Abstract
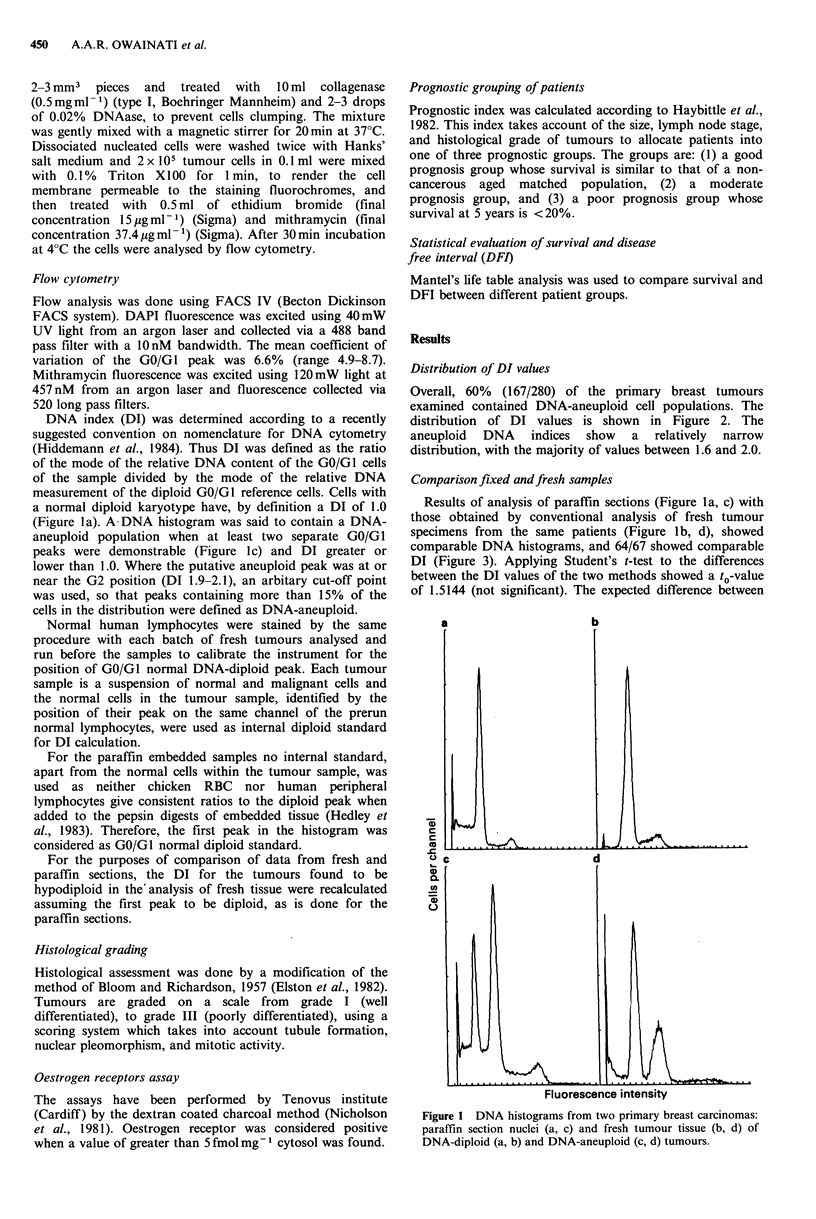
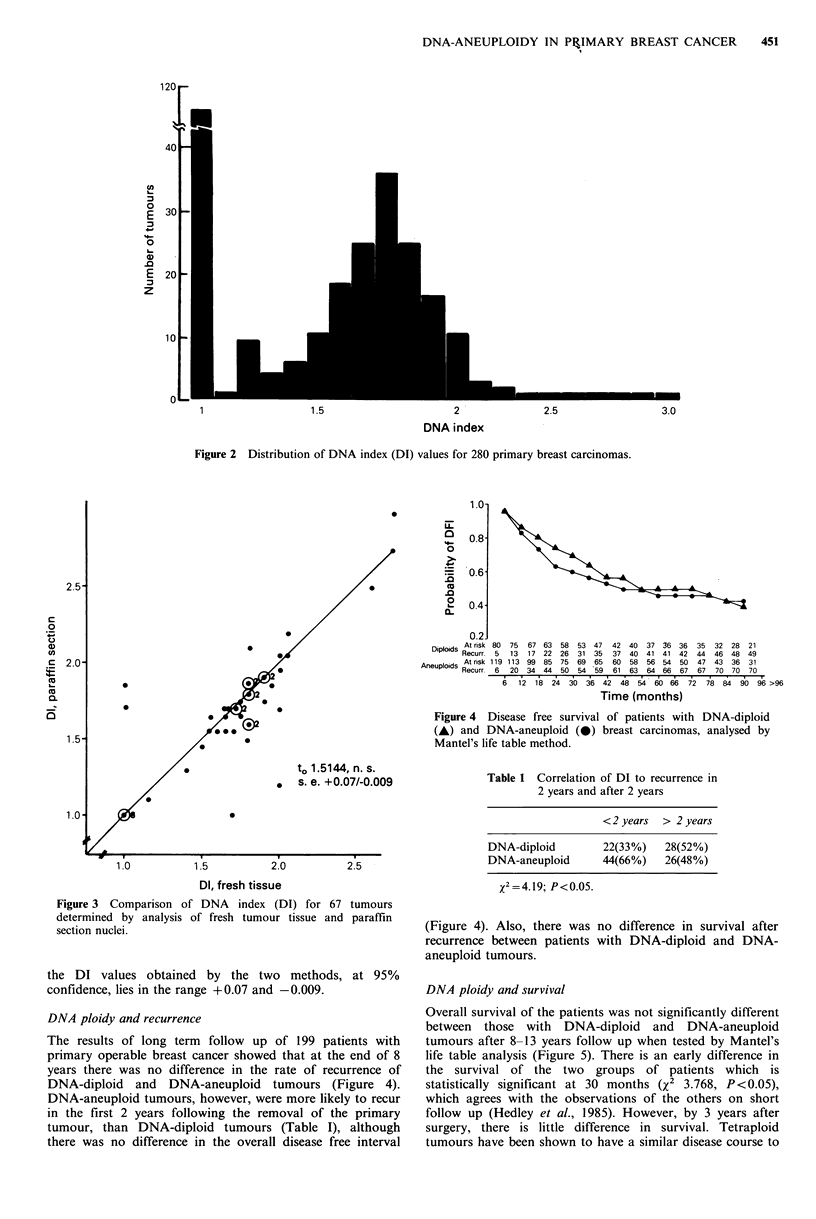
Cellular DNA content of primary tumours from 280 patients with operable breast cancer was determined by flow cytometry using nuclei from paraffin sections stained with DAPI, and 199 of these patients were followed for 8-13 years after surgery. Tumours from 67 patients have also been analyzed for their DNA content using single cell suspensions from fresh tumour tissue stained with mithramycin and ethidium bromide, and the results compared with those obtained from paraffin blocks of the same tumours. Overall 60% of the tumours contained cells with abnormal DNA content (DNA-aneuploid populations). Survival and disease free interval were not significantly different in patients with DNA-diploid and DNA-aneuploid tumours when analysed by Mantel's life table method. There was however, an early advantage for patients with DNA-diploid tumours: during the first 30 months after surgery DNA-aneuploidy was associated with higher rate of recurrence and shorter survival. DNA-aneuploidy was strongly related to histological grade. Thus 11/49 (22%) grade I, 60/102 (59%) grade II, and 96/129 (74%) grade III tumours were DNA-aneuploid. Although there was no significant difference in survival of patients with DNA-diploid and DNA-aneuploid tumours overall, there appears to be an unexpected association between DNA-aneuploidy and better survival in grade II patients (P less than 0.01); a similar trend was observed for grade I patients. Although the proportion of DNA-aneuploid tumours was similar in oestrogen receptor positive and negative tumours, DNA-aneuploidy was associated with lower levels of oestrogen receptors in comparison to DNA-diploid tumours. Comparison between the modal DNA values of fresh and paraffin embedded samples showed high rate of comparability (64/67, P less than 0.0001).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKIN N. B., RICHARDS B. M. Deoxyribonucleic acid in human tumours as measured by microspectrophotometry of Feulgen stain: a comparison of tumours arising at different sites. Br J Cancer. 1956 Dec;10(4):769–786. doi: 10.1038/bjc.1956.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin N. B., Kay R. Prognostic significance of modal DNA value and other factors in malignant tumours, based on 1465 cases. Br J Cancer. 1979 Aug;40(2):210–221. doi: 10.1038/bjc.1979.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin N. B. Modal deoxyribonucleic acid value and survival in carcinoma of the breast. Br Med J. 1972 Jan 29;1(5795):271–272. doi: 10.1136/bmj.1.5795.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer G. U., Caspersson T. O., Wallgren A. S. DNA content and survival in mammary carcinoma. Anal Quant Cytol. 1980 Sep;2(3):161–165. [PubMed] [Google Scholar]
- Auer G., Eriksson E., Azavedo E., Caspersson T., Wallgren A. Prognostic significance of nuclear DNA content in mammary adenocarcinomas in humans. Cancer Res. 1984 Jan;44(1):394–396. [PubMed] [Google Scholar]
- BLOOM H. J., RICHARDSON W. W. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957 Sep;11(3):359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson P. B., Thornthwaite J. T., Woolley T. W., Sugarbaker E. V., Seckinger D. Prognostic indicators including DNA histogram type, receptor content, and staging related to human breast cancer patient survival. Cancer Res. 1984 Sep;44(9):4187–4196. [PubMed] [Google Scholar]
- Dowle C. S., Owainati A., Robins A., Burns K., Ellis I. O., Elston C. W., Blamey R. W. Prognostic significance of the DNA content of human breast cancer. Br J Surg. 1987 Feb;74(2):133–136. doi: 10.1002/bjs.1800740221. [DOI] [PubMed] [Google Scholar]
- Elston C. W., Gresham G. A., Rao G. S., Zebro T., Haybittle J. L., Houghton J., Kearney G. The cancer research campaign (King's/Cambridge trial for early breast cancer: clinico-pathological aspects. Br J Cancer. 1982 May;45(5):655–669. doi: 10.1038/bjc.1982.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers S. B., Långström E., Baldetorp B., Killander D. Flow-cytometric DNA analysis in primary breast carcinomas and clinicopathological correlations. Cytometry. 1984 Jul;5(4):408–419. doi: 10.1002/cyto.990050419. [DOI] [PubMed] [Google Scholar]
- Fosså S. D., Marton P. F., Knudsen O. S., Kaalhus O., Børmer O., Vaage S. Nuclear feulgen DNA content and nuclear size in human breast carcinoma. Hum Pathol. 1982 Jul;13(7):626–630. doi: 10.1016/s0046-8177(82)80004-x. [DOI] [PubMed] [Google Scholar]
- Haybittle J. L., Blamey R. W., Elston C. W., Johnson J., Doyle P. J., Campbell F. C., Nicholson R. I., Griffiths K. A prognostic index in primary breast cancer. Br J Cancer. 1982 Mar;45(3):361–366. doi: 10.1038/bjc.1982.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W. Application of DNA flow cytometry to paraffin-embedded archival material for the study of aneuploidy and its clinical significance. Cytometry. 1985 Jul;6(4):327–333. doi: 10.1002/cyto.990060409. [DOI] [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W., Rugg C. A., Musgrove E. A. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem. 1983 Nov;31(11):1333–1335. doi: 10.1177/31.11.6619538. [DOI] [PubMed] [Google Scholar]
- Hedley D. W., Rugg C. A., Ng A. B., Taylor I. W. Influence of cellular DNA content on disease-free survival of Stage II breast cancer patients. Cancer Res. 1984 Nov;44(11):5395–5398. [PubMed] [Google Scholar]
- Nicholson R. I., Campbell F. C., Blamey R. W., Elston C. W., George D., Griffiths K. Steroid receptors in early breast cancer: value in prognosis. J Steroid Biochem. 1981 Dec;15:193–199. doi: 10.1016/0022-4731(81)90275-2. [DOI] [PubMed] [Google Scholar]
- Olszewski W., Darzynkiewicz Z., Rosen P. P., Schwartz M. K., Melamed M. R. Flow cytometry of breast carcinoma: I. Relation of DNA ploidy level to histology and estrogen receptor. Cancer. 1981 Aug 15;48(4):980–984. doi: 10.1002/1097-0142(19810815)48:4<980::aid-cncr2820480421>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Stuart-Harris R., Hedley D. W., Taylor I. W., Levene A. L., Smith I. E. Tumour ploidy, response and survival in patients receiving endocrine therapy for advanced breast cancer. Br J Cancer. 1985 Apr;51(4):573–576. doi: 10.1038/bjc.1985.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor I. W., Musgrove E. A., Friedlander M. L., Foo M. S., Hedley D. W. The influence of age on the DNA ploidy levels of breast tumours. Eur J Cancer Clin Oncol. 1983 May;19(5):623–628. doi: 10.1016/0277-5379(83)90178-5. [DOI] [PubMed] [Google Scholar]
- Thorud E., Fosså S. D., Vaage S., Kaalhus O., Knudsen O. S., Børmer O., Shoaib M. C. Primary breast cancer. Flow cytometric DNA pattern in relation to clinical and histopathologic characteristics. Cancer. 1986 Feb 15;57(4):808–811. doi: 10.1002/1097-0142(19860215)57:4<808::aid-cncr2820570421>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]


