Abstract
The chemokine receptors CXCR4 and CCR5 function as coreceptors for HIV-1 entry into CD4+ cells. During the early stages of HIV infection, viral isolates tend to use CCR5 for viral entry, while later isolates tend to use CXCR4. The pattern of expression of these chemokine receptors on T cell subsets and their regulation has important implications for AIDS pathogenesis and lymphocyte recirculation. A mAb to CXCR4, 12G5, showed partial inhibition of chemotaxis and calcium influx induced by SDF-1, the natural ligand of CXCR4. 12G5 stained predominantly the naive, unactivated CD26low CD45RA+ CD45R0− T lymphocyte subset of peripheral blood lymphocytes. In contrast, a mAb specific for CCR5, 5C7, stained CD26high CD45RAlow CD45R0+ T lymphocytes, a subset thought to represent previously activated/memory cells. CXCR4 expression was rapidly up-regulated on peripheral blood mononuclear cells during phytohemagglutinin stimulation and interleukin 2 priming, and responsiveness to SDF-1 increased simultaneously. CCR5 expression, however, showed only a gradual increase over 12 days of culture with interleukin 2, while T cell activation with phytohemagglutinin was ineffective. Taken together, the data suggest distinct functions for the two receptors and their ligands in the migration of lymphocyte subsets through lymphoid and nonlymphoid tissues. Furthermore, the largely reciprocal expression of CXCR4 and CCR5 among peripheral blood T cells implies distinct susceptibility of T cell subsets to viral entry by T cell line-tropic versus macrophage-tropic strains during the course of HIV infection.
Chemokines and their receptors are postulated to direct migration of distinct leukocyte subsets to sites of inflammation and to their specific niches in lymphoid organs (1, 2). Lymphocyte migration experiments in vivo have revealed preferential pathways of recirculation for certain subsets of T cells (3). Among T lymphocytes, the naive CD45RA+ subset is the predominant lymph node homing subset, whereas CD45R0+ memory type T cells are the predominant population migrating through peripheral tissues (1, 4, 5). Virtually all T cell chemoattractants described to date selectively attract memory/activated T cells (6–9). We recently have described a CXC-chemokine, SDF-1, that attracts unactivated, freshly isolated peripheral blood lymphocytes with high efficacy (10). SDF-1 attracts lymphocytes and monocytes, but not neutrophils, in vitro and in vivo (10). SDF-1 is unusually well conserved between mouse and man (11), and its gene is located on chromosome 10, while the other CXC- and CC-chemokines cluster on chromosomes 4 and 17, respectively. SDF-1 functions in the development of nonhematopoietic organs, including the heart, and in B lymphocyte development, and its knockout is lethal (12). SDF-1 signals through a seven-transmembrane-domain receptor previously known as LESTR/fusin, now designated CXC-chemokine receptor 4 (CXCR4) (13, 14).
The importance of chemokine receptors for HIV entry and AIDS pathogenesis has been illustrated by numerous publications over the last year. Before its identity as a chemokine receptor was known, CXCR4 was shown to mediate entry of T cell line-tropic (T-tropic) HIV-1 strains (15), a process that subsequently was shown to be inhibited by SDF-1 (13, 14). The CC-chemokine receptors CCR5, and to a lesser extent CCR3 and CCR2b, mediate entry of macrophage-tropic (M-tropic) viral strains (16–20). The importance of chemokine receptors in HIV-1 pathogenesis is underscored by the observation that individuals deficient in CCR5 and peripheral blood mononuclear cells (PBMC) from these individuals are resistant to infection by HIV-1 (19, 21–24).
Thus, the expression of chemokine receptors and their regulation is thought to influence lymphocyte migration, as well as HIV infection and AIDS pathogenesis. Here, using a specific mAb, we report on the expression of CXCR4 on leukocytes and compare this expression with that of CCR5. Our findings show distinct expression patterns as well as differential regulation during phytohemagglutinin (PHA) stimulation and interleukin 2 (IL-2) priming of the two coreceptors on T lymphocytes.
MATERIALS AND METHODS
Cells, Cell Lines, and Cell Culture.
Human PBMC, neutrophils, and eosinophils were obtained from healthy donors as described (10, 25). For lymphocyte stimulation experiments, freshly prepared PBMC were seeded into 24 well-plates (Corning) at 2 × 106 cells per ml in RPMI 1640 medium with 10% fetal calf serum containing either 150 units/ml recombinant IL-2 (kindly provided by Antonio Lanzavecchia, Basel, Switzerland) or 2.5 μg/ml PHA (Sigma). Half the volume was replaced with fresh medium supplemented with IL-2 and PHA, respectively, on days 6 and 9. The stably CXCR4-transfected Chinese hamster ovary cell line 1C2 was grown as described (26).
mAbs.
A mAb to CXCR4, termed 12G5, was generated by immunizing BALB/c mice with Sup-T1 cells that were chronically infected with the SIVmac variant CP-MAC (27). mAbs to CCR5 were generated in C57/BL6 mice using CCR5-transfected L1.2 cells (L.W. and C.R.M., unpublished results), two of which (5C7 and biotinylated 3D8) were used in this study. These mAbs specifically stain L1.2 cells transfected with CCR5 but not other CC-chemokine receptors. In addition, these mAbs are unreactive against leukocytes from CCR5-deficient individuals. Phycoerythrin (PE)-conjugated mAbs to CD20 (L27), CD26 (L272), CD56 (MY31), CD69 (L78), CD45R0 (UCHL-1), and a peridinin-chlorophyll-protein (PerCP)-conjugated mAb to CD3 (SK7) as well as PE- and PerCP-labeled control antibodies were obtained from Becton Dickinson. A PE-conjugated mAb to CD45RA (MEM56) was purchased from Caltag Laboratories (Burlingame, CA). Three-color flow cytometry was carried out by incubating 2 × 105 cells in PBS/1% BSA/5 mM EDTA for 30 min with supernatant or purified antibody at a final concentration of 5 μg/ml. Cells were washed, incubated with a 1:50 dilution of a fluorescein isothiocyanate-conjugated goat anti-mouse Ig F(ab′)2 (Caltag Laboratories) for 20 min and washed again before incubation with 10% normal mouse serum in PBS to block unoccupied sites on the goat anti-mouse Ig F(ab′)2. Finally, the appropriate directly conjugated mAb(s) were added. Cells were washed and analyzed using a FACScan (Becton Dickinson). Fluorescence intensity of chemokine receptor-expressing cells was quantitated in terms of number of molecules of equivalent soluble fluorochrome (see Table 1) using Quantum 26 beads (Flow Cytometry Standards, San Juan, PR) according to the manufacturer’s recommendations.
Table 1.
Surface expression of CXCR4 is restricted to mononuclear cells
| Leukocyte subset | CXCR4 expression, % positive* | Specific fluorescence intensity of 12G5-positive cells, MESF† |
|---|---|---|
| Neutrophils | 0.4 ± 0.3 | — |
| Eosinophils | 0.0 ± 0.2 | — |
| T lymphocytes (CD3+ cells) | 41.6 ± 16.1 | 3580 ± 1423 |
| B lymphocytes (CD20+ cells) | 53.5 ± 18.4 | 4989 ± 2264 |
| Natural killer cells (CD56+ cells) | 0.2 ± 0.3 | — |
| Monocytes (CD14+ cells) | 20.4 ± 9.4 | 1525 ± 537 |
| Jurkat | 90 | 9369 |
The percentage of cells stained with control mAb was <3%, and it was subtracted. Results are mean ± SD of eight experiments.
The fluorescence intensity (MESF, molecules of equivalent soluble fluorochrome) of leukocytes stained with a control mAb was 37 for lymphocytes and 1622 for monocytes, and these values were subtracted. Results are mean ± SD.
Chemokines and Chemotaxis Assay.
Synthetic full-length human SDF-1α-(1–67) and its N-terminal truncation form SDF-1α-(6–67) (10, 13) were a kind gift of Ian Clark-Lewis (Vancouver, Canada). MIP-1β was purchased from Genzyme. Chemotaxis assays were carried out as described (10, 13) with the exception that PBMC were migrated for 2 h. Briefly, 5 × 105 PBMC or T cell blasts in 100 μl of RPMI 1640 medium containing 0.25% human serum albumin were transmigrated through 5-μm pore-size bare filter Transwell inserts (Costar). Migrated cells were counted by FACS analysis scatter-gating on lymphocytes. For antibody inhibition, cells were incubated for 15 min with differing concentrations of mAb to CXCR4 (12G5) or CCR3 (7B11) before addition to the top chamber of the chemotaxis assay. Chemotaxis then was carried out in the presence of mAb to an optimal concentration of 1.5 μg/ml SDF-1α-(1–67).
Calcium Fluorimetry.
Measurement of intracellular calcium was carried out as described (13). Briefly, CXCR4 stably transfected CHO cells, termed 1C2, were loaded with fura-2 AM (Molecular Probes) and added in the presence or absence of mAb to a stirred cuvette of a Hitachi F2000 spectrometer (Hitachi Instruments, Danbury, CT). Chemokines were added to a final concentration of 1 μg/ml.
RESULTS
A CXCR4-Specific mAb, 12G5, Inhibits SDF-1-Mediated Cellular Responses in Lymphocytes and CXCR4 Transfectants.
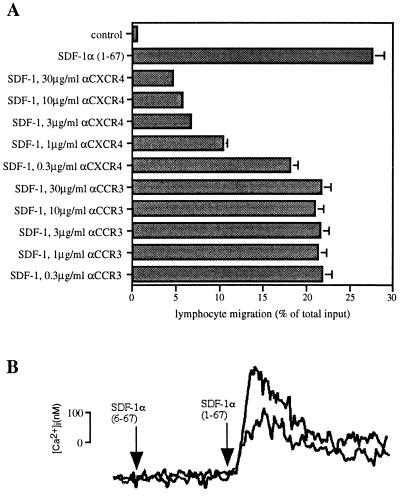
A mAb, termed 12G5, that had been generated by immunizing BALB/c mice with Sup-T1 cells chronically infected with the SIVmac variant CP-MAC was shown to specifically bind the HIV-1 coreceptor CXCR4 (27). Because the CXC-chemokine SDF-1 uses this receptor, we tested the 12G5 mAb for inhibition of cellular responses mediated by SDF-1. mAb 12G5 inhibited SDF-1-induced chemotaxis of freshly purified lymphocytes (Fig. 1A) and monocytes (data not shown) in a concentration-dependent manner, while a mAb to the CC-chemokine receptor 3 (CCR3) (28), termed 7B11, was ineffective in the same assay. However, inhibition was consistently incomplete in multiple experiments. This could be explained by the presence of additional receptors for SDF-1 on lymphocytes or by incomplete interference by mAb 12G5 of SDF-1 signaling through CXCR4. To address this question we tested 12G5 for inhibition of SDF-1-mediated increases in intracellular calcium concentrations in CHO cells stably transfected with CXCR4 (26). At 10 μg/ml, 12G5 mAb inhibited about 50% of SDF-1-induced calcium influx, and 50 μg/ml of 12G5 mAb gave no further inhibition (Fig. 1B). Because untransfected CHO cells are unresponsive to SDF-1 (13, 14) this experiment shows that the 12G5 mAb inhibits CXCR4-mediated responses to SDF-1 only partially. Therefore, only partial inhibition by 12G5 mAb of lymphocyte chemotaxis to SDF-1 would be expected, although the presence of an additional receptor on lymphocytes cannot be discounted.
Figure 1.
The CXCR4-specific mAb 12G5 partially blocks SDF-1-induced cellular responses. (A) mAb 12G5 blocks the majority of SDF-1-induced lymphocyte chemotaxis. Freshly isolated PBMC were preincubated with the indicated concentrations of mAbs to CXCR4 (12G5) and CCR3 (7B11) for 15 min and subsequently added to the top chamber of bare filter Transwell inserts. SDF-1 at the optimal concentration of 1.5 μg/ml was added to the bottom chamber. Transmigrated cells were counted by flow cytometry scatter-gating on the lymphocytes. Results are shown as percentage of input. The experiment shown was representative of four independent experiments. (B) 12G5 partially blocks SDF-1-induced increases in intracellular calcium. CXCR4 stably transfected CHO cells (1C2) loaded with fura-2 AM were exposed to 1 μg/ml of full-length SDF-1α-(1–67) or truncated inactive SDF-1α-(6–67) (negative control) in the presence (lower reading) or absence (upper reading) of 10 μg/ml 12G5 mAb. Using the mAb at 50 μg/ml produced identical results.
Expression of CXCR4 on Leukocyte Subsets.
CXCR4 mRNA has been reported to be widely expressed in leukocytes and related cell lines (26, 29), yet SDF-1 acts only on certain leukocyte subsets (10). Expression of CXCR4 was measured on leukocyte subsets by flow cytometry using mAb 12G5. CXCR4 was expressed on monocytes and lymphocyte subsets with some donor variation and not on granulocytes (Table 1). This pattern of expression coincides with our previous finding that SDF-1 is an efficacious chemoattractant for lymphocytes and monocytes but not neutrophils (10).
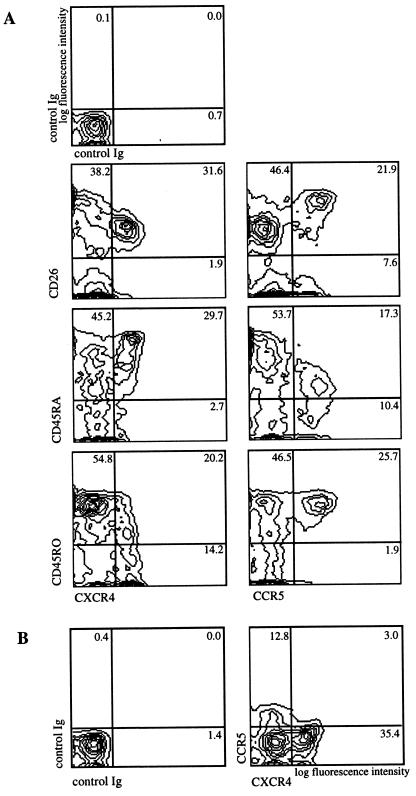
Chemokines are thought to direct lymphocyte subset specific migration (1, 2). Functional subsets among T lymphocytes, namely the so-called naive and memory subsets, can be distinguished by the expression of surface antigens such as CD45RA, CD45R0, CD2, and CD11a (30). These subsets have been shown to be biased for distinct routes of recirculation in vivo (3, 31) and to show different chemotactic properties in vitro (6–9). We therefore carried out three-color flow cytometry using PBMC to determine the expression pattern of CXCR4 on CD3+ T lymphocyte subsets. Fig. 2A, Left shows that CXCR4 is expressed predominantly on CD26low CD45RA+ CD45R0− T lymphocytes, the T cell subset referred to as naive (32), although CD45RA expression may be more a marker for resting, unactivated cells (4). CXCR4 was evenly distributed on CD4+ and CD8+ subsets (data not shown). We compared this novel expression pattern of CXCR4 with that of CCR5, the other major HIV-1 coreceptor (16–20) using a mAb generated against CCR5 L1.2 transfectants, 5C7 (L.W. and C.R.M., unpublished results). CCR5 is expressed on a reciprocal subset of T lymphocytes compared with CXCR4-expressing cells (Fig. 2A). The CCR5+ subset was CD26high CD45RAlow CD45R0+, corresponding to an activated/memory subset (32, 33). In an attempt to demonstrate that CXCR4- and CCR5-expressing T lymphocytes in the peripheral blood belong predominantly to mutually exclusive subsets, three-color flow cytometry was carried out using mAb to CXCR4 and CCR5. Fig. 2B shows that the HIV coreceptors CXCR4 and CCR5 are largely expressed on distinct, mutually exclusive subsets of freshly isolated peripheral blood lymphocytes.
Figure 2.
The HIV coreceptors CXCR4 and CCR5 are expressed on distinct T lymphocyte subsets. (A) Three-color flow cytometry of freshly isolated PBMC shows expression on distinct T lymphocyte subsets. Two-dimensional contour plots show CXCR4 (12G5 mAb) and CCR5 (5C7 mAb) expression versus CD26, CD45RA, and CD45R0. (Top Left) Staining using irrelevant control antibodies. Lymphocytes were gated according to their forward and side scatter, and CD3-PerCP staining. (B) Two-dimensional contour plot shows expression of CXCR4 (12G5 mAb) versus CCR5 (biotinylated 3D8 mAb) on T cells. T cells were analyzed as in A. Percentages of cells in the respective quadrants are indicated.
The HIV Coreceptors CXCR4 and CCR5 Are Differentially Regulated After T Lymphocyte Activation and IL-2 Priming.
Because CXCR4 and CCR5 are expressed predominantly on resting naive and activated/memory type T lymphocytes, respectively, we determined receptor expression during T cell activation using three-color immunofluorescence flow cytometry. Freshly prepared PBMC were cultured in media containing either PHA (2.5 μg/ml) or IL-2 (150 units/ml) for up to 12 days. Responsiveness of stimulated lymphocytes to SDF-1 and MIP-1β as well as receptor expression was determined on days 0, 3, 6, 9, and 12. Although there is promiscuity among CC-chemokines and their receptors, MIP-1β is thought to be specific for CCR5, because PBMC from individuals deficient in CCR5 are unresponsive to MIP-1β while responses to other CC-chemokines are normal (22).
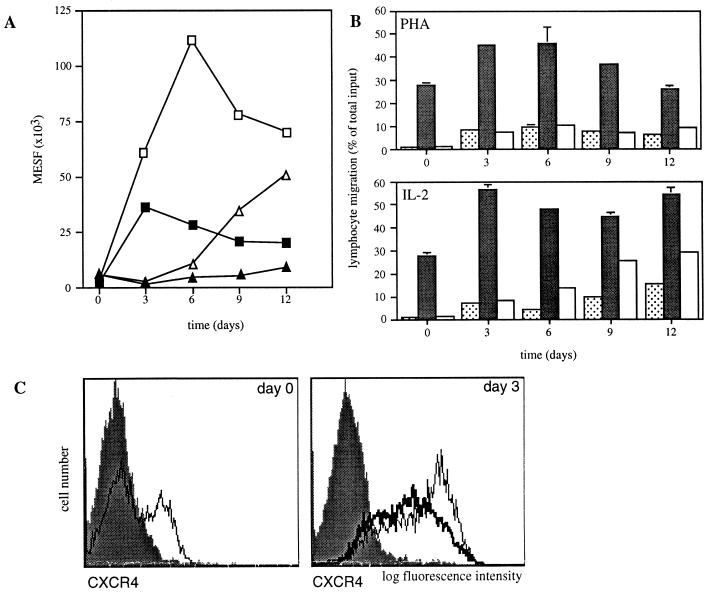
PHA stimulation induced a rapid up-regulation of CXCR4 within the first 72 h with >80% of CD3+ blasts staining positive (Fig. 3 A and C). This increase in receptor expression correlated with an increased number of lymphocytes responding to SDF-1 in the chemotaxis assay (Fig. 3B). In contrast, CCR5 expression was consistently unchanged during the first 12 days of PHA stimulation (Fig. 3A). In fact, on day 3 during PHA stimulation, decreased expresssion of CCR5 compared with freshly isolated PBMC was observed. Similar results were obtained for T cell activation using immobilized anti-CD3 (data not shown).
Figure 3.
CXCR4 and CCR5 are differentially regulated during PHA stimulation and IL-2 priming. (A) Expression of CXCR4 (squares) and CCR5 (triangles) changes during PHA stimulation (filled symbols) and IL-2 priming (open symbols). Immunofluorescent staining with 12G5 mAb to CXCR4 and 5C7 mAb to CCR5 is expressed for the entire population as number of molecules of equivalent soluble fluorochrome (MESF) from one representative experiment of three. Fluorescent intensity of the entire population for CCR5 decreased on day 3 as the CCR5-positive subset of T cells was markedly reduced. T cells were gated according to their forward and side scatter, and CD3-PerCP staining in A and C. (B) Chemotactic response to SDF-1 and MIP-1β correlates with expression of CXCR4 and CCR5, respectively. T cells migrated to control medium (dotted columns), SDF-1α-(1–67) (1.5 μg/ml) (filled columns), and MIP-1β (100 ng/ml) (open columns). Shown is chemotaxis of PBMC stimulated with PHA (Upper) or primed with IL-2 (Lower) for the indicated number of days. Bars indicate the range of duplicates. (C) CXCR4 is rapidly up-regulated during PHA stimulation and IL-2 priming. CXCR4 expression was measured in freshly isolated lymphocytes (thin line, Left), in day 3 PHA-stimulated T cell blast (thick line, Right), and in day 3 IL-2 stimulated T cells (thin line, Right). The filled bar graphs indicate staining with an unrelated control mAb.
IL-2 recently was found to be a potent inducer of CC-chemokine receptor mRNA and to increase responsiveness to CC-chemokines in CD45R0+ T lymphocytes (34). Consistent with these findings, CCR5 expression gradually increased over 12 days in the presence of 150 units/ml IL-2, and specific migration to MIP-1β above background became detectable (Fig. 3 A and B). CXCR4 expression also was rapidly up-regulated by IL-2, but peaked earlier on day 6 after a 32-fold increase in the number of molecules of equivalent soluble fluorochrome as determined for the entire population of CD3+ lymphocytes (mean of three independent experiments) (Fig. 3A). In long-term (>3 weeks) cultures of CD3 activated, IL-2-stimulated T cells, CXCR4 expression gradually declined to levels approaching background, whereas CCR5 expression on these cells was high (data not shown).
DISCUSSION
Chemokine receptors play an important role during the early events of HIV-1 infection of CD4+ cells. CXCR4 is the fusogenic receptor that promotes entry of T-tropic HIV-1 strains, while CCR5 allows entry of M-tropic HIV-1 strains (13–20). The importance of these findings recently has been underscored by the finding that individuals deficient in CCR5 remain uninfected in the face of high-risk exposure to virus (22–24). CCR5 appears to be important during early stages of infection, whereas CXCR4-using T-tropic viruses emerge later in the progression to AIDS. Knowledge of the expression and regulation of these receptors is of prime importance for understanding HIV pathogenesis. We investigated the functional activity of a mAb to CXCR4, expression of CXCR4 and its relation to that of CCR5 on blood leukocytes, and the regulation of CXCR4 and CCR5 after T cell activation.
A mAb specific for CXCR4, termed 12G5, partially blocked SDF-1-induced chemotaxis and increases of intracellular calcium. These findings further support reports on the specificity of this mAb for CXCR4 (27). Complete inhibition of SDF-1-mediated cellular responses could not be reached even at high concentrations of antibody. Our previous work has shown that function-blocking antibodies to chemokine receptors are difficult to generate (28), and indeed many mAbs produced in our laboratory block only about 90% of functional responses (35).
CXCR4 expression on T cells and on macrophages and its absence from neutrophilic and eosinophilic granulocytes mirrors the chemotactic activity of SDF-1 for leukocyte subsets. We recently have reported that SDF-1 is an efficacious chemoattractant for lymphocytes and monocytes but is inactive on neutrophils (10). The same experiments demonstrated that SDF-1 attracts 10-fold more freshly isolated, unactivated lymphocytes than previously described lymphocyte chemoattractants, such as MIP-1α, MIP-1β, RANTES, and IL-8 (10). Here we show that the SDF-1 receptor CXCR4 is predominantly expressed on the naive, unstimulated T lymphocyte subset, a distribution for a chemokine receptor that to our knowledge has not been previously described. Our previous studies have shown that CD45RA+ and CD45R0+ T cell subsets migrate along an SDF-1 gradient with comparable efficacy (10), which indicates that the lower expression of CXCR4 on the CD45R0+ subset is sufficient to induce efficient migration. These findings indicate that receptor number is not the only determinant for efficient chemotaxis in the Transwell chemotaxis assay and that other factors that differ among subsets, like migratory capacity, also may play a role.
The expression of SDF-1 in the absence of inflammation and its high efficacy previously have lead us to propose that SDF-1 may play a role in lymphocyte recirculation (10). Studies in sheep have shown that CD45RA+ T cells are generally excluded from peripheral tissues, but migrate effectively through organized lymphoid tissues, such as lymph nodes (1, 3, 4). Migration through high endothelial venules is dependent on G-protein-coupled receptors, possibly chemokine receptors (36). The unique expression of CXCR4 on the CD26low CD45RA+ CD45R0− naive lymphocyte subset further substantiates the notion that SDF-1 may be important in lymphocyte recirculation.
We found that CCR5 is expressed on the CD26high CD45RAlow, CD45R0+ lymphocyte subset. This coincides with the expression of the receptor for the CC-chemokine MCP-1 (9). Both receptors are expressed on the activated/memory subset.
CXCR4 and CCR5 expression are differentially regulated during T cell activation and IL-2 priming. IL-2 recently has been described to potently up-regulate CCR1 and CCR2 mRNA in CD45R0+ T lymphocytes as well as the chemotactic potential to CC-chemokines in these cells (34). T cell activation, on the other hand, using PHA or anti-CD3/anti-CD28 did not induce responsiveness to CC-chemokines in these studies, and it actually counteracted the effect of IL-2 (34). Our results confirm these findings, now on the level of surface expression of CCR5, that prolonged incubation with IL-2 increases CC-chemokine receptor expression and responsiveness to CC-chemokines in T lymphocytes while T cell activation is ineffective. T cell activation, simulated in vitro by PHA stimulation, may be a way to rapidly immobilize a lymphocyte at the site of activation while exposure to IL-2 may be the mechanism to enhance its ability to migrate to this site. In contrast, CXCR4 expression during T cell activation follows a distinct pattern. CXCR4 is rapidly up-regulated after both PHA stimulation and IL-2 priming, reaching a peak within 3–6 days. Interestingly, CXCR4 expression and responsiveness to SDF-1 in day 12 PHA blasts remains high, indicating that this receptor–ligand pair may participate not only in the localization of resting lymphocytes but also in events during lymphocyte activation. Only long-term (>3 weeks) CD3/IL-2-stimulated T cells showed decreased expression of CXCR4. These long-term cultured T cells resemble in many ways circulating CD45R0+ T cells, which also express low levels of CXCR4.
CXCR4 and CCR5 are the predominant chemokine receptors used as coreceptors in HIV-1 entry, and as such their expression pattern is important for determining viral tropism. CXCR4 and CCR5 can be used by a wide variety of HIV-1 strains from different clades (37). Thus, although some HIV-1 strains can use other chemokine receptors for entry, CXCR4 and CCR5 represent the model coreceptors for T-tropic and M-tropic HIV-1 strains, respectively (15–20). In the course of human HIV infection, M-tropic virus strains predominate during the early phase of infection, while dual-tropic and T-tropic strains occur late during disease progression to AIDS. In this report, we identified the T lymphocyte subsets expressing the two coreceptors and found them to be largely mutually exclusive. These findings suggest that in progression to AIDS during the shift from M- to dual- and finally T-tropic virus strains, the target cell population will shift to a different lymphocyte subset. This crucial point in AIDS pathogenesis is still poorly understood, and further work will be necessary to elucidate the importance of our in vitro findings. CXCR4 usage as a coreceptor may render a much larger population of lymphocytes susceptible to HIV-1 late in the course of infection.
Coreceptor expression may limit viral entry in quiescent lymphocytes. It has been appreciated by many groups that freshly purified, unstimulated T lymphocytes are not susceptible to productive infection with HIV-1 and its associated cytopathology (38–41). While this may be due to post-entry blocks (39), data from Engleman’s group suggest that fusion of a cell line expressing env glycoproteins of the T-tropic HXBc2 strain with unactivated CD4+ T lymphocytes as measured in a syncytium formation assay occurs at a significantly lower rate than with PHA- or anti-CD3-activated T cells (38, 40). In other words, membrane fusion using a cell line constitutively expressing env glycoproteins of a T-tropic HIV-1 strain was enhanced when the CD4+ T lymphocyte fusion partner previously was activated. These results may relate to our finding that CXCR4 is efficiently up-regulated after T cell activation. Furthermore, the apparent down-modulation of CCR5 in stimulated T lymphocytes may explain the recently observed resistance of CD3/CD28 stimulated CD4+ T cells to infection by a M-tropic HIV-1 strain (42).
The characterization of functionally important chemokine receptors in T cells with mAbs opens up many avenues for future work. Understanding the precise role of CXCR4 and CCR5 in lymphocyte recirculation in vivo and in AIDS pathogenesis are important goals for further work.
Acknowledgments
We are indebted to Nasim Kassam and Heidi Heath for excellent technical assistance. We thank Dr. Ian Clark-Lewis for supplying SDF-1 protein and Drs. Joe Sodroski and Walter Newman for critical comments on the manuscript. C.C.B. was supported by a grant from the Deutsche Forschungsgemeinschaft. This work was also supported by National Institutes of Health grant HL 48675.
ABBREVIATIONS
- CXCR4
CXC-chemokine receptor 4
- CCR5
CC-chemokine receptor 5
- T-tropic
T cell line-tropic
- M-tropic
macrophage-tropic
- PBMC
peripheral blood mononuclear cells
- PHA
phytohemagglutinin
- IL-2
interleukin 2
- PE
phycoerythrin
References
- 1.Springer T A. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 2.Mackay C R. J Exp Med. 1996;184:799–802. doi: 10.1084/jem.184.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mackay C R, Marston W L, Dudler L. J Exp Med. 1990;171:801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackay C R. Adv Immunol. 1993;53:217–265. doi: 10.1016/s0065-2776(08)60501-5. [DOI] [PubMed] [Google Scholar]
- 5.Butcher E C, Picker L J. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 6.Schall T J, Bacon K, Toy K J, Goeddel D V. Nature (London) 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 7.Taub D D, Conlon K, Lloyd A R, Oppenheim J J, Kelvin D J. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 8.Carr M W, Roth S J, Luther E, Rose S S, Springer T A. Proc Natl Acad Sci USA. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin S, LaRosa G, Campbell J J, Smith-Heath H, Kassam N, Shi X, Zeng L, Butcher E C, Mackay C R. Eur J Immunol. 1996;26:640–647. doi: 10.1002/eji.1830260320. [DOI] [PubMed] [Google Scholar]
- 10.Bleul C C, Fuhlbrigge R C, Casasnovas J M, Aiuti A, Springer T A. J Exp Med. 1996;184:1101–1110. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirozu M, Nakano T, Inazawa J, Tashiro K, Tada H, Shinohara T, Honjo T. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 12.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S-I, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Nature (London) 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 13.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. Nature (London) 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 14.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J-L, Arenzana-Seisdedos A, Schwartz O, Heard J-M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. Nature (London) 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 16.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 17.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Nature (London) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 18.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 19.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 20.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 21.Paxton W A, Martin S R, Tse D, O’Brien T R, Skurnick J, VanDevanter N L, Padian N, Braun J F, Kotler D P, Wolinsky S M, Koup R A. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 22.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 23.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, et al. Nature (London) 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 24.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, et al. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 25.Ponath P D, Qin S, Ringler D J, Clark-Lewis I, Wang J, Kassam N, Smith H, Shi X, Gonzalo J-A, Newman W, Gutierrez-Ramos J-C, Mackay C R. J Clin Invest. 1996;97:604–612. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loetscher M, Geiser T, O’Reilly T, Zwahlen R, Baggiolini M, Moser B. J Biol Chem. 1994;269:232–237. [PubMed] [Google Scholar]
- 27.Endres M J, Clapham P R, Marsh M, Ahuja M, Davis-Turner J, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N C, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 28.Heath H, Qin S, Rao P, Wu L, LaRosa G, Kassam N, Ponath P D, Mackay C R. J Clin Invest. 1997;99:178–184. doi: 10.1172/JCI119145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reppert S M, Weaver D R, Stehle J H, Rivkees S A, Grabbe S, Granstein R D. Mol Cell Neurosci. 1992;3:206–214. doi: 10.1016/1044-7431(92)90040-9. [DOI] [PubMed] [Google Scholar]
- 30.Sanders M E, Makgoba M W, Sharrow S O, Stephany D, Springer T A, Young H A, Shaw S. J Immunol. 1988;140:1401–1407. [PubMed] [Google Scholar]
- 31.Mackay C. Curr Opin Immunol. 1993;5:423–427. doi: 10.1016/0952-7915(93)90063-x. [DOI] [PubMed] [Google Scholar]
- 32.Akbar A N, Terry L, Timms A, Beverley P C L, Janossy G. J Immunol. 1988;140:2171–2178. [PubMed] [Google Scholar]
- 33.Morimoto C, Torimoto Y, Levinson G, Rudd C E, Schreiber M, Dang N H, Letvin N L, Schlossman S F. J Immunol. 1989;143:3430–3439. [PubMed] [Google Scholar]
- 34.Loetscher P, Seitz M, Baggiolini M, Moser B. J Exp Med. 1996;184:569–577. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu L, Ruffing N, Shi X, Newman W, Soler D, Mackay C R, Qin S. J Biol Chem. 1996;271:31202–31209. doi: 10.1074/jbc.271.49.31202. [DOI] [PubMed] [Google Scholar]
- 36.Bargatze R F, Butcher E C. J Exp Med. 1993;178:367–372. doi: 10.1084/jem.178.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Huang Y, He T, Cao Y, Ho D D. Nature (London) 1996;383:768. doi: 10.1038/383768a0. [DOI] [PubMed] [Google Scholar]
- 38.Gowda S D, Stein B S, Mohagheghpour N, Benike C J, Engleman E G. J Immunol. 1989;142:773–780. [PubMed] [Google Scholar]
- 39.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S Y. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 40.Mohagheghpour N, Chakrabarti R, Stein B S, Gowda S D, Engleman E G. J Biol Chem. 1991;266:7233–7238. [PubMed] [Google Scholar]
- 41.von Schwedler U, Kornbluth R S, Trono D. Proc Natl Acad Sci USA. 1994;91:6992–6996. doi: 10.1073/pnas.91.15.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levine B L, Mosca J D, Riley J L, Carroll R G, Vahey M T, Jagodzinski L L, Wagner K F, Mayers D L, Burke D S, Weislow O S, St. Louis D C, June C H. Science. 1996;272:1939–1943. doi: 10.1126/science.272.5270.1939. [DOI] [PubMed] [Google Scholar]