Abstract
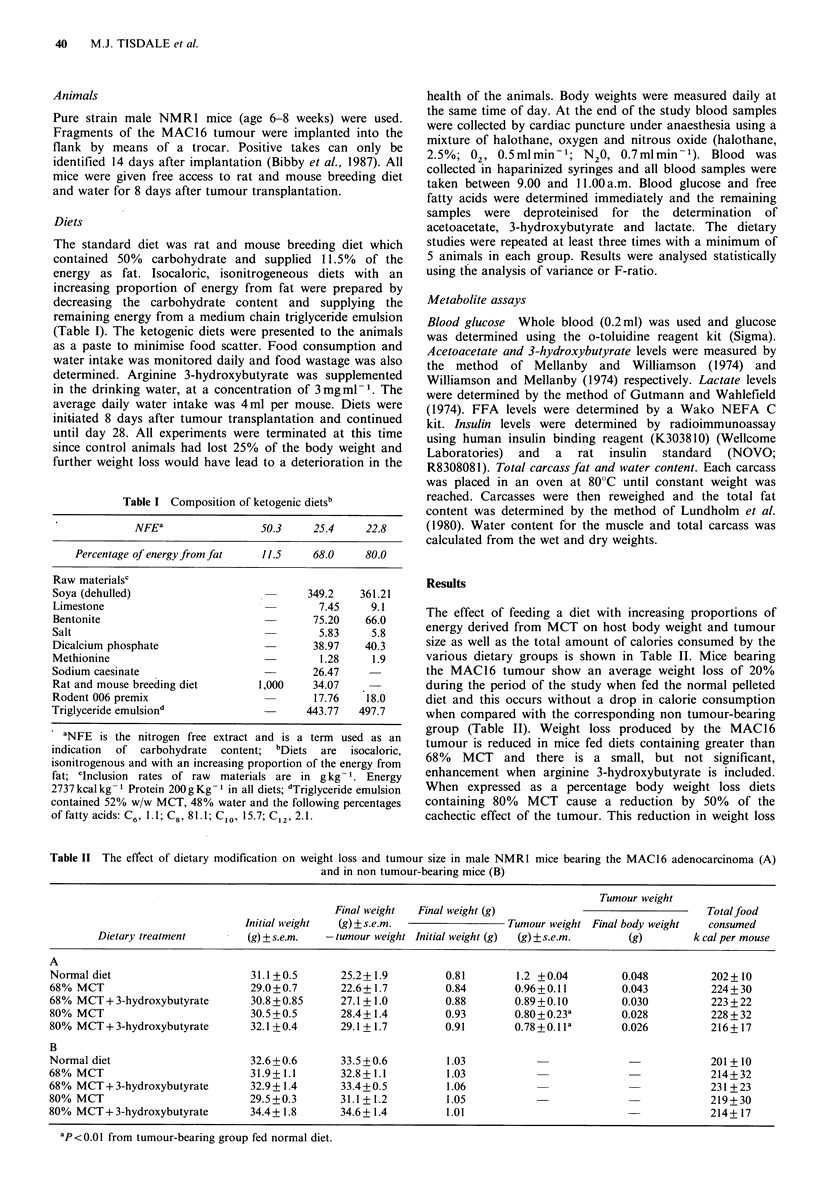
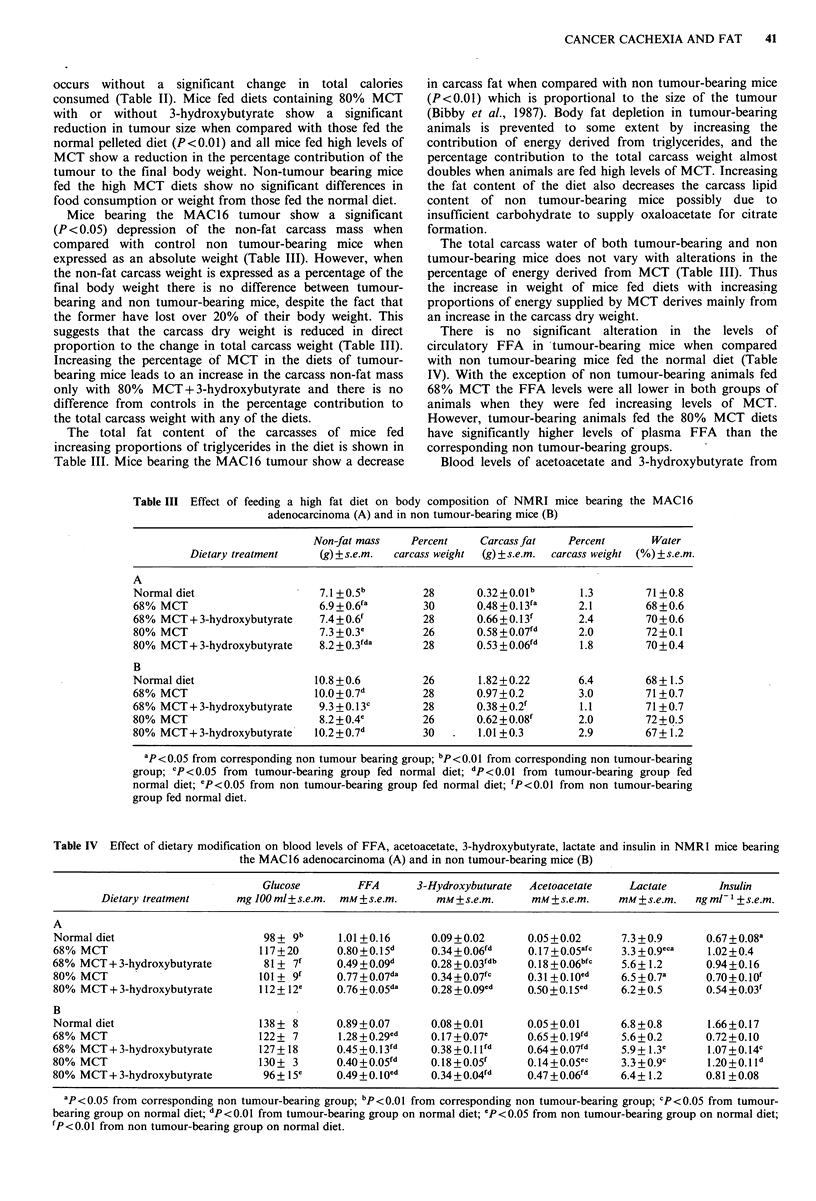
An attempt has been made to reverse cachexia and to selectively deprive the tumour of metabolic substrates for energy production by feeding a ketogenic regime, since ketone bodies are considered important in maintaining homeostasis during starvation. As a model we have used a transplantable mouse adenocarcinoma of the colon (MAC 16) which produces extensive weight loss without a reduction in food intake. When mice bearing the MAC16 tumour were fed on diets in which up to 80% of the energy was supplied as medium chain triglycerides (MCT) with or without arginine 3-hydroxybutyrate host weight loss was reduced in proportion to the fat content of the diet, and there was also a reduction in the percentage contribution of the tumour to the final body weight. The increase in carcass weight in tumour-bearing mice fed high levels of MCT was attributable to an increase in both the fat and the non-fat carcass mass. Blood levels of free fatty acids (FFA) were significantly reduced by MCT addition. The levels of both acetoacetate and 3-hydroxybutyrate were elevated in mice fed the high fat diets, and tumour-bearing mice fed the normal diet did not show increased plasma levels of ketone bodies over the non-tumour-bearing group despite the loss of carcass lipids. Both blood glucose and plasma insulin levels were reduced in mice bearing the MAC16 tumour and this was not significantly altered by feeding the high fat diets. The elevation in ketone bodies may account for the retention of both the fat and the non-fat carcass mass. This is the first example of an attempt to reverse cachexia by a diet based on metabolic differences between tumour and host tissues, which aims to selectively feed the host at the expense of the tumour.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibby M. C., Double J. A., Ali S. A., Fearon K. C., Brennan R. A., Tisdale M. J. Characterization of a transplantable adenocarcinoma of the mouse colon producing cachexia in recipient animals. J Natl Cancer Inst. 1987 Mar;78(3):539–546. [PubMed] [Google Scholar]
- Buzby G. P., Mullen J. L., Stein T. P., Miller E. E., Hobbs C. L., Rosato E. F. Host-tumor interaction and nutrient supply. Cancer. 1980 Jun 15;45(12):2940–2948. doi: 10.1002/1097-0142(19800615)45:12<2940::aid-cncr2820451208>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Conyers R. A., Need A. G., Durbridge T., Harvey N. D., Potezny N., Rofe A. M. Cancer, ketosis and parenteral nutrition. Med J Aust. 1979 May 5;1(9):398–399. doi: 10.5694/j.1326-5377.1979.tb126984.x. [DOI] [PubMed] [Google Scholar]
- Conyers R. A., Need A. G., Rofe A. M., Potezny N., Kimber R. J. Nutrition and cancer. Br Med J. 1979 Apr 28;1(6171):1146–1146. doi: 10.1136/bmj.1.6171.1146-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon K. C., Tisdale M. J., Preston T., Plumb J. A., Calman K. C. Failure of systemic ketosis to control cachexia and the growth rate of the Walker 256 carcinosarcoma in rats. Br J Cancer. 1985 Jul;52(1):87–92. doi: 10.1038/bjc.1985.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor H., Hillyard L. A., Abraham S. Effect of dietary fat on growth kinetics of transplantable mammary adenocarcinoma in BALB/c mice. J Natl Cancer Inst. 1985 Jun;74(6):1299–1305. [PubMed] [Google Scholar]
- Goodlad G. A., Mitchell A. J., McPhail L., Clark C. M. Serum insulin and somatomedin levels in the tumour-bearing rat. Eur J Cancer. 1975 Oct;11(10):733–737. doi: 10.1016/0014-2964(75)90048-1. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Alberti K. G., Houghton C. R., Williamson D. H., Krebs H. A. The effect of acetoacetate on plasma insulin concentration. Biochem J. 1971 Nov;125(2):541–544. doi: 10.1042/bj1250541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber D., Chlebowski R. T., Ishibashi D. E., Herrold J. N., Block J. B. Abnormalities in glucose and protein metabolism in noncachectic lung cancer patients. Cancer Res. 1982 Nov;42(11):4815–4819. [PubMed] [Google Scholar]
- Lundholm K., Edström S., Karlberg I., Ekman L., Scherstén T. Glucose turnover, gluconeogenesis from glycerol, and estimation of net glucose cycling in cancer patients. Cancer. 1982 Sep 15;50(6):1142–1150. doi: 10.1002/1097-0142(19820915)50:6<1142::aid-cncr2820500618>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- MIDER G. B. Some aspects of nitrogen and energy metabolism in cancerous subjects: a review. Cancer Res. 1951 Nov;11(11):821–829. [PubMed] [Google Scholar]
- Magee B. A., Potezny N., Rofe A. M., Conyers R. A. The inhibition of malignant cell growth by ketone bodies. Aust J Exp Biol Med Sci. 1979 Oct;57(5):529–539. doi: 10.1038/icb.1979.54. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr Brain metabolism during fasting. J Clin Invest. 1967 Oct;46(10):1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney S. D., Bistrian B. R., Wolfe R. R., Blackburn G. L. The human metabolic response to chronic ketosis without caloric restriction: physical and biochemical adaptation. Metabolism. 1983 Aug;32(8):757–768. doi: 10.1016/0026-0495(83)90105-1. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Hendler R. G., Felig P. Effect of ketone infusions on amino acid and nitrogen metabolism in man. J Clin Invest. 1975 Jun;55(6):1382–1390. doi: 10.1172/JCI108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologides A. Cancer cachexia. Cancer. 1979 May;43(5 Suppl):2004–2012. doi: 10.1002/1097-0142(197905)43:5+<2004::aid-cncr2820430708>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Tisdale M. J., Brennan R. A. Loss of acetoacetate coenzyme A transferase activity in tumours of peripheral tissues. Br J Cancer. 1983 Feb;47(2):293–297. doi: 10.1038/bjc.1983.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale M. J., Brennan R. A. Metabolic substrate utilization by a tumour cell line which induces cachexia in vivo. Br J Cancer. 1986 Oct;54(4):601–606. doi: 10.1038/bjc.1986.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATERHOUSE C., NYE W. H. Metabolic effects of infused triglyceride. Metabolism. 1961 May;10:403–414. [PubMed] [Google Scholar]
- Waterhouse C., Jeanpretre N., Keilson J. Gluconeogenesis from alanine in patients with progressive malignant disease. Cancer Res. 1979 Jun;39(6 Pt 1):1968–1972. [PubMed] [Google Scholar]


