Abstract
Decreased membrane rigidity is one of the characteristics of malignant cells, resulting in part from the desaturation of stearic acid into oleic acid. In this study we investigated the influence of stearic acid on tumour cell inhibition in vitro and tumour development in vivo. Stearic acid inhibited the colony-forming ability of 4 out of 5 rat and two human tumour continuous cell lines in vitro. In contrast, the colony-forming ability of rat fibroblasts was not inhibited and that of human foetal lung fibroblasts was inhibited at a higher dose than that required to inhibit human tumour cell lines. Using a model of rat mammary carcinoma induced by nitroso-methyl urea (NMU) the subcutaneous injection of stearic acid at weekly intervals prevented tumour development in 5 to 10 rats. Using iodostearic acid twice weekly, 11 of 19 rats were alive and tumour free at week 22 whilst all of 14 animals injected with NMU alone had died of tumour by the 16th week. The ratio of stearic to oleic acids in erythrocyte membranes was significantly reduced in the tumour-bearing rats, but was normal in tumour-free animals treated with stearic or iodostearic acid. These preliminary data indicate that stearic acid inhibits tumour development in rats.
Full text
PDF
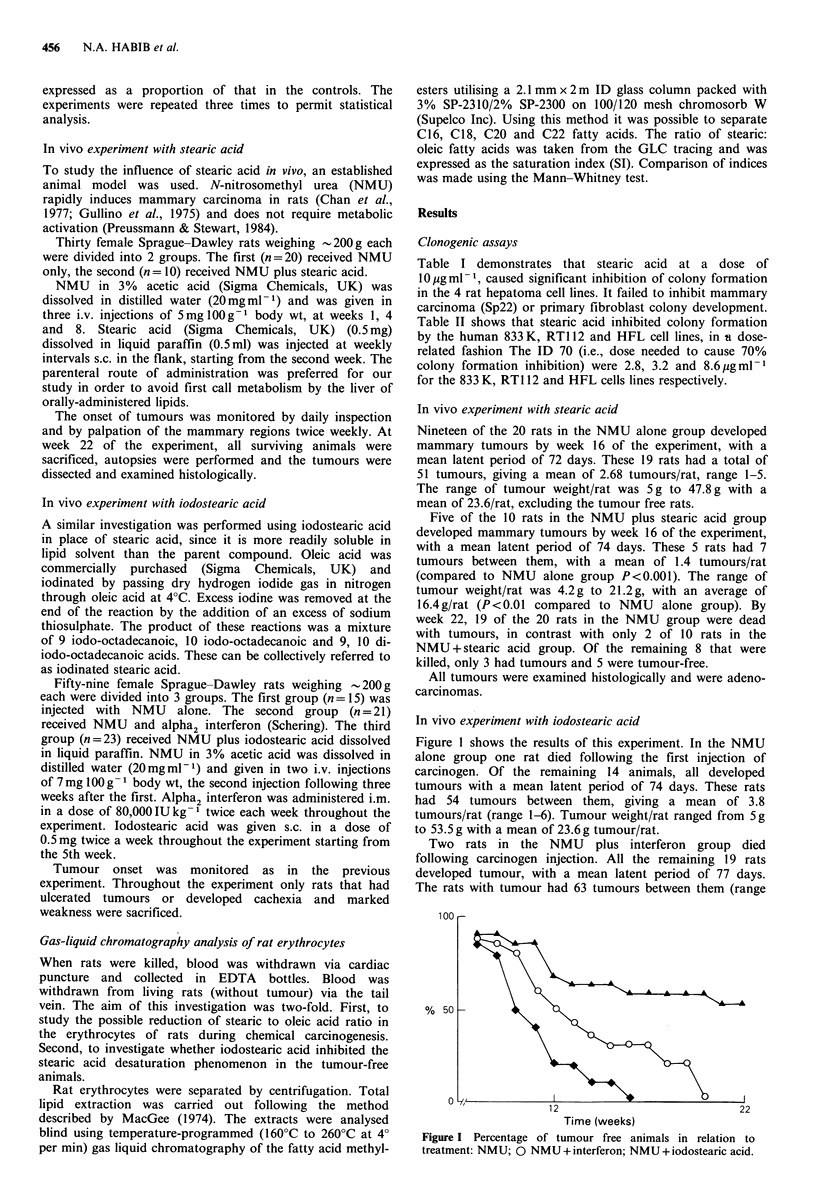
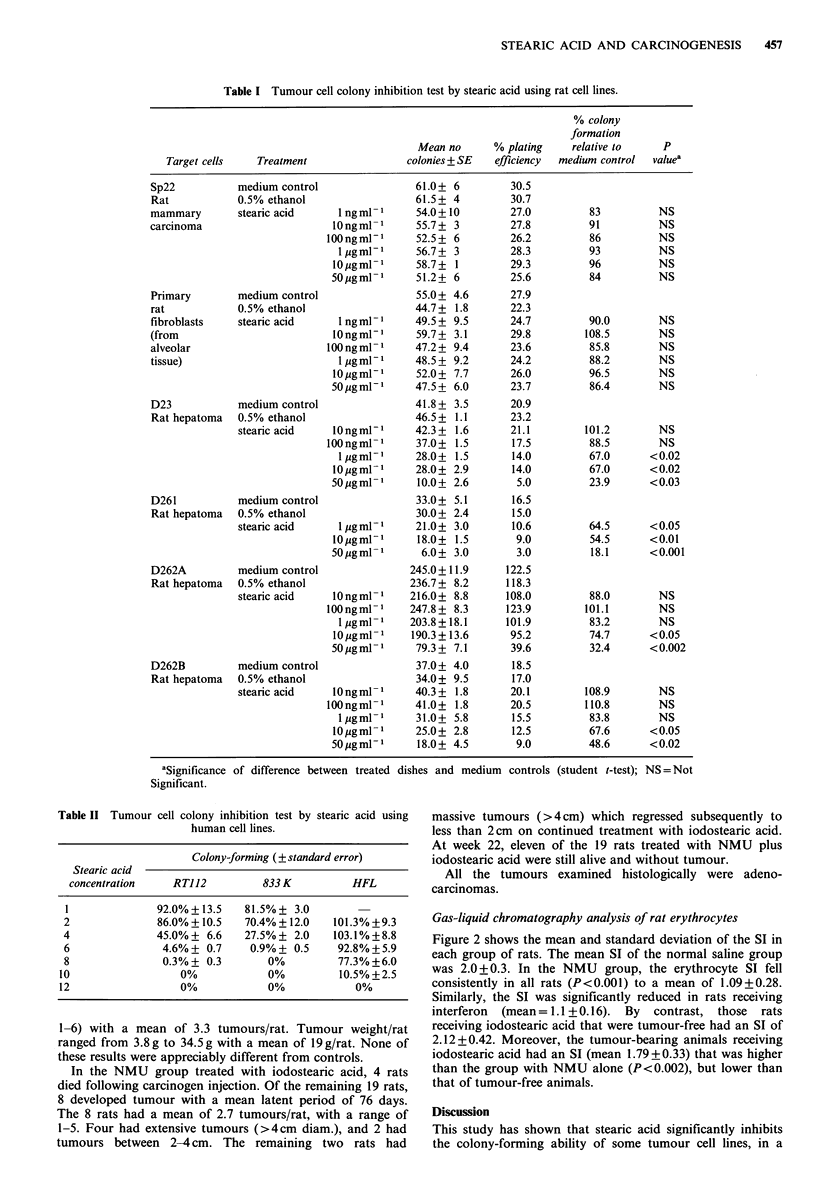
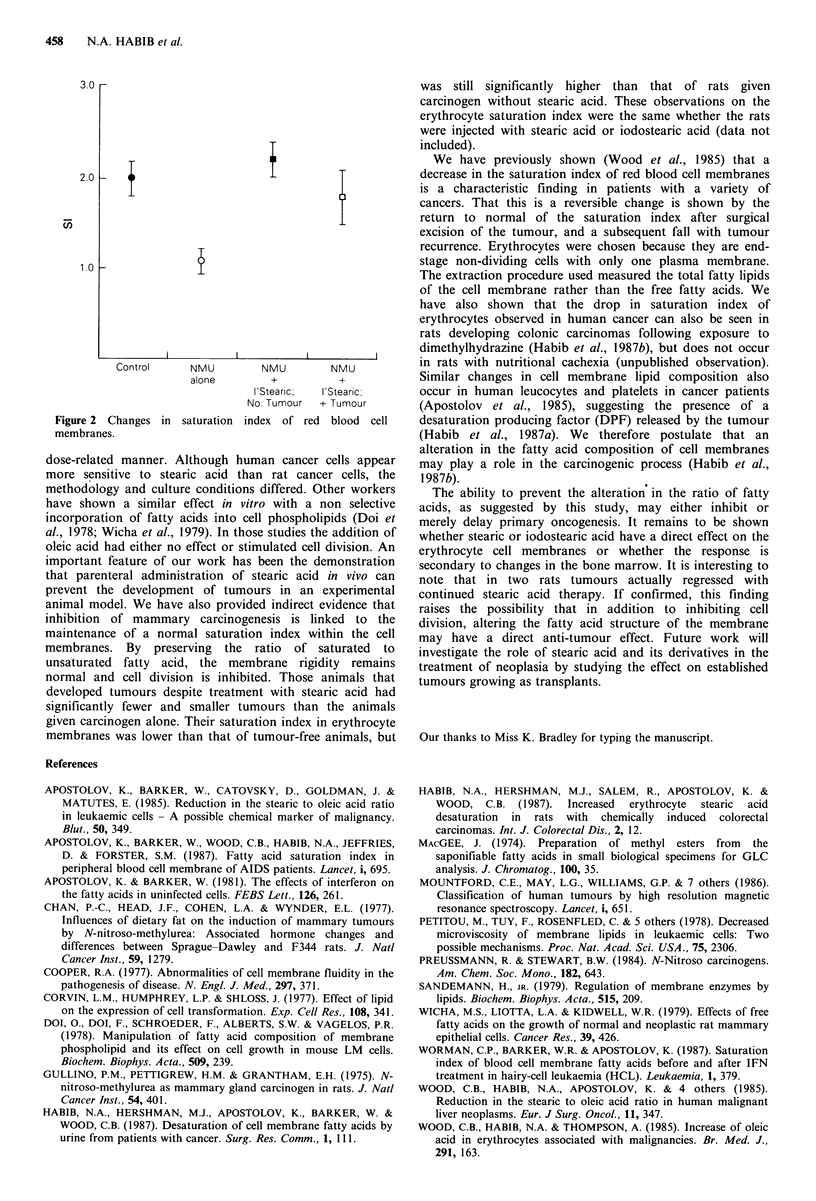
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apostolov K., Barker W., Catovsky D., Goldman J., Matutes E. Reduction in the stearic to oleic acid ratio in leukaemic cells--a possible chemical marker of malignancy. Blut. 1985 Jun;50(6):349–354. doi: 10.1007/BF00320928. [DOI] [PubMed] [Google Scholar]
- Apostolov K., Barker W. The effects of interferon on the fatty acids in uninfected cells. FEBS Lett. 1981 Apr 20;126(2):261–264. doi: 10.1016/0014-5793(81)80256-6. [DOI] [PubMed] [Google Scholar]
- Apostolov K., Barker W., Wood C. B., Habib N. A., Jeffries D., Forster S. M. Fatty acid saturation index in peripheral blood cell membranes of AIDS patients. Lancet. 1987 Mar 21;1(8534):695–695. doi: 10.1016/s0140-6736(87)90469-7. [DOI] [PubMed] [Google Scholar]
- Chan P. C., Head J. F., Cohen L. A., Wynder E. L. Influence of dietary fat on the induction of mammary tumors by N-nitrosomethylurea: associated hormone changes and differences between Sprague-Dawley and F344 rats. J Natl Cancer Inst. 1977 Oct;59(4):1279–1283. doi: 10.1093/jnci/59.4.1279. [DOI] [PubMed] [Google Scholar]
- Cooper R. A. Abnormalities of cell-membrane fluidity in the pathogenesis of disease. N Engl J Med. 1977 Aug 18;297(7):371–377. doi: 10.1056/NEJM197708182970707. [DOI] [PubMed] [Google Scholar]
- Corwin L. M., Humphrey L. P., Shloss J. Effect of lipids on the expression of cell transformation. Exp Cell Res. 1977 Sep;108(2):341–347. doi: 10.1016/s0014-4827(77)80041-4. [DOI] [PubMed] [Google Scholar]
- Doi O., Doi F., Schroeder F., Alberts A. W., Vagelos P. R. Manipulation of fatty acid composition of membrane phospholipid and its effects on cell growth in mouse LM cells. Biochim Biophys Acta. 1978 May 18;509(2):239–250. doi: 10.1016/0005-2736(78)90044-5. [DOI] [PubMed] [Google Scholar]
- Gullino P. M., Pettigrew H. M., Grantham F. H. N-nitrosomethylurea as mammary gland carcinogen in rats. J Natl Cancer Inst. 1975 Feb;54(2):401–414. [PubMed] [Google Scholar]
- Habib N. A., Hershman M. J., Salem R., Barker W., Apostolov K., Wood C. B. Increased erythrocyte stearic acid desaturation in rats with chemically induced colorectal carcinomas. Int J Colorectal Dis. 1987 Feb;2(1):12–14. doi: 10.1007/BF01648990. [DOI] [PubMed] [Google Scholar]
- MacGee J., Allen K. G. Preparation of methyl esters from the saponifiable fatty acids in small biological specimens for gas-liquid chromatographic analysis. J Chromatogr. 1974 Nov 13;100(1):35–42. doi: 10.1016/s0021-9673(00)86037-9. [DOI] [PubMed] [Google Scholar]
- Mountford C. E., Saunders J. K., May G. L., Holmes K. T., Williams P. G., Fox R. M., Tattersall M. H., Barr J. R., Russell P., Smith I. C. Classification of human tumours by high-resolution magnetic resonance spectroscopy. Lancet. 1986 Mar 22;1(8482):651–653. doi: 10.1016/s0140-6736(86)91727-7. [DOI] [PubMed] [Google Scholar]
- Petitou M., Tuy F., Rosenfeld C., Mishal Z., Paintrand M., Jasnin C., Mathe G., Inbar M. Decreased microviscosity of membrane lipids in leukemic cells: two possible mechanisms. Proc Natl Acad Sci U S A. 1978 May;75(5):2306–2310. doi: 10.1073/pnas.75.5.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Kidwell W. R. Effects of free fatty acids on the growth of normal and neoplastic rat mammary epithelial cells. Cancer Res. 1979 Feb;39(2 Pt 1):426–435. [PubMed] [Google Scholar]
- Wood C. B., Habib N. A., Apostolov K., Thompson A., Barker W., Hershman M., Blumgart L. H. Reduction in the stearic to oleic acid ratio in human malignant liver neoplasms. Eur J Surg Oncol. 1985 Dec;11(4):347–348. [PubMed] [Google Scholar]
- Wood C. B., Habib N. A., Thompson A., Bradpiece H., Smadja C., Hershman M., Barker W., Apostolov K. Increase of oleic acid in erythrocytes associated with malignancies. Br Med J (Clin Res Ed) 1985 Jul 20;291(6489):163–165. doi: 10.1136/bmj.291.6489.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman C. P., Barker W. R., Apostolov K. Saturation index of blood cell membrane fatty acids before and after IFN treatment in hairy cell leukemia. Leukemia. 1987 Apr;1(4):379–382. [PubMed] [Google Scholar]


