Abstract
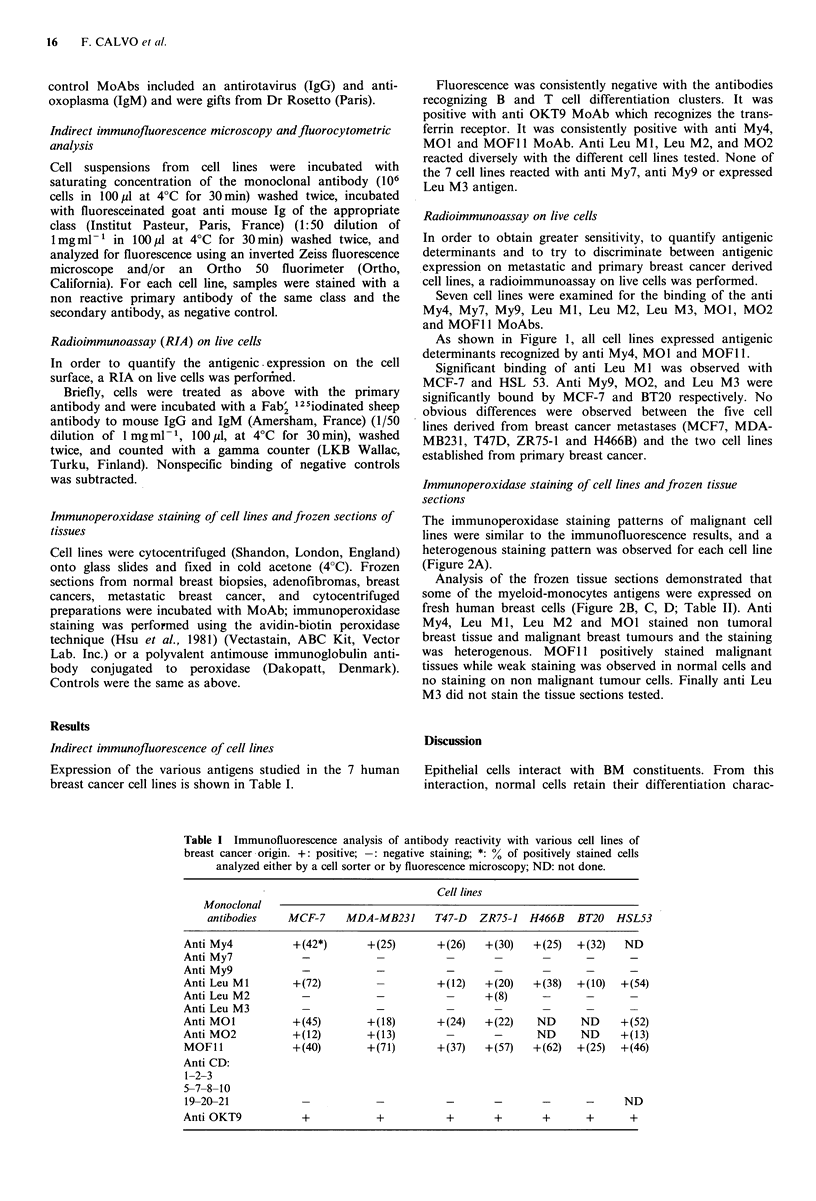
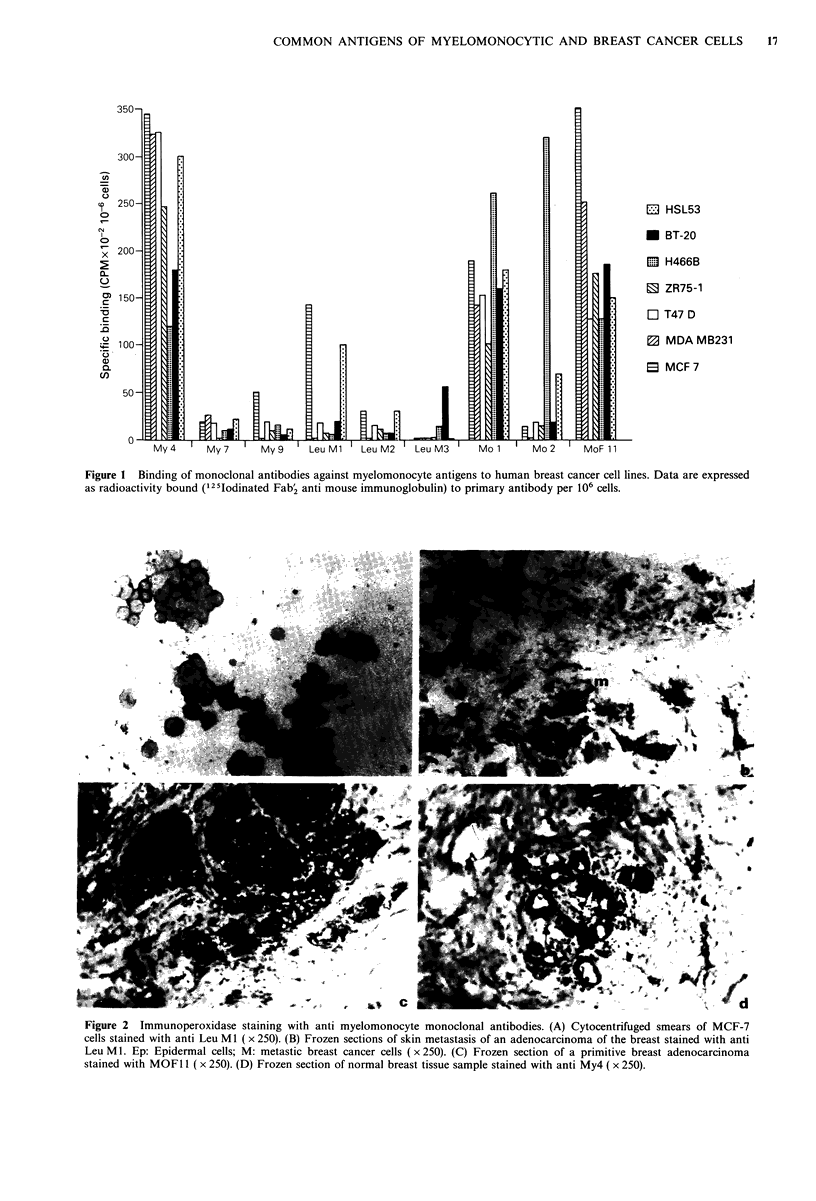
We have examined the expression of several myeloid cell associated antigens, some of which are involved in myelomonocyte adhesion, in seven well characterized human breast cancer cell lines, since common properties of adhesiveness and migration are found in haemopoietic cells and epithelial cancer cells. Five of these cell lines were of metastatic origin and two were derived from primary breast carcinoma. Antigenic expression was evaluated by immunofluorescence (IF), flow cytometry (FCM), radioimmunoassay on live cells (RIA) and immunoperoxidase staining. None of these cell lines expressed T or B lymphoid specific antigens. Myeloid antigens My4, MO1, and MOF11 (derived from the hybridization of mouse X63 - Ag8 cells with spleen cells from Balb/c mice immunized with purified human monocytes) were expressed in the 7 cell lines. Leu M1, Leu M3, My9, and MO2 antigens were expressed in some of the cell lines. Leu M2 and My7 antigens were not expressed or at very low levels. The expression of these myeloid antigens was also tested by immunoperoxidase staining, and found on frozen sections of normal mammary gland, fibroadenoma of the breast, primary breast cancer, and lymph node and skin metastases of breast tumours. This common expression in epithelial breast cells and in myeloid cells might be related to common biological functions such as interaction with extracellular matrix which precedes cell migration, a normal function of macrophages and an abnormal function expressed or amplified in human cancer epithelial cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaout M. A., Hakim R. M., Todd R. F., 3rd, Dana N., Colten H. R. Increased expression of an adhesion-promoting surface glycoprotein in the granulocytopenia of hemodialysis. N Engl J Med. 1985 Feb 21;312(8):457–462. doi: 10.1056/NEJM198502213120801. [DOI] [PubMed] [Google Scholar]
- Atkin N. B. Premature chromosome condensation in carcinoma of the bladder: presumptive evidence for fusion of normal and malignant cells. Cytogenet Cell Genet. 1979;23(3):217–219. doi: 10.1159/000131329. [DOI] [PubMed] [Google Scholar]
- Ball E. D., Sorenson G. D., Pettengill O. S. Expression of myeloid and major histocompatibility antigens on small cell carcinoma of the lung cell lines analyzed by cytofluorography: modulation by gamma-interferon. Cancer Res. 1986 May;46(5):2335–2339. [PubMed] [Google Scholar]
- Bunn P. A., Jr, Linnoila I., Minna J. D., Carney D., Gazdar A. F. Small cell lung cancer, endocrine cells of the fetal bronchus, and other neuroendocrine cells express the Leu-7 antigenic determinant present on natural killer cells. Blood. 1985 Mar;65(3):764–768. [PubMed] [Google Scholar]
- Cailleau R., Young R., Olivé M., Reeves W. J., Jr Breast tumor cell lines from pleural effusions. J Natl Cancer Inst. 1974 Sep;53(3):661–674. doi: 10.1093/jnci/53.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo F., Brower M., Carney D. N. Continuous culture and soft agarose cloning of multiple human breast carcinoma cell lines in serum-free medium. Cancer Res. 1984 Oct;44(10):4553–4559. [PubMed] [Google Scholar]
- Charpin C., Lissitzky J. C., Jacquemier J., Lavaut M. N., Kopp F., Pourreau-Schneider N., Martin P. M., Toga M. Immunohistochemical detection of laminin in 98 human breast carcinomas: a light and electron microscopic study. Hum Pathol. 1986 Apr;17(4):355–365. doi: 10.1016/s0046-8177(86)80458-0. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Mirski S., McGarry R. C., Cheng R., Campling B. G., Roder J. C. Differential expression of the Leu-7 antigen on human lung tumor cells. Cancer Res. 1985 Sep;45(9):4285–4290. [PubMed] [Google Scholar]
- Dana N., Todd R. F., 3rd, Pitt J., Springer T. A., Arnaout M. A. Deficiency of a surface membrane glycoprotein (Mo1) in man. J Clin Invest. 1984 Jan;73(1):153–159. doi: 10.1172/JCI111186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efremidis A. P., Bekesi J. G. Anti-common acute lymphoblastic leukemia antibody (CALLA) (J5) reactivity by small cell lung cancer (SCLC) cells. Blood. 1986 Jan;67(1):252–253. [PubMed] [Google Scholar]
- Engel L. W., Young N. A., Tralka T. S., Lippman M. E., O'Brien S. J., Joyce M. J. Establishment and characterization of three new continuous cell lines derived from human breast carcinomas. Cancer Res. 1978 Oct;38(10):3352–3364. [PubMed] [Google Scholar]
- Gazdar A. F., Bunn P. A., Jr, Minna J. D., Baylin S. B. Origin of human small cell lung cancer. Science. 1985 Aug 16;229(4714):679–680. doi: 10.1126/science.2992083. [DOI] [PubMed] [Google Scholar]
- Giavazzi R., Hart I. R. Mononuclear phagocyte adherence in the presence of laminin. A possible marker of cellular differentiation. Exp Cell Res. 1983 Jul;146(2):391–399. doi: 10.1016/0014-4827(83)90141-6. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Birdwell C. R. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978 Nov;38(11 Pt 2):4155–4171. [PubMed] [Google Scholar]
- Hand P. H., Thor A., Schlom J., Rao C. N., Liotta L. Expression of laminin receptor in normal and carcinomatous human tissues as defined by a monoclonal antibody. Cancer Res. 1985 Jun;45(6):2713–2719. [PubMed] [Google Scholar]
- Huang L. C., Brockhaus M., Magnani J. L., Cuttitta F., Rosen S., Minna J. D., Ginsburg V. Many monoclonal antibodies with an apparent specificity for certain lung cancers are directed against a sugar sequence found in lacto-N-fucopentaose III. Arch Biochem Biophys. 1983 Jan;220(1):318–320. doi: 10.1016/0003-9861(83)90417-4. [DOI] [PubMed] [Google Scholar]
- Huard T. K., Malinoff H. L., Wicha M. S. Macrophages express a plasma membrane receptor for basement membrane laminin. Am J Pathol. 1986 May;123(2):365–370. [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. S., Lagarde A. E., Dennis J. W., Donaghue T. P. Spontaneous fusion in vivo between normal host and tumor cells: possible contribution to tumor progression and metastasis studied with a lectin-resistant mutant tumor. Mol Cell Biol. 1983 Apr;3(4):523–538. doi: 10.1128/mcb.3.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keydar I., Chen L., Karby S., Weiss F. R., Delarea J., Radu M., Chaitcik S., Brenner H. J. Establishment and characterization of a cell line of human breast carcinoma origin. Eur J Cancer. 1979 May;15(5):659–670. doi: 10.1016/0014-2964(79)90139-7. [DOI] [PubMed] [Google Scholar]
- LASFARGUES E. Y., OZZELLO L. Cultivation of human breast carcinomas. J Natl Cancer Inst. 1958 Dec;21(6):1131–1147. [PubMed] [Google Scholar]
- Larizza L., Schirrmacher V., Graf L., Pflüger E., Peres-Martinez M., Stöhr M. Suggestive evidence that the highly metastatic variant ESb of the T-cell lymphoma Eb is derived from spontaneous fusion with a host macrophage. Int J Cancer. 1984 Nov 15;34(5):699–707. doi: 10.1002/ijc.2910340518. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Tryggvason K., Garbisa S., Hart I., Foltz C. M., Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980 Mar 6;284(5751):67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases: role of the basement membrane. Warner-Lambert Parke-Davis Award lecture. Am J Pathol. 1984 Dec;117(3):339–348. [PMC free article] [PubMed] [Google Scholar]
- Lipinski M., Braham K., Caillaud J. M., Carlu C., Tursz T. HNK-1 antibody detects an antigen expressed on neuroectodermal cells. J Exp Med. 1983 Nov 1;158(5):1775–1780. doi: 10.1084/jem.158.5.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff M. R., Pert C. B. Small cell carcinoma of the lung: macrophage-specific antigens suggest hemopoietic stem cell origin. Science. 1984 Sep 7;225(4666):1034–1036. doi: 10.1126/science.6089338. [DOI] [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., DiFlorio R., Hujanen E. S., Lyall R. M., Liotta L. A., Thorgeirsson U., Siegal G. P., Schiffmann E. Laminin promotes rabbit neutrophil motility and attachment. J Clin Invest. 1986 Apr;77(4):1180–1186. doi: 10.1172/JCI112419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Hujanen E. S., Martin G. R. Basement membrane and the invasive activity of metastatic tumor cells. J Natl Cancer Inst. 1986 Aug;77(2):311–316. [PubMed] [Google Scholar]
- Thorgeirsson U. P., Turpeenniemi-Hujanen T., Liotta L. A. Cancer cells, components of basement membranes, and proteolytic enzymes. Int Rev Exp Pathol. 1985;27:203–234. [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. Secretory responses of human neutrophils: exocytosis of specific (secondary) granules by human neutrophils during adherence in vitro and during exudation in vivo. J Immunol. 1979 Jul;123(1):285–294. [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]