Abstract
Cardiac neural crest ablation results in depressed myocardial calcium transients and elevated proliferation in myocardium at a stage when cardiac neural crest cells are not in contact with the myocardium. To test the hypothesis that cardiac neural crest–derived cells, which migrate into the caudal, ventral pharynx at stage 14, block a signal from the ventral pharynx, we cultured stage 12 chick heart tube or myocardial strips in the presence or absence of ventral pharynx. We found that myocardium cultured with ventral pharynx that had not yet contacted neural crest cells had significantly reduced calcium transients and an increased rate of proliferation. Ventral pharynx from intact embryos at a stage when neural crest–derived cells had reached the pharynx had no effect on myocardial calcium transients. Ventral pharynx from neural crest–ablated embryos continued to suppress myocardial calcium transients at this later stage. Myocardium cultured with FGF-2 also showed a significant reduction in calcium transients. An FGF-2–neutralizing Ab reversed the deleterious effect of the ventral pharynx on myocardial calcium transients and proliferation. We therefore examined the expression of FGF-2 and similar FGFs in the ventral pharynx. Only FGF-8 was expressed in a temporospatial pattern that made it a viable candidate for altering the myocardial calcium transient during stages 14–18. In explant cultures, neutralizing Ab for FGF-8 rescued development of the myocardial calcium transient in neural crest–ablated chick embryos.
Introduction
Ablation of the cardiac neural crest in chick embryos results in a characteristic set of defects that include hypoplasia of the pharyngeal glands, mispatterning of the great arteries, and persistent truncus arteriosus, failure of the embryonic outflow tract to divide to form the aorta and the pulmonary trunk (1, 2). These morphological anomalies are accompanied by ventricular function deficiencies that were once thought to be caused by altered hemodynamic properties in the aortic arch arteries. Morphological and functional defects that mirror those found in the chick neural crest ablation model are found in children that have neural crest–related congenital heart disease (3).
In chick embryos, the cardiac neural crest originates at the dorsal aspect of the neural tube, between the level of the mid-otic placode and the third somite (2, 4). At stage 10 (Hamburger-Hamilton stages), these cells begin migrating ventrally and laterally (2). By stage 12, cardiac neural crest–derived cells populate the circumpharyngeal region where they pause briefly before proceeding into the pharyngeal arches (2). At late stage 13, neural crest–derived cells enter the caudal pharyngeal arches, first populating the third arch, then the fourth arch, and finally the sixth arch region (2). By stage 14, the neural crest cells in arch 3 lie in intimate contact with endothelial strands that are opening to form the third-arch artery (2, 5, 6). Neural crest–derived cells are also in intimate contact with the ventral pharyngeal endoderm and ectoderm at this stage. It is at stage 14 that slight deficiencies can first be detected in ventricular function in neural crest–ablated embryos (7).
The most striking functional abnormality that is observed in neural crest–ablated chick embryos at this early stage is decreased ventricular contractility (8, 9). In addition, these embryos often exhibit incomplete looping of the cardiac tube, altered conotruncal shape, and dilated ventricles at stage 18 (8, 9). These features support the view that there is a primary alteration in the myocardium prior to the time neural crest–derived cells would normally enter the outflow tract of the heart. Almost 48 hours elapses before neural crest cells would migrate into the outflow tract (10).
Recently, two important aspects of early myocardial development, altered excitation-contraction coupling and increased myocardial proliferation accompanied by myofibrillar disarray, have been demonstrated in cardiac neural crest–ablated embryos, clearly indicating that early myocardial development is influenced by the cardiac neural crest (7). The onset of this myocardial dysfunction is both temporally and spatially isolated from outflow tract septation. At stage 14, 24 hours after cardiac neural crest ablation, myocardial calcium transients are depressed by more than 30% (7). The myofibrillar disorganization observed in these embryos may be associated with increased proliferation rather than delayed development (7). In contrast, endocardial development appears to be normal, as suggested by the normal expression of fibrillin 2 and the normal formation of cushion mesenchyme and trabeculae (7). Although mesenchyme from delaminating endocardial cells seeds the atrioventricular canal and outflow tract cushions at a typical rate, the thickness of the cardiac jelly, another indicator of myocardial development, is uneven (7). The atypical heart morphology in cardiac neural crest–ablated embryos caused by the irregular deposition of cardiac jelly allows them to be recognized readily.
These deficiencies are sustained throughout development. In myocytes isolated at day 11 and day 15 from hearts of cardiac neural crest–ablated chick embryos, the myocardial calcium transient, a necessary component of excitation-contraction coupling, is significantly depressed compared with that in myocytes from control hearts (11, 12). Reduced calcium transients are due, in part, to a reduction in L-type calcium current, but caffeine-stimulated calcium transients are also suppressed, indicating that sarcoplasmic reticulum function is impaired (11). The high mortality rates caused by heart failure in these embryos may be related to impaired cardiac excitation-contraction coupling.
Since the neural crest supports development of the aortic arch arteries, which are conduits for the entire cardiac output early in development, it seemed reasonable to assume that myocardial dysfunction was the result of hemodynamic alterations in the aortic arch arteries. However, no alterations in the hemodynamics of the aortic arch arteries and peripheral circulation in neural crest–ablated chick embryos have been found. Sophisticated methods to measure pressure, pressure gradients, impedance, vascular resistance, wall stresses, and flow have all failed to detect any deficiencies in these parameters in the aortic arch arteries of cardiac neural crest–ablated embryos (13). In addition, a systolic pressure gradient across the aortic arch arteries, first described by Hu and Clark (14), is unaffected by neural crest ablation. Although hemodynamic abnormalities cannot be completely excluded as a cause of myocardial dysfunction in these embryos, other factors must play a more prominent role in generating the ventricular functional changes that have been observed.
Based on a meticulously constructed time line of cardiac neural crest cell migration, we determined that neural crest–derived cells first enter the ventral pharynx just before the stage at which myocardial dysfunction is first noted in neural crest–ablated embryos (2). Our hypothesis is that cardiac neural crest–derived cells may inhibit a signal such as a growth factor, produced in the ventral pharynx, which affects myocardial development. In the absence of cardiac neural crest cells, continued inappropriate signaling from the ventral pharynx may cause defective myocardial development.
To test whether the ventral pharynx can cause alterations in myocardial development similar to those observed in neural crest–ablated embryos, we cultured stage 12 myocardium in the presence or absence of stage 13 ventral pharynx (without heart) for 24 hours, then measured calcium transients and proliferation rates in the cultured myocardium. Changes in these parameters are hallmarks of myocardial dysfunction in neural crest–ablated chick embryos. We found a 34% decrease in calcium transients and elevated proliferation in myocardium cocultured with ventral pharynx. This decrease is similar in magnitude to that found in cardiac neural crest–ablated embryos at stage 14.
To identify growth factors that might mediate this effect, we cultured myocardium in the presence or absence of FGF-2. We found that FGF-2 had an effect similar to ventral pharynx on the myocardial calcium transients. A polyclonal anti–FGF-2-neutralizing Ab reversed the effect of the ventral pharynx on myocardial calcium transients and also inhibited proliferation in the myocardium, leading to the conclusion that an FGF-2–like molecule is released by the ventral pharynx and causes a decrease in the calcium transient of cocultured myocardium. A survey of several similar members of the FGF family showed robust expression of FGF-8 in the ventrolateral pharyngeal endoderm and ectoderm. In the absence of cardiac neural crest, FGF-8–neutralizing Ab restored the myocardial calcium transient to a normal level.
Methods
Animal surgery and explant incubation.
To obtain chick embryonic myocardium, fertilized Arbor Acre chicken eggs were incubated at 37°C and 70% humidity for about 48 hours or until they reached Hamburger-Hamilton stage 12. Each embryo was removed through a window in the egg and placed in PBS. The heart was excised as a single piece, the ventral foregut was removed, and the myocardium was dissected into two or three longitudinal strips of equal size. Each strip was incubated for 24 hours at 37°C with 5% CO2 in 50 μl of DMEM containing 6% FBS. Following this incubation, each strip of myocardium was washed in chick Ringer’s solution and prepared for calcium transient measurements.
In one series of experiments, the ventral pharynx was obtained by removing the heart and dissecting the ventral foregut free by making a bilateral cut at the anterior intestinal portal and transverse incision at pharyngeal pouch 2. The heart tube was removed from this piece, which was cut into two pieces of equal size and cultured, with and without anti–FGF-2– or anti–FGF-8–neutralizing Ab (Atlanta Biologicals Inc., Norcross, Georgia, USA), under the conditions described above. Another well containing only culture medium was maintained under identical conditions for 24 hours. Strips of myocardium from a single chick heart were cultured under each condition for an additional 24 hours.
In a second series of experiments, the heart was removed from the embryo. For one set of hearts, the ventral pharynx was dissected free as described. In a second set, the heart remained attached to the ventral pharynx. All hearts were cultured for 24 hours at 37°C with 5% CO2 in 50 μl of DMEM with 6% FBS. After incubation, the hearts were dissected free from the ventral pharynx (if necessary), washed in chick Ringer’s solution, and prepared for calcium transient measurements.
In a third series, explants of the ventral pharynx and heart were removed from embryos that had undergone cardiac neural crest ablation alone or combined cardiac neural crest and nodose placode ablations at stage 10. The ablations were performed by laser microsurgery, as reported previously (15, 16).
Myocardial calcium transient measurements.
Calcium transients were elicited by electrical field stimulation and measured as described previously (7, 11). Each heart tube or myocardial strip was placed on a 25-mm diameter glass coverslip (no. 1 thickness; Fisher Scientific Co., Suwanee, Georgia, USA) that was mounted in a perfusion chamber (Harvard Apparatus, Holliston, Massachusetts, USA) containing 1 ml of Ringer’s solution (in mM: 142 NaCl, 4 KCl, 5 HEPES, 1.8 MgCl2 · 6H2O, 1.8 CaCl2, 10 dextrose). After washing gently several times with Ringer’s solution, the tissue sample was loaded with fura-2. A Photon Technology International Inc., Lawrenceville, New Jersey, USA microspectrofluorometer with dual monochromators (Delta Scan) was used to collect the fura-2 calcium transients. In most experiments, myocardium was electrically stimulated at 1 Hz by applying 50 V for 50 ms with a pulse stimulator (model II; Hewlett-Packard, Palo Alto, California, USA) through two platinum electrodes placed on either side of a single piece of tissue, as described previously by Creazzo et al. (11). In other experiments, the myocardium was stimulated at 0.2 Hz or 0.5 Hz. At the stages examined, the amplitudes of the calcium transients were not affected by the rate of stimulation in the range of 0.2–1 Hz. Statistical analysis of myocardial calcium transients was performed using ANOVA with appropriate post hoc testing for significantly different groups (SigmaStat 2.0 software; SPSSScience, Chicago, Illinois, USA).
Immunohistochemistry.
For HNK1/MF-20 immunohistochemistry, embryos were fixed overnight in ethanol with 2% acetic acid, then dehydrated, cleared, and embedded in paraffin. The blocks were sectioned at 7 μm and mounted on plus slides (VWR Scientific, Bridgeport, New Jersey, USA). The slides were blocked with 10% goat serum, then incubated in undiluted HNK1 supernatant (American Type Culture Collection, Rockville, Maryland, USA) for 1 hour. After visualizing with VIP (Vector Laboratories, Burlingame, California, USA) the slides were incubated in desmin Ab (Accurate Chemical & Scientific Corp., Westbury, New York, USA) at 1:500 for 1 hour, followed by the rabbit ABC Elite kit (Vector Laboratories), and visualized with 3,3-diaminobenzidine (Vector Laboratories). The slides were washed briefly in water, dehydrated, cleared in xylene, and mounted with Cytoseal (Thomas Scientific). A counterstain was not necessary.
For double staining with proliferating cell nuclear antigen (PCNA) Ab and 4′,6-diamidine-2-phenylindole dihydrochloride (DAPI), cultured myocardium was fixed briefly, then implanted into the cerebral vesicle of a stage 18 chick embryo, which thus served as a carrier. These carrier embryos had been fixed previously as noted above. The embryo carrier containing the myocardium was then embedded in paraffin, sectioned, and mounted. Slides were blocked with 10% goat serum and incubated overnight in PCNA Ab. After washing 1–3 hours in Tris and 5 minutes in Tris with 2% FBS, the slides were incubated in secondary Ab for 1 hour. After washing, the slides were stained with DAPI (Roche Diagnostics Corp., Roche Molecular Biochemicals, Indianapolis, Indiana, USA), viewed on an Olympus BX-40 epifluorescence microscope (Olympus barrier filter, WABI and WU; Olympus America Inc., Melville, New York, USA), and photographed.
Cloning and in situ hybridization.
A 225-bp fragment of FGF-2 was cloned using the primers as described by Désiré et al. (17) and cDNA from stage 18 whole embryos.
A 239-bp fragment of FGF-4 (accession number U14654) was made using primers spanning the region of 321–559 bp and cDNA from stage 18 whole embryos. A 393-bp fragment of FGF-8 (accession number U55189) was made using primers spanning the region of 17–409 bp and cDNA from hearts from stage 14 to stage 18 embryos. The PCR fragments of FGF-2, -4, and -8 were cloned into the vector PCRII (Invitrogen Corp., San Diego, California, USA). PCR fragments containing one of the promoter primers and one of the gene-specific primers were generated and labeled with digoxigenin. A 220-bp clone of chick int-2 cloned previously in our lab was used (18). A PCR fragment was generated using the T3 and T7 primers. This fragment was labeled with digoxigenin.
In situ hybridization was done with digoxigenin-labeled riboprobes following Wilkinson’s protocol (19). The whole-mount embryos were visualized and photographed after clearing in graded glycerols. The embryos were then dehydrated and embedded in paraffin, sectioned transversely at 12 μm, and mounted.
Results
Neural crest–derived cells interpose between myocardium and the ventral pharynx.
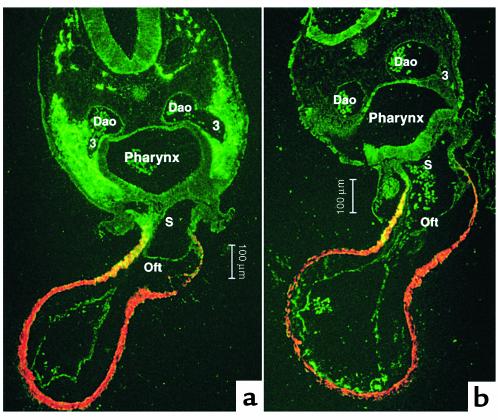
To determine the relationship between the neural crest, the ventral pharynx, and the myocardium, cross sections of the pharynx at stage 15 were double-stained for MF-20, to identify myocardium and HNK-1, a neural crest–specific marker. Significant differences have been observed first in ventricular function of sham-operated and cardiac neural crest–ablated embryos at stage 14 (7). HNK-1/MF-20 double-staining showed that at this stage the neural crest lies in intimate contact with the pharyngeal endoderm and is beginning to interpose between the ventrolateral pharyngeal endoderm and the ectoderm of arch 3 (Figure 1a). By stages 15 and 16, the neural crest cells surround a functional third arch artery and a nascent fourth arch artery and have interposed between the ventral endoderm of the foregut and the splanchnic mesoderm. In neural crest–ablated embryos, the pharyngeal endoderm, ectoderm, and splanchnic mesoderm remain in close proximity (Figure 1b). The splanchnic mesoderm is continuous with the myocardium. The neural crest–derived cells later form a substantial barrier between the ventral pharynx and the myocardium proper (10).
Figure 1.
Transverse sections of stage 16 chick embryos at the level of the third pharyngeal arch where the cardiac outflow tract joins the pharynx. The sections are double-stained with MF-20 (myosin heavy chain marker; red) to show the cardiac muscle of the outflow tract and HNK-1 (neural crest marker; green) to illustrate the location of cardiac neural crest cells. The muscular wall of the outflow tract is continuous with the splanchnic mesoderm underlying the ventral pharyngeal endoderm. Some of these cells express HNK-1 in both the sham and neural crest–ablated embryo. (a) The cardiac neural crest migrating from the circumpharyngeal region into arch 3 in a sham-operated embryo. The neural crest cells interpose between the endoderm and ectoderm of the pharyngeal arch and ventrally migrate between the splanchnic mesoderm and pharyngeal endoderm. (b) The altered relationship of pharyngeal endoderm with the ectoderm and splanchnic mesoderm in a neural crest–ablated embryo. Dao, dorsal aorta; 3, third aortic arch artery; Oft, cardiac outflow track; S, aortic sac.
Ventral pharynx causes suppression of myocardial calcium transients.
To determine whether exposure to ventral pharynx can suppress calcium transients in developing myocardium similar to the suppression found in neural crest–ablated embryos, we employed a culture system. Ventral pharynx excised from stage 13 embryos was cultured under standard conditions for 24 hours. Myocardium excised from stage 12 embryos was dissected into strips of roughly equal size. One-half of the myocardium from each embryo was cultured alone, the other was cocultured with ventral pharynx that had been cultured for the previous 24 hours to allow accumulation of factors that might influence myocardial development. Strips of myocardium were cultured under both conditions for 24 hours, harvested, and transferred to a perfusion chamber where they were loaded with fura-2.
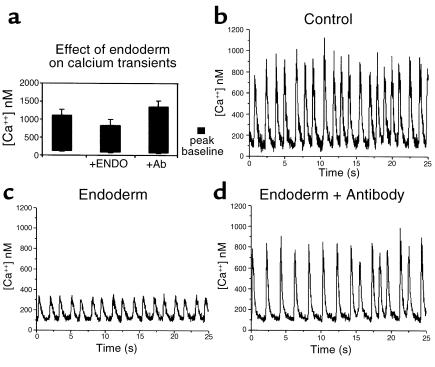
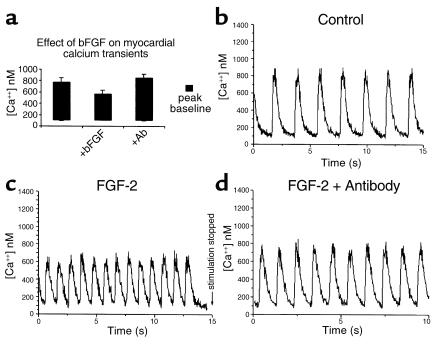
Figure 2, b and c, show examples of calcium transients that were obtained for myocardium excised from a single, stage 12 chick embryo and cultured for 24 hours. A significant reduction can be seen in the calcium transient obtained from the myocardium cocultured with ventral pharynx. Calcium transients for ten paired pieces of myocardium were determined in this manner (Figure 2a). On average, the calcium transients were 34% lower for myocardium that was cocultured with ventral pharynx compared with those cultured alone. The depressed calcium transient was not due to a pharmacological effect because culturing myocardium with ventral pharynx for 1 hour had no significant effect on myocardial calcium transients (data not shown).
Figure 2.
Calcium transients are suppressed significantly when myocardium is cultured with ventral pharynx. (a) The average calcium transients produced in myocardium that was cultured for 24 hours, alone or in the presence of ventral pharynx with and without anti–FGF-2-neutralizing Ab. Also shown are typical calcium transients elicited from myocardial strips derived from a single heart tube cultured alone (b), in the presence of ventral pharynx (c), or in the presence of ventral pharynx and anti–FGF-2-neutralizing Ab (d). The effect of ventral pharynx on development of myocardial calcium transients was significant (P < 0.05).
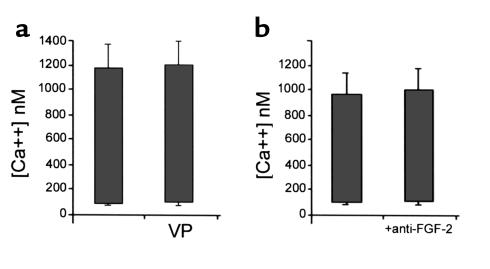
If the arrival of neural crest cells in the caudal pharynx alters the ability of the ventral pharynx to disrupt development of the myocardial calcium transients, then ventral pharynx removed after the neural crest cells have arrived should not alter the calcium transient in coculture with myocardium. Experiments similar to the one described above were performed using ventral pharynx excised from stage 16 embryos. We found that exposure of stage 12 myocardium to stage 16 ventral pharynx for 24 hours had no significant effect on development of the calcium transient (Figure 3a).
Figure 3.
Stage 16 ventral pharynx or anti–FGF-2-neutralizing Ab alone had no effect on development of myocardial calcium transients. Each bar shows the average peak diastolic and peak systolic calcium. (a) The effect of stage 16 ventral pharynx (VP) on myocardial calcium transients. (b) The effect of anti–FGF-2-neutralizing Ab alone on myocardial calcium transients.
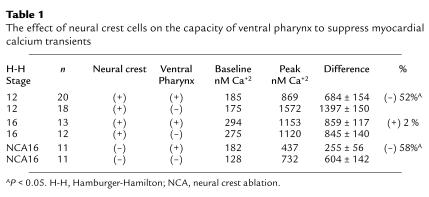
To determine the effect of the neural crest on the ability of ventral pharynx to suppress myocardial calcium transients, we wanted to maintain the architecture of the ventral pharynx. Thus, heart tubes from stage 12 embryos with intact cardiac neural crest were explanted, attached to the ventral pharynx, and cultured as a single piece for 24 hours. The myocardium in this preparation maintained its normal relationship with the ventral pharynx while in culture. After 24 hours in culture, the heart tubes were dissected free for calcium transient measurement. The myocardial calcium transient was significantly depressed (Table 1). This same preparation was used to compare the calcium transients in stage 16 sham-operated versus cardiac neural crest–ablated chick embryo explants cultured for 24 hours. The myocardial calcium transients in the ventral pharynx-myocardium explants from embryos with cardiac neural crest ablations were also significantly depressed (Table 1).
Table 1.
The effect of neural crest cells on the capacity of ventral pharynx to suppress myocardial calcium transients
Basic fibroblast growth factor causes suppression of myocardial calcium transients.
Since FGF-2 has been shown to depress calcium transients in immature myocardium and is known to increase proliferation, we determined the effect of FGF-2 on the stage 12 myocardium cultured for 24 hours. One-half of the myocardium from individual hearts was cultured in the presence of FGF-2 while the other half was cultured without supplementary FGF-2. FGF-2 concentrations of 10 ng/ml, 100 ng/ml, and 200 ng/ml were used in these experiments. At 24 hours, the myocardium was harvested and calcium transients were determined. Calcium transients were significantly suppressed in myocardium cultured in the presence of FGF-2 with maximal effect at a concentration of 100 ng/ml. A typical set of myocardial calcium transients is shown in Figure 4, b and c. The average calcium transients obtained from ten pairs of myocardium showed that those cultured with 100 ng/ml of FGF-2 were decreased by 53.2% (Figure 4a). This is similar to the decrease observed in myocardium that was cocultured with ventral pharynx. The myocardial calcium transient was protected from the deleterious effect of FGF-2 by a neutralizing Ab to FGF-2 (Figure 4d). Culturing the myocardium with 100 ng/ml FGF-2 for 1 hour had no significant effect on myocardial calcium transients, indicating that the effect is developmental rather than pharmacologic (data not shown). Myocardium cultured for 24 hours in the presence or absence of the FGF-2–neutralizing Ab showed normal calcium transients suggesting further that the FGF-like factor is not autonomous to the myocardium (Figure 3b). In contrast to the results obtained with FGF-2, culturing stage 12 myocardium for 24 hours with endothelin had no effect on the calcium transients.
Figure 4.
Development of myocardial calcium transients is significantly suppressed by FGF-2. (a) The average calcium transients elicited from myocardium cultured for 24 hours alone, with FGF-2 (50 ng/ml), or with FGF-2 and 50-fold excess of anti–FGF-2–neutralizing Ab. Also shown are typical calcium transients elicited from cultured myocardial strips from a single heart tube alone (b), with FGF-2 (c), or with FGF-2 and anti–FGF-2-neutralizing Ab (d). The effect of FGF-2 on development of myocardial calcium transients was significant (P < 0.05).
Neutralizing anti–FGF-2 Ab rescues calcium transients in myocardium cultured with ventral pharynx.
To determine whether the ventral pharynx produced an FGF-2–like signal that suppresses myocardial calcium transients, FGF-2–neutralizing Ab was added to myocardium cultured in the presence or absence of ventral pharynx. Calcium transients from these sets of myocardium showed that the neutralizing Ab reversed the effect of the ventral pharynx (Figure 2d).
Ventral pharynx elicits enhanced cell proliferation in cocultured myocardium.
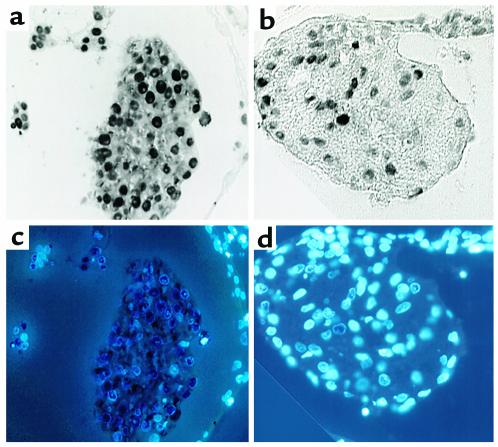
FGF-2 has been shown to increase the rate of proliferation of cardiac myocytes. To determine whether ventral pharynx had a similar effect on proliferation, myocardium obtained from stage 13 embryos was cut into two equal strips and cultured for 8 hours. One strip was cultured alone, the other strip was cultured in the presence of ventral pharynx from stage 13 embryos. At 8 hours, the myocardium was harvested and prepared for immunohistochemistry. An Ab against the PCNA antigen, a cell-cycle marker, was used to determine the rate of proliferation in the myocardium (Figure 5, a and b). The nuclei were also stained with DAPI, so that PCNA-negative nuclei might be more easily identified (Figure 5, c and d).
Figure 5.
Ventral pharynx produces a factor that enhances myocardial proliferation. PCNA staining shows proliferating myocytes in strips derived from a single heart cultured in the presence of ventral pharynx (a) or in the presence of ventral pharynx and anti–FGF-neutralizing Ab (b). DAPI staining labeled nuclei in each myocyte allowing determination of the percentage of myocytes that were proliferating in the presence of ventral pharynx (c) or ventral pharynx and anti–FGF-neutralizing Ab (d).
The rate of proliferation was determined by dividing the number of PCNA-positive myocardial cells by the total number of myocardial cells. The percent of cells that were PCNA-positive in myocardium that was cocultured with ventral pharynx was over 90%, indicating a very high rate of proliferation. The percent of PCNA-positive cells in myocardium that had been cultured with ventral pharynx and an FGF-2–neutralizing Ab was less than 40%.
FGF-8 in the ventral pharynx disrupts development of the myocardial calcium transient after neural crest ablation.
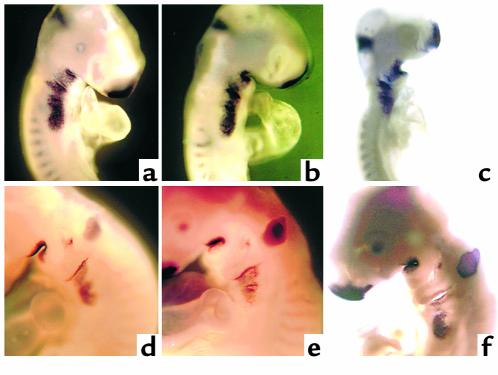
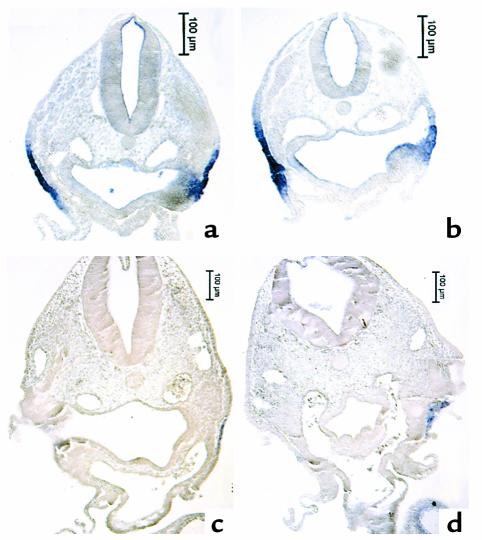
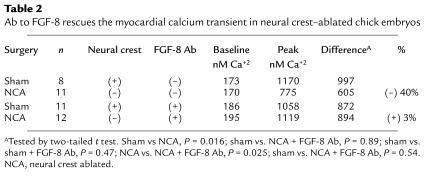
The most likely FGF family members to disrupt development of the myocardial calcium transient are FGF-2, -4, and –8. FGF-3 (int-2) expression has also been reported in the ventral pharynx. We cloned these members of the FGF family and performed whole-mount in situ hybridization to gain some idea of which of these FGFs might be expressed in a temporospatial pattern consistent with eliciting the altered myocardial calcium transient in neural crest–ablated embryos. Only FGF-8 message was expressed in the pharynx at a time and in a place that could alter development of the calcium transient. FGF-8 message expression was most prominent in the ventrolateral pharyngeal ectoderm, but it could also be seen in the pharyngeal endoderm and was prominent in the pharyngeal pouches (Figure 6, a and d, and Figure 7, a and c). Expression of FGF-8 message was not altered after neural crest ablation or neural crest and nodose placode ablation (Figure 6, b, c, e, and f, and Figure 7, b and d), suggesting that the same amount of FGF-8 protein might be available after neural crest ablation as in a normal embryo. Addition of FGF-8–neutralizing Ab to explant cultures of the ventral pharynx and heart from neural crest–ablated embryos between stages 13 and 18 led to complete restoration of the myocardial calcium transient (Table 2). In contrast, addition of FGF-8–neutralizing Ab to the same type of cultures from embryos with intact neural crest did not alter development of the myocardial calcium transient.
Figure 6.
In situ hybridization showing pharyngeal FGF-8 message expression in stage 14 (a–c) and stage 18 (d–f) chick embryos. (a and d) Sham-operated embryos with prominent FGF-8 message expression in the frontonasal process, midbrain-hindbrain boundary, and all of the pharyngeal arches and pouches. The expression in arches 1 and 2 appears to be less than that in arches 3, 4, and 6. (b and e) Embryos that have undergone cardiac neural crest ablations. The expression of FGF-8 message appears to be identical to that in the sham-operated embryos. (c and f) Embryos with double ablations of the cardiac neural crest and nodose placodes. These embryos show overall growth retardation although the expression of FGF-8 message in the pharyngeal region appears to be approximately the same as neural crest–ablated and sham-operated embryos at both stages.
Figure 7.
Paraffin sections of whole-mount embryos in Figure 6 showing the location of FGF-8 message in pharyngeal arch 3. (a and c) Sham-operated embryos at stages 14 (a) and 18 (c). (b and d) Neural crest–ablated embryos at stages 14 (b) and 18 (d). (a) FGF-8 message is expressed strongly in the ectoderm covering the arch and weakly in the pharyngeal endoderm. (b) A similar pattern and level of expression of FGF-8 message after neural crest ablation to that of the sham-operated control. (c) Dramatic decrease in FGF-8 message expression at stage 18 in both the endoderm and ectoderm of a sham-operated embryo. (d) A similar decrease in expression in a neural crest–ablated embryo.
Table 2.
Ab to FGF-8 rescues the myocardial calcium transient in neural crest–ablated chick embryos
Discussion
In vertebrates, the heart field is established through an interaction of the lateral plate mesoderm with the anterior endoderm (20). This first occurs in the anterior portion of the cardiogenic plate and progresses in an anterior to posterior manner until all cardiac myocyte progenitors have been converted to cardiac myocytes. The anterior endoderm has been implicated in this process by in vitro experiments, which have shown that isolated lateral plate mesoderm undergoes myocardial differentiation in the presence of anterior endoderm (21). Even noncardiac mesoderm can be induced to express cardiac markers by the anterior endoderm (22). Indeed, if the anterior endoderm is removed from a newt embryo before the tail-bud stage, heart formation is completely blocked (22). This failure of cardiac morphogenesis can be partially reversed by the simultaneous removal of the neural plate, especially the anterior plate and folds, where the premigratory cardiac neural crest lies (2, 22).
Many factors have been identified that are essential to this process, and these factors play similar roles in the induction of myocardium from lateral plate mesoderm throughout the vertebrate phylum. Growth factors produced by the pharyngeal endoderm that can induce aspects of myocardial development in the lateral plate mesoderm include activin-A, FGF-2, FGF-4, and TGF-β (23). The endoderm has the capacity to induce myocardial differentiation long after stable sarcomere expression, myofibril organization, and beating have been established in the heart (ref. 24; Farrell and Kirby, unpublished data). In fact, pharyngeal endoderm maintains this inductive capacity at least until the formation of the third pharyngeal pouch at about stage 14 in chicks (Farrell and Kirby, unpublished data) and until a comparable stage in frogs (24). This coincides with the stage at which cardiac neural crest–derived cells begin to populate pharyngeal arch 3 (6).
It was this reasoning that led us to hypothesize that the pharyngeal endoderm continued to release factors that are detrimental to myocardial development after cardiac neural crest ablation. However, it is difficult to obtain a pure preparation of ventral pharyngeal endoderm between stages 13 and 18, and so the “endoderm” preparations that were tested initially always contained some splanchnic mesoderm and pharyngeal ectoderm. We have subsequently found that FGF-8 is expressed in the endoderm and ectoderm of the ventrolateral pharynx where cardiac neural crest cells first enter the caudal pharyngeal arches. Because the FGF-8 originating from both layers is most probably available for neural crest cells in normal embryos and in elevated concentrations in neural crest–ablated embryos, both endoderm and ectoderm should be considered as sources of the FGF-8 that is detrimental to myocardial development in the absence of cardiac neural crest cells. In the absence of the cardiac neural crest cells, this FGF-8 causes development of suppressed myocardial calcium transients, an effect that can be reversed by the addition of anti–FGF-2 or FGF-8–neutralizing Ab’s. The FGF-2 Ab in these studies has been shown to cross-react with FGF-8a.
The expression pattern of FGF-8 in the chick embryo supports the view that pharyngeal endoderm/ectoderm is a source of FGF-8 during the stages of development that we have examined. FGF-2 has been detected in the chick myocardium, from stage 9 on, in punctate cytoplasmic aggregates (25). However the FGF-2 expression pattern does not extend into the inflow or outflow tracts (26). Thus FGF-8 is the most likely candidate to disrupt development of the myocardial calcium transient.
While detailed studies have not been done with FGF-8, it is known that FGF-2 can repress expression of the ryanodine receptor, a mediator of calcium release from the sarcoplasmic reticulum, in a myogenic cell line (27). FGF-2 has been shown to suppress calcium transients in adult rat cardiac myocytes (28). FGF-2 also markedly increases the rate of proliferation of cardiac myocytes (29, 30, 31). These effects are in line with the effects of pharyngeal endoderm on myocardium that we have observed.
In cardiac neural crest–ablated embryos, morphological and functional defects in the myocardium can be observed as early as stage 14 (8). The mechanism by which neural crest might mediate maturation of the myocardium is unknown. However, at least two distinct mechanisms are possible. First, neural crest–derived cells that interpose between the pharyngeal endoderm/ectoderm and the myocardium may provide a physical barrier that limits growth factor diffusion between these tissues. Second, the neural crest–derived cells may release factors that specifically inhibit transmission of a signal from the ventral pharynx to the myocardium.
It is interesting to note changes in gene expression that coincide with neural crest migration into the pharyngeal arches. For instance, concentrated deposits of fibroblast growth factor receptor-2 (FGFR-2), which have been detected in chick embryonic endothelial and myocardial cells, diminish in the myocardium, but not in the endothelium, after stage 14, around the time that neural crest cells contact the pharyngeal endoderm (26). Neural crest–derived cells in the pharyngeal arches also express FGFR-2 (32). The arrival of neural crest–derived cells in the pharyngeal arches has also been reported to be coincident with the expression of FGF-3, an FGF-2–related growth factor (33). However, we did not see expression of FGF-3 in the ventral pharynx at an appropriate time by in situ hybridization.
We have shown that, before contact with neural crest–derived cells, the pharyngeal endoderm/ectoderm produces FGF-8 that appears to suppress development of myocardial calcium transients when neural crest cells do not populate the caudal pharyngeal arches at the appropriate time. The degree to which the myocardial calcium transients are suppressed by exposure to pharyngeal endoderm/ectoderm is similar to the suppression found in myocardial calcium transients obtained from intact cardiac neural crest–ablated embryos at stage 14. At this stage, neural crest–derived cells attain intimate contact with the pharyngeal endoderm. After stage 16, the ventral pharynx normally has no effect on myocardial calcium transients. However, we have found that ventral pharynx from stage 16 neural crest–ablated embryos maintains the ability to suppress myocardial calcium transients.
Our results support the view that the neural crest plays a role in the maturation of the myocardium by blocking FGF-8 signaling between the ventral pharynx and the myocardium. We have shown that prolonged exposure of the myocardium to the pharynx significantly impairs the development of excitation-contraction coupling. This effect may also be important in vivo, where the myocardium is inappropriately exposed to the ventral pharynx in the cardiac neural crest–ablated embryos during the stages in development when neural crest–derived cells would normally be populating the region between the ventral pharynx and the myocardium.
Because of the potential for variability based on slight differences in embryonic age, we decided in the original myocardial testing that being able to do paired statistical analysis would give a more definitive answer to the hypothesis. The only way to have paired samples was to use a single heart for the different treatments. We chose to cut the strips longitudinally to minimize the differences in the chambers included, hoping that the greater-lesser curvature (which is also left-right at this stage) difference would factor out across both experimental and sham groups. The strips were randomized so that lesser-versus-greater curvature strips would be in different treatment groups in each experiment. While we cannot guarantee that greater or lesser curvature strips were evenly represented in each treatment group, the differences are quite significant between treatments in the sham-operated and experimental groups. Later experiments with specific neutralizing Ab’s were done in explant cultures in which only a single heart tube in one treatment group was compared with a single heart tube in another treatment group. While this disallowed paired statistics, we still had significant differences between the treatment groups.
Recent ultrasound studies have shown that the fraction of diastolic blood that is ejected during systole in human fetuses with persistent truncus arteriosus is reduced (34). It may be that the high level of mortality found in children with neural crest–related congenital heart disease is due primarily to impaired myocardial function, rather than the associated structural defects. A better understanding of the factors involved in producing the functional defects in these children may ultimately lead to more effective treatments.
Acknowledgments
The authors thank Karen Waldo for assistance with the figures, Karen Waldo and Linda Leatherbury for comments on the experiments and results, and NIH for continued support (HD17063, HL36059).
References
- 1.Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to aorticopulmonary septation. Science. 1983;220:1059–1061. doi: 10.1126/science.6844926. [DOI] [PubMed] [Google Scholar]
- 2.Kuratani SC, Kirby ML. Migration and distribution of circumpharyngeal crest cells in the chick embryo. Formation of the circumpharyngeal ridge and E/C8+ crest cells in the vertebrate head region. Anat Rec. 1992;234:263–280. doi: 10.1002/ar.1092340213. [DOI] [PubMed] [Google Scholar]
- 3.Goldmuntz E, Emanuel BS. Genetic disorders of cardiac morphogenesis. The DiGeorge and velocardiofacial syndromes. Circ Res. 1997;80:437–443. doi: 10.1161/01.res.80.4.437. [DOI] [PubMed] [Google Scholar]
- 4.LeDouarin, N.M. 1992. The neural crest. Cambridge University Press. Cambridge, United Kingdom.
- 5.Bockman DE, Redmond ME, Kirby ML. Alteration of early vascular development after ablation of cranial neural crest. Anat Rec. 1989;225:209–217. doi: 10.1002/ar.1092250306. [DOI] [PubMed] [Google Scholar]
- 6.Waldo KL, Kumiski D, Kirby ML. Cardiac neural crest is essential for the persistence rather than the formation of an arch artery. Dev Dyn. 1996;205:281–292. doi: 10.1002/(SICI)1097-0177(199603)205:3<281::AID-AJA8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 7.Waldo K, et al. A novel role for cardiac neural crest in heart development. J Clin Invest. 1999;103:1499–1507. doi: 10.1172/JCI6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leatherbury L, Connuck DM, Gauldin HE, Kirby ML. Hemodynamic changes and compensatory mechanisms during early cardiogenesis after neural crest ablation in chick embryos. Pediatr Res. 1991;30:509–512. doi: 10.1203/00006450-199112000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Tomita H, Connuck DM, Leatherbury L, Kirby ML. Relation of early hemodynamic changes to final cardiac phenotype and survival after neural crest ablation in chick embryos. Circulation. 1991;84:1289–1295. doi: 10.1161/01.cir.84.3.1289. [DOI] [PubMed] [Google Scholar]
- 10.Waldo K, Miyagawa-Tomita S, Kumiski D, Kirby ML. Cardiac neural crest cells provide new insight into septation of the cardiac outflow tract: aortic sac to ventricular septal closure. Dev Biol. 1998;196:129–144. doi: 10.1006/dbio.1998.8860. [DOI] [PubMed] [Google Scholar]
- 11.Creazzo TL, Brotto MA, Burch J. Excitation-contraction coupling in the day 15 embryonic chick heart with persistent truncus arteriosus. Pediatr Res. 1997;42:731–737. doi: 10.1203/00006450-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Creazzo TL. Reduced “L” type calcium current in the embryonic chick heart with persistent truncus arteriosus. Circ Res. 1990;66:1491–1498. doi: 10.1161/01.res.66.6.1491. [DOI] [PubMed] [Google Scholar]
- 13.Leatherbury L, Braden DS, Tomita H, Gauldin HE, Jackson WF. Hemodynamic changes. Wall stresses and pressure gradients in neural crest-ablated chick embryos. Ann NY Acad Sci. 1990;588:305–313. doi: 10.1111/j.1749-6632.1990.tb13220.x. [DOI] [PubMed] [Google Scholar]
- 14.Hu N, Clark EB. Hemodynamics of the stage 12 to stage 29 chick embryo. Circ Res. 1989;65:1665–1670. doi: 10.1161/01.res.65.6.1665. [DOI] [PubMed] [Google Scholar]
- 15.Kirby ML, Kumiski DH, Myers T, Cerjan C, Mishima N. Backtransplantation of chick cardiac neural crest cells cultured in LIF rescues heart development. Dev Dyn. 1993;198:296–311. doi: 10.1002/aja.1001980407. [DOI] [PubMed] [Google Scholar]
- 16.Harrison TA, Stadt HA, Kumiski D, Kirby ML. Compensatory responses and development of the nodose ganglion following ablation of placodal precursors in the embryonic chick (Gallus domesticus) Cell Tissue Res. 1995;281:379–385. doi: 10.1007/BF00583407. [DOI] [PubMed] [Google Scholar]
- 17.Désiré L, Head MW, Fayein NA, Courtois Y, Jeanny JC. Suppression of fibroblast growth factor 2 expression by antisense oligonucleotides inhibits embryonic chick neural retina cell differentiation and survival in vivo. Dev Dyn. 1998;212:63–74. doi: 10.1002/(SICI)1097-0177(199805)212:1<63::AID-AJA6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Qin F, Kirby ML. Int-2 influences the development of the nodose ganglion. Pediatr Res. 1995;38:485–492. doi: 10.1203/00006450-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson, D.G. 1992. In situ hybridization. In In situ hybridization: a practical approach. D. Rickwood and B.D. Hames, editors. IRS Press. Oxford, United Kingdom. 75–83.
- 20.Fishman MC, Chien KR. Fashioning the vertebrate heart: earliest embryonic decisions. Development. 1997;124:2099–2117. doi: 10.1242/dev.124.11.2099. [DOI] [PubMed] [Google Scholar]
- 21.Schultheiss TM, Xydas S, Lassar AB. Induction of avian cardiac myogenesis by anterior endoderm. Development. 1995;121:4203–4214. doi: 10.1242/dev.121.12.4203. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson AG, Duncan JT. Heart induction in salamanders. J Exp Zool. 1968;167:79–103. doi: 10.1002/jez.1401670106. [DOI] [PubMed] [Google Scholar]
- 23.Gannon M, Bader D. Initiation of cardiac differentiation occurs in the absence of anterior endoderm. Development. 1995;121:2439–2450. doi: 10.1242/dev.121.8.2439. [DOI] [PubMed] [Google Scholar]
- 24.Sater AK, Jacobson AG. The restriction of the heart morphogenetic field in Xenopus laevis. Dev Biol. 1990;140:328–336. doi: 10.1016/0012-1606(90)90083-u. [DOI] [PubMed] [Google Scholar]
- 25.Han JK. Expression of the fibroblast growth factor-2 gene during chick development. Mol Cells. 1997;7:208–213. [PubMed] [Google Scholar]
- 26.Parlow MH, Bolender DL, Kokan-Moore NP, Lough J. Localization of bFGF-like proteins as punctate inclusions in the preseptation myocardium of the chicken embryo. Dev Biol. 1991;146:139–147. doi: 10.1016/0012-1606(91)90454-b. [DOI] [PubMed] [Google Scholar]
- 27.Marks AR, Taubman MB, Saito A, Dai Y, Fleischer S. The ryanodine receptor/junctional channel complex is regulated by growth factors in a myogenic cell line. J Cell Biol. 1991;114:303–312. doi: 10.1083/jcb.114.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishibashi Y, et al. Negative inotropic effect of basic fibroblast growth factor on adult rat cardiac myocyte. Circulation. 1997;96:2501–2504. doi: 10.1161/01.cir.96.8.2501. [DOI] [PubMed] [Google Scholar]
- 29.Pasumarthi KB, Kardami E, Cattini PA. High and low molecular weight fibroblast growth factor-2 increase proliferation of neonatal rat cardiac myocytes but have differential effects on binucleation and nuclear morphology. Evidence for both paracrine and intracrine actions of fibroblast growth factor-2. Circ Res. 1996;78:126–136. doi: 10.1161/01.res.78.1.126. [DOI] [PubMed] [Google Scholar]
- 30.Sugi Y, Sasse J, Lough J. Inhibition of precardiac mesoderm cell proliferation by antisense oligodeoxynucleotide complementary to fibroblast growth factor-2 (FGF-2) Dev Biol. 1993;157:28–37. doi: 10.1006/dbio.1993.1109. [DOI] [PubMed] [Google Scholar]
- 31.Mima T, Ueno H, Fischman DA, Williams LT, Mikawa T. Fibroblast growth factor receptor is required for in vivo cardiac myocyte proliferation at early embryonic stages of heart development. Proc Natl Acad Sci USA. 1995;92:467–471. doi: 10.1073/pnas.92.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patstone G, Pasquale EB, Maher PA. Different members of the fibroblast growth factor receptor family are specific to distinct cell types in the developing chicken embryo. Dev Biol. 1993;155:107–123. doi: 10.1006/dbio.1993.1011. [DOI] [PubMed] [Google Scholar]
- 33.Mahmood R, Kiefer P, Guthrie S, Dickson C, Mason I. Multiple roles for FGF-3 during cranial neural development in the chicken. Development. 1995;121:1399–1410. doi: 10.1242/dev.121.5.1399. [DOI] [PubMed] [Google Scholar]
- 34.Lutin WA, et al. Hemodynamic abnormalities in fetuses with congenital heart disease. Pediatr Cardiol. 1999;20:390–395. doi: 10.1007/s002469900497. [DOI] [PubMed] [Google Scholar]