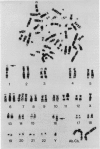
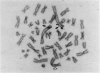
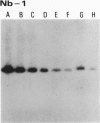
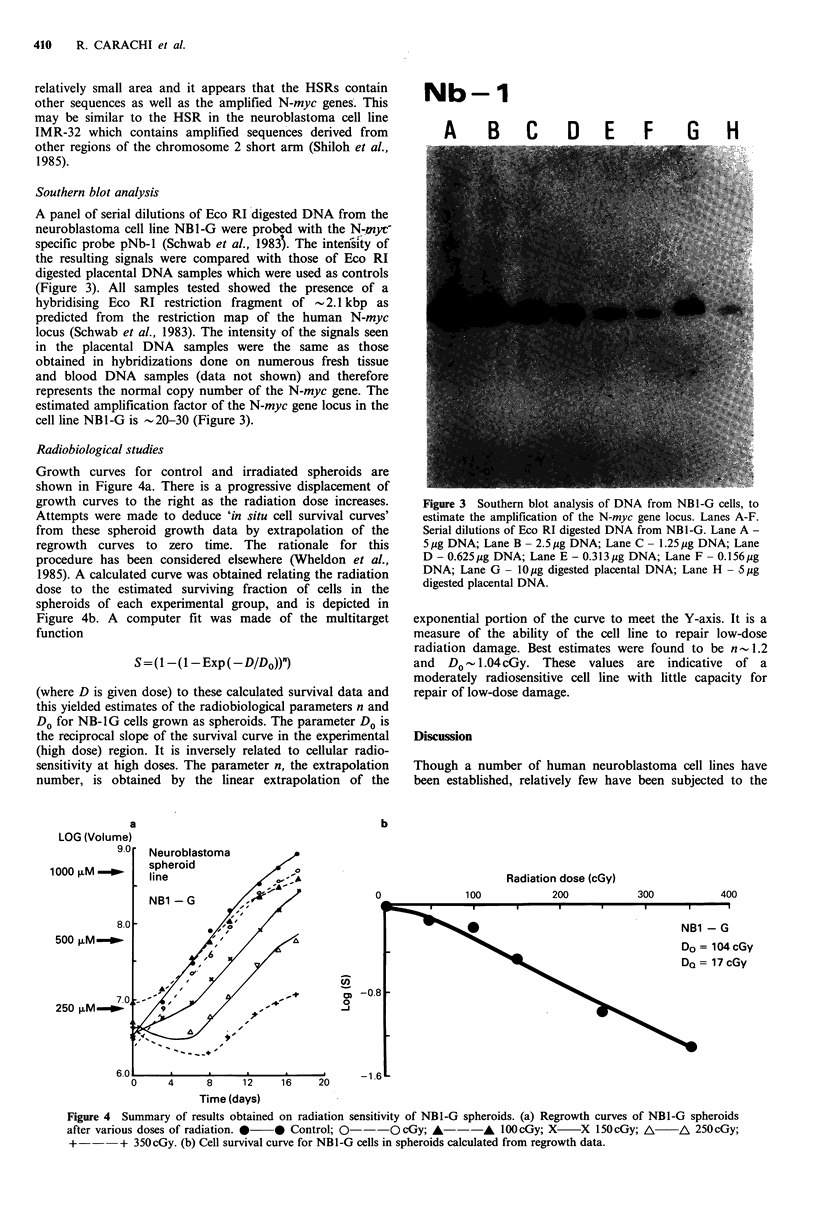
Abstract
The properties of a new tumour cell line (NB1-G) derived from human neuroblastoma by xenografting in nude rats followed by adaptation to tissue culture are described. Studies using a panel of monoclonal antibodies demonstrate the neuro-ectodermal nature of the cells and support the diagnosis of the primary tumour as neuroblastoma. Cytogenetic studies have revealed a human karyotype with several chromosomal abnormalities. Genetic analysis by in situ DNA hybridization has demonstrated the presence of multiple copies of the N-myc gene. Approximately 20-30 fold amplification of the gene is observed on Southern blot analysis. The cell line has been adapted to growth as multicellular tumour spheroids as well as monolayer culture. Radiobiological studies on spheroids show the cells to be radiosensitive with low capacity for sub-lethal damage accumulation and repair. The cell line should be useful for fundamental studies of human neuroblastoma as well as experimental therapy in vitro.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breslow N., McCann B. Statistical estimation of prognosis for children with neuroblastoma. Cancer Res. 1971 Dec;31(12):2098–2103. [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Schwab M., Varmus H. E., Bishop J. M. Amplification of N-myc sequences in primary human neuroblastomas: correlation with advanced disease stage. Prog Clin Biol Res. 1985;175:105–113. [PubMed] [Google Scholar]
- Gallimore P. H., Richardson C. R. An improved banding technique exemplified in the karyotype analysis of two strains of rat. Chromosoma. 1973;41(3):259–263. doi: 10.1007/BF00344020. [DOI] [PubMed] [Google Scholar]
- Jaffe N. Neuroblastoma: review of the literature and an examination of factors contributing to its enigmatic charcter. Cancer Treat Rev. 1976 Jun;3(2):61–82. doi: 10.1016/s0305-7372(76)80005-9. [DOI] [PubMed] [Google Scholar]
- Joseph A. M., Gosden J. R., Chandley A. C. Estimation of aneuploidy levels in human spermatozoa using chromosome specific probes and in situ hybridisation. Hum Genet. 1984;66(2-3):234–238. doi: 10.1007/BF00286608. [DOI] [PubMed] [Google Scholar]
- Mitchell A. R., Gosden J. R., Miller D. A. A cloned sequence, p82H, of the alphoid repeated DNA family found at the centromeres of all human chromosomes. Chromosoma. 1985;92(5):369–377. doi: 10.1007/BF00327469. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Klempnauer K. H., Varmus H. E., Bishop J. M., Gilbert F., Brodeur G., Goldstein M., Trent J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983 Sep 15;305(5931):245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- Shiloh Y., Shipley J., Brodeur G. M., Bruns G., Korf B., Donlon T., Schreck R. R., Seeger R., Sakai K., Latt S. A. Differential amplification, assembly, and relocation of multiple DNA sequences in human neuroblastomas and neuroblastoma cell lines. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3761–3765. doi: 10.1073/pnas.82.11.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sugimoto T., Sawada T., Arakawa S., Matsumura T., Sakamoto I., Takeuchi Y., Reynolds C. P., Kemshead J. T., Helson L. Possible differential diagnosis of neuroblastoma from rhabdomyosarcoma and Ewing's sarcoma by using a panel of monoclonal antibodies. Jpn J Cancer Res. 1985 Apr;76(4):301–307. [PubMed] [Google Scholar]
- Treuner J., Feine U., Niethammer D., Müller-Schaumburg W., Meinke J., Eibach E., Dopfer R., Klingebiel T., Grumbach S. Scintigraphic imaging of neuroblastoma with [131-I]iodobenzylguanidine. Lancet. 1984 Feb 11;1(8372):333–334. doi: 10.1016/s0140-6736(84)90375-1. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R. Growth delay in small EMT6 spheroids induced by cytotoxic drugs and its modification by misonidazole pretreatment under hypoxic conditions. Br J Cancer. 1982 Apr;45(4):565–570. doi: 10.1038/bjc.1982.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheldon T. E., Livingstone A., Wilson L., O'Donoghue J., Gregor A. The radiosensitivity of human neuroblastoma cells estimated from regrowth curves of multicellular tumour spheroids. Br J Radiol. 1985 Jul;58(691):661–664. doi: 10.1259/0007-1285-58-691-661. [DOI] [PubMed] [Google Scholar]
- Wheldon T. E., O'Donoghue J., Gregor A., Livingstone A., Wilson L. Radiobiological considerations in the treatment of neuroblastoma by total body irradiation. Radiother Oncol. 1986 Aug;6(4):317–326. doi: 10.1016/s0167-8140(86)80199-2. [DOI] [PubMed] [Google Scholar]
- Yuhas J. M., Li A. P., Martinez A. O., Ladman A. J. A simplified method for production and growth of multicellular tumor spheroids. Cancer Res. 1977 Oct;37(10):3639–3643. [PubMed] [Google Scholar]