Abstract
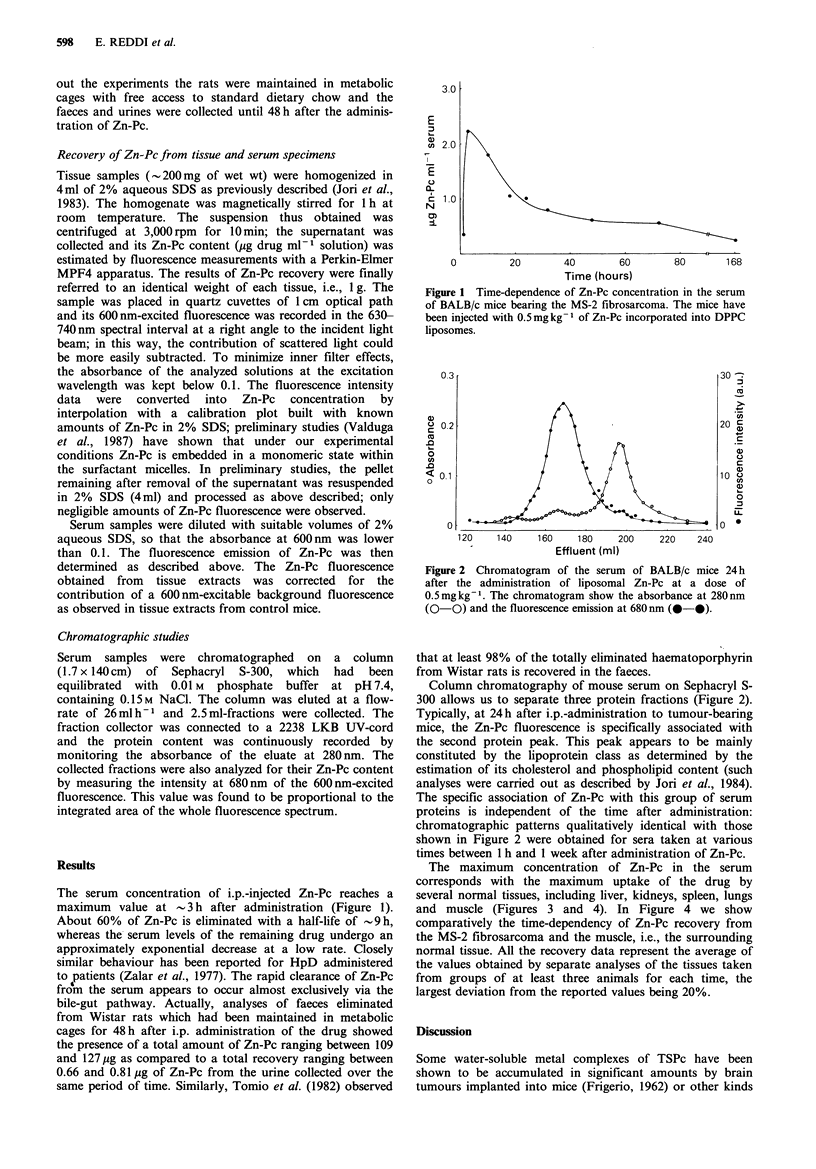
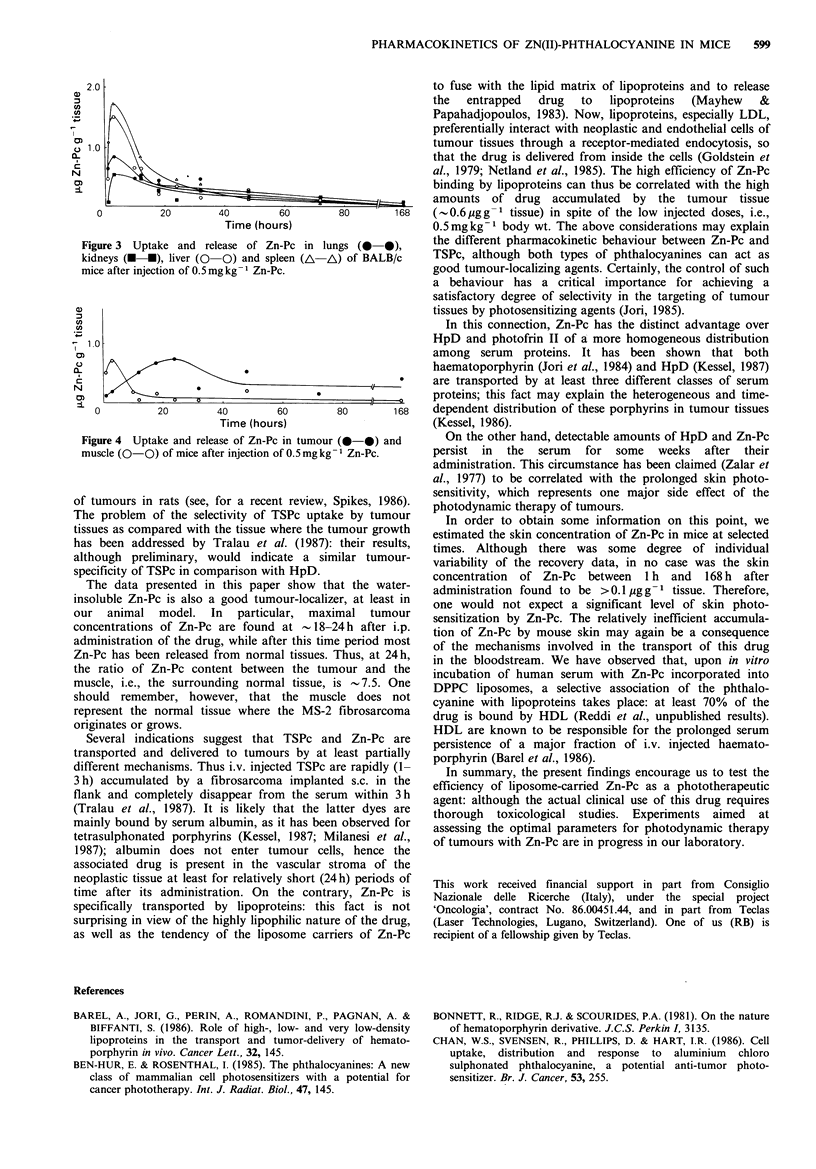
Zn(II)-phthalocyanine (Zn-Pc) incorporated into unilamellar liposomes of dipalmitoylphosphatidylcholine has been injected intraperitoneally (0.5 mg kg-1) to BALB/c mice bearing a transplanted MS-2 fibrosarcoma. The drug is specifically transported by serum lipoproteins and cleared from the serum via the bile-gut pathway in a biphasic process: approximately 60% of Zn-Pc is eliminated with a serum half-life of approximately 9 hours, while the remaining aliquot is eliminated at a very slow rate. Several normal tissues take up the drug within 3 hours after administration but release it almost completely after 24-48 hours. On the other hand, the tumour shows a maximum concentration of Zn-Pc (approximately 0.6 microgram g-1 of tissue) after 18-24 hours; at this time, the ratio between the Zn-Pc levels in the tumour and the muscle (which represents the surrounding normal tissue) is approximately 7.5. The results are discussed in terms of a possible use of Zn-Pc as a photosensitizer in the photodynamic therapy of tumours.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barel A., Jori G., Perin A., Romandini P., Pagnan A., Biffanti S. Role of high-, low- and very low-density lipoproteins in the transport and tumor-delivery of hematoporphyrin in vivo. Cancer Lett. 1986 Aug;32(2):145–150. doi: 10.1016/0304-3835(86)90112-6. [DOI] [PubMed] [Google Scholar]
- Ben-Hur E., Rosenthal I. The phthalocyanines: a new class of mammalian cells photosensitizers with a potential for cancer phototherapy. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Feb;47(2):145–147. doi: 10.1080/09553008514550211. [DOI] [PubMed] [Google Scholar]
- Chan W. S., Svensen R., Phillips D., Hart I. R. Cell uptake, distribution and response to aluminium chloro sulphonated phthalocyanine, a potential anti-tumour photosensitizer. Br J Cancer. 1986 Feb;53(2):255–263. doi: 10.1038/bjc.1986.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J. Hematoporphyrin as a photosensitizer of tumors. Photochem Photobiol. 1983 Sep;38(3):377–379. doi: 10.1111/j.1751-1097.1983.tb02687.x. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J. Photodynamic therapy (PDT) of malignant tumors. Crit Rev Oncol Hematol. 1984;2(2):83–116. doi: 10.1016/s1040-8428(84)80016-5. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Jori G., Beltramini M., Reddi E., Salvato B., Pagnan A., Ziron L., Tomio L., Tsanov T. Evidence for a major role of plasma lipoproteins as hematoporphyrin carriers in vivo. Cancer Lett. 1984 Oct;24(3):291–297. doi: 10.1016/0304-3835(84)90025-9. [DOI] [PubMed] [Google Scholar]
- Jori G., Tomio L., Reddi E., Rossi E., Corti L., Zorat P. L., Calzavara F. Preferential delivery of liposome-incorporated porphyrins to neoplastic cells in tumour-bearing rats. Br J Cancer. 1983 Aug;48(2):307–309. doi: 10.1038/bjc.1983.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel D. Sites of photosensitization by derivatives of hematoporphyrin. Photochem Photobiol. 1986 Oct;44(4):489–493. doi: 10.1111/j.1751-1097.1986.tb04697.x. [DOI] [PubMed] [Google Scholar]
- LIPSON R. L., BALDES E. J., OLSEN A. M. The use of a derivative of hematoporhyrin in tumor detection. J Natl Cancer Inst. 1961 Jan;26:1–11. [PubMed] [Google Scholar]
- Moan J. Porphyrin photosensitization and phototherapy. Photochem Photobiol. 1986 Jun;43(6):681–690. doi: 10.1111/j.1751-1097.1986.tb05647.x. [DOI] [PubMed] [Google Scholar]
- Netland P. A., Zetter B. R., Via D. P., Voyta J. C. In situ labelling of vascular endothelium with fluorescent acetylated low density lipoprotein. Histochem J. 1985 Dec;17(12):1309–1320. doi: 10.1007/BF01002528. [DOI] [PubMed] [Google Scholar]
- Rousseau J., Ali H., Lamoureux G., Lebel E., van Lier J. E. Synthesis, tissue distribution and tumor uptake of 99mTc- and 67Ga-tetrasulfophthalocyanine. Int J Appl Radiat Isot. 1985 Sep;36(9):709–716. doi: 10.1016/0020-708x(85)90041-9. [DOI] [PubMed] [Google Scholar]
- Spikes J. D., Bommer J. C. Zinc tetrasulphophthalocyanine as a photodynamic sensitizer for biomolecules. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 Jul;50(1):41–45. doi: 10.1080/09553008614550421. [DOI] [PubMed] [Google Scholar]
- Spikes J. D. Phthalocyanines as photosensitizers in biological systems and for the photodynamic therapy of tumors. Photochem Photobiol. 1986 Jun;43(6):691–699. doi: 10.1111/j.1751-1097.1986.tb05648.x. [DOI] [PubMed] [Google Scholar]
- Tomio L., Zorat P. L., Jori G., Reddi E., Salvato B., Corti L., Calzavara F. Elimination pathway of hematoporphyrin from normal and tumor-bearing rats. Tumori. 1982 Aug;68(4):283–286. doi: 10.1177/030089168206800402. [DOI] [PubMed] [Google Scholar]
- Tralau C. J., MacRobert A. J., Coleridge-Smith P. D., Barr H., Bown S. G. Photodynamic therapy with phthalocyanine sensitisation: quantitative studies in a transplantable rat fibrosarcoma. Br J Cancer. 1987 Apr;55(4):389–395. doi: 10.1038/bjc.1987.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valduga G., Reddi E., Jori G. Spectroscopic studies on Zn(II)-phthalocyanine in homogeneous and microheterogeneous systems. J Inorg Biochem. 1987 Jan;29(1):59–65. doi: 10.1016/0162-0134(87)80012-0. [DOI] [PubMed] [Google Scholar]
- Zalar G. L., Poh-Fitzpatrick M., Krohn D. L., Jacobs R., Harber L. C. Induction of drug photosensitization in man after parenteral exposure to hematoporphyrin. Arch Dermatol. 1977 Oct;113(10):1392–1397. [PubMed] [Google Scholar]


