<1455::aid-cncr2820500803>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Tveit K. M., Fodstad O., Lotsberg J., Vaage S., Pihl A. Colony growth and chemosensitivity in vitro of human melanoma biopsies. Relationship to clinical parameters. Int J Cancer. 1982 May 15;29(5):533–538. doi: 10.1002/ijc.2910290508. [DOI] [PubMed] [Google Scholar]
- Tveit K. M., Fodstad O., Olsnes S., Pihl A. In vitro sensitivity of human melanoma xenografts to cytotoxic drugs. Correlation with in vivo chemosensitivity. Int J Cancer. 1980 Dec 15;26(6):717–722. doi: 10.1002/ijc.2910260604. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R. Predictive chemosensitivity testing. Br J Cancer. 1985 Mar;51(3):295–299. doi: 10.1038/bjc.1985.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelcker G., Wagner T., Wientzek C., Hohorst H. J. Pharmacokinetics of "activated" cyclophosphamide and therapeutic efficacies. Cancer. 1984 Sep 15;54(6 Suppl):1179–1186. doi: 10.1002/1097-0142(19840915)54:1+<1179::aid-cncr2820541315>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Von Hoff D. D., Clark G. M., Stogdill B. J., Sarosdy M. F., O'Brien M. T., Casper J. T., Mattox D. E., Page C. P., Cruz A. B., Sandbach J. F. Prospective clinical trial of a human tumor cloning system. Cancer Res. 1983 Apr;43(4):1926–1931. [PubMed] [Google Scholar]
- Zirvi K. A., Masui H., Giuliani F. C., Kaplan N. O. Correlation of drug sensitivity on human colon adenocarcinoma cells grown in soft agar and in athymic mice. Int J Cancer. 1983 Jul 15;32(1):45–51. doi: 10.1002/ijc.2910320108. [DOI] [PubMed] [Google Scholar]


