Abstract
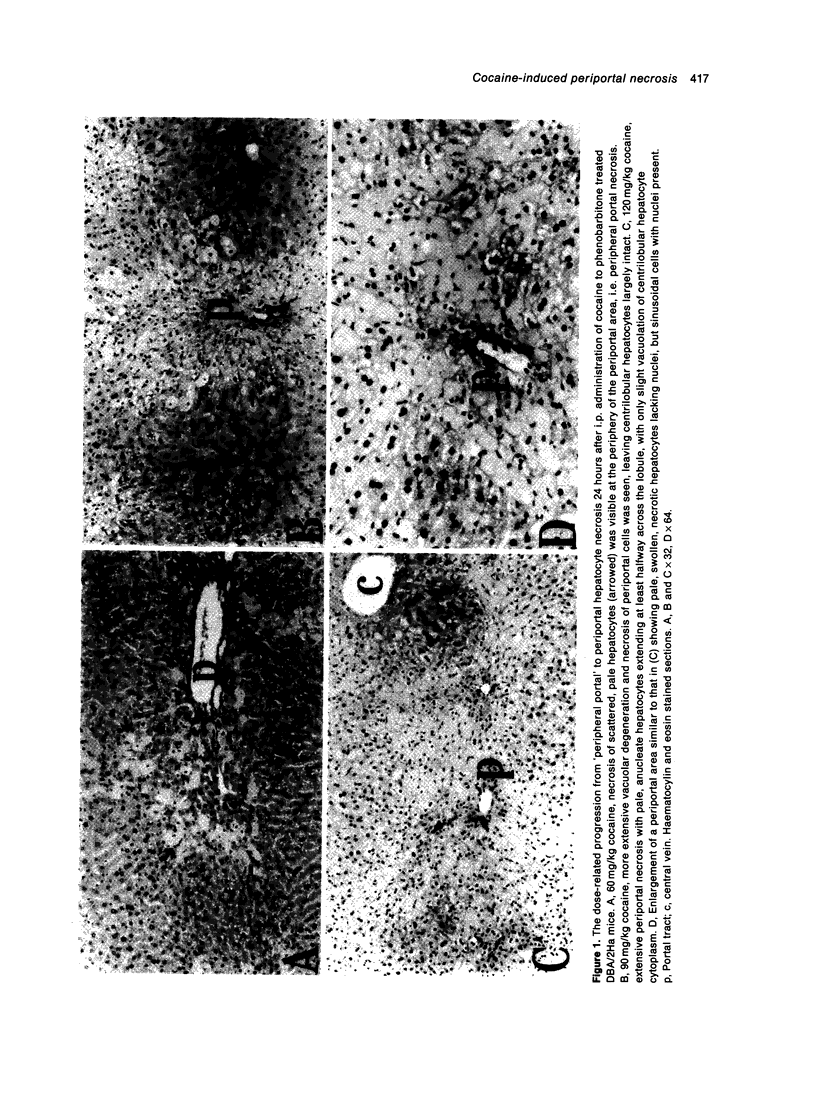
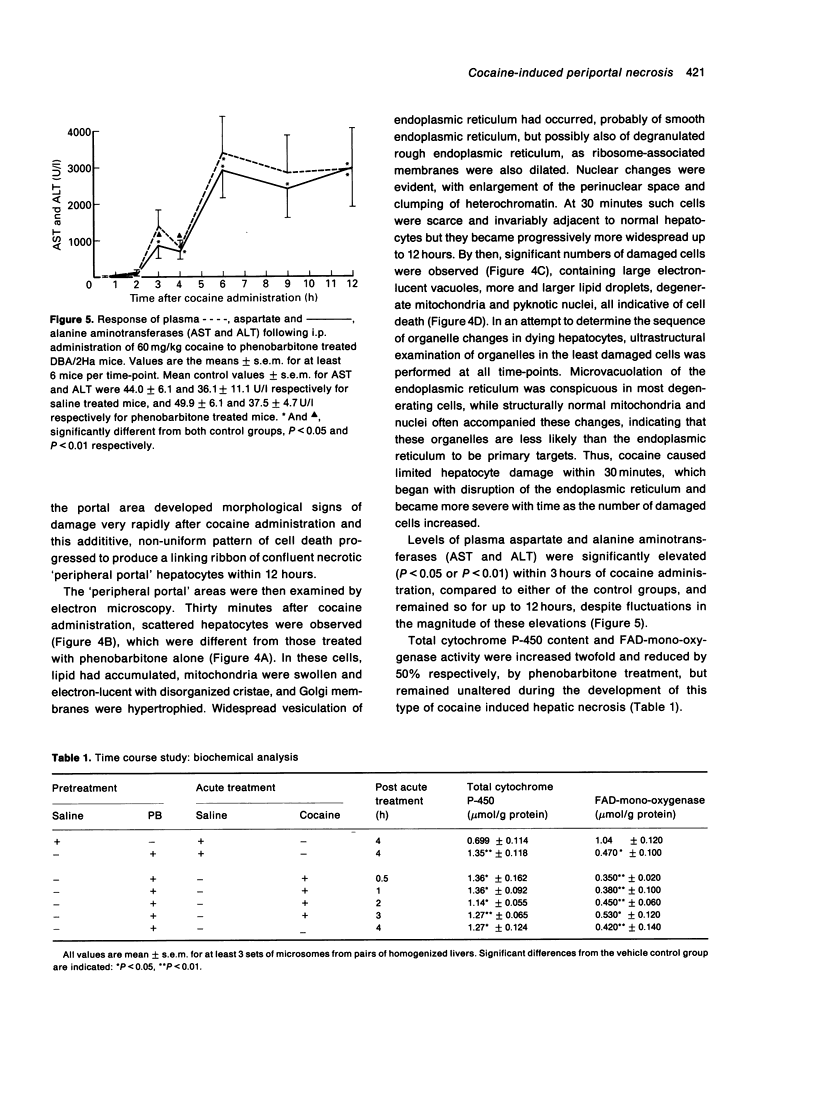
Cocaine is reported to produce either periportal or mid-zonal necrosis in mice pretreated with the enzyme inducer phenobarbitone (James et al. 1987; Powell et al. 1991; Charles & Powell 1992). Dose-response and time course experiments were performed in phenobarbitone treated male DBA/2Ha mice to study the pathogenesis of this unusual cocaine induced lesion. An increase in the dose of cocaine from 60 to 90 or 120 mg/kg produced more extensive and severe periportal and linking portal damage and elevated plasma aspartate (AST) and alanine (ALT) aminotransferases in a dose dependent manner. Scattered hepatocyte degeneration began at the edge of the periportal region and was detectable by electron microscopy within 30 minutes of administration of 60 mg/kg of cocaine, with conspicuous disorganization of the endoplasmic reticulum being one of the earliest changes. Significant elevations of plasma AST and ALT were observed 3 hours after cocaine administration and were sustained for 12 hours, at which time progressive hepatocyte damage had developed into a network of confluent necrosis at the periphery of the periportal region. The rapidity of organelle derangement and subsequent cell death, and absence of any effect on total cytochrome P-450 or FAD-mono-oxygenase levels, appear to distinguish this periportal lesion from previous reports of cocaine induced centrilobular necrosis in non-enzyme induced mice, suggesting that the two types of damage may develop by different mechanisms. The observation that periportal lesions commence at the periphery of the periportal area, progressing portalwards with increasing dose and time, offers an explanation for the previously conflicting reports of cocaine induced mid-zonal and/or periportal lesions in phenobarbitone treated mice.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boelsterli U. A., Göldlin C. Biomechanisms of cocaine-induced hepatocyte injury mediated by the formation of reactive metabolites. Arch Toxicol. 1991;65(5):351–360. doi: 10.1007/BF02284256. [DOI] [PubMed] [Google Scholar]
- Charles S. J., Powell C. J. Rapidly developing cocaine-induced peripheral portal liver damage. Toxicol Lett. 1992 Dec;64-65 Spec No:729–737. doi: 10.1016/0378-4274(92)90254-h. [DOI] [PubMed] [Google Scholar]
- Evans M. A., Harbison R. D. Cocaine-induced hepatotoxicity in mice. Toxicol Appl Pharmacol. 1978 Sep;45(3):739–754. doi: 10.1016/0041-008x(78)90167-9. [DOI] [PubMed] [Google Scholar]
- Gottfried M. R., Kloss M. W., Graham D., Rauckman E. J., Rosen G. M. Ultrastructure of experimental cocaine hepatotoxicity. Hepatology. 1986 Mar-Apr;6(2):299–304. doi: 10.1002/hep.1840060224. [DOI] [PubMed] [Google Scholar]
- Hadley M. R., Oldham H. G., Camilleri P., Murphy J., Hutt A. J., Damani L. A. Stereoselective microsomal N-oxidation of N-ethyl-N-methylaniline. Biochem Pharmacol. 1993 May 5;45(9):1739–1742. doi: 10.1016/0006-2952(93)90428-y. [DOI] [PubMed] [Google Scholar]
- Izor-Povenmire K., House M. A. Acute crack cocaine intoxication: a case study. Focus Crit Care. 1989 Apr;16(2):112–119. [PubMed] [Google Scholar]
- James R. C., Schiefer M. A., Roberts S. M., Harbison R. D. Antagonism of cocaine-induced hepatotoxicity by the alpha adrenergic antagonists phentolamine and yohimbine. J Pharmacol Exp Ther. 1987 Aug;242(2):726–732. [PubMed] [Google Scholar]
- Jover R., Ponsoda X., Gómez-Lechón J., Castell J. V. Cocaine hepatotoxicity: two different toxicity mechanisms for phenobarbital-induced and non-induced rat hepatocytes. Biochem Pharmacol. 1993 Dec 3;46(11):1967–1974. doi: 10.1016/0006-2952(93)90638-d. [DOI] [PubMed] [Google Scholar]
- Kanel G. C., Cassidy W., Shuster L., Reynolds T. B. Cocaine-induced liver cell injury: comparison of morphological features in man and in experimental models. Hepatology. 1990 Apr;11(4):646–651. doi: 10.1002/hep.1840110418. [DOI] [PubMed] [Google Scholar]
- Kloss M. W., Rosen G. M., Rauckman E. J. Acute cocaine-induced hepatotoxicity in DBA/2Ha male mice. Toxicol Appl Pharmacol. 1982 Aug;65(1):75–83. doi: 10.1016/0041-008x(82)90364-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landon E. J., Naukam R. J., Rama Sastry B. V. Effects of calcium channel blocking agents on calcium and centrilobular necrosis in the liver of rats treated with hepatotoxic agents. Biochem Pharmacol. 1986 Feb 15;35(4):697–705. doi: 10.1016/0006-2952(86)90369-2. [DOI] [PubMed] [Google Scholar]
- Mann A. H., Price S. C., Mitchell F. E., Grasso P., Hinton R. H., Bridges J. W. Comparison of the short-term effects of di(2-ethylhexyl) phthalate, di(n-hexyl) phthalate, and di(n-octyl) phthalate in rats. Toxicol Appl Pharmacol. 1985 Jan;77(1):116–132. doi: 10.1016/0041-008x(85)90273-x. [DOI] [PubMed] [Google Scholar]
- Mehanny S. Z., Abdel-Rahman M. S. Cocaine hepatotoxicity in mice: histologic and enzymatic studies. Toxicol Pathol. 1991;19(1):24–29. doi: 10.1177/019262339101900103. [DOI] [PubMed] [Google Scholar]
- Misra A. L., Pontani R. B., Vadlamani N. L. Metabolism of norcocaine, N-hydroxy norcocaine and cocaine-N-oxide in the rat. Xenobiotica. 1979 Mar;9(3):189–199. doi: 10.3109/00498257909038720. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Tayama K., Nakao T., Hiraga K. On the mechanism of butylated hydroxytoluene-induced hepatic toxicity in rats. Biochem Pharmacol. 1984 Aug 15;33(16):2669–2674. doi: 10.1016/0006-2952(84)90643-9. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Perino L. E., Warren G. H., Levine J. S. Cocaine-induced hepatotoxicity in humans. Gastroenterology. 1987 Jul;93(1):176–180. doi: 10.1016/0016-5085(87)90331-3. [DOI] [PubMed] [Google Scholar]
- Powell C. J., Connolly A. K., Charles S. J. Shifting necrosis: butylated hydroxytoluene (BHT) and phenobarbital move cocaine-induced hepatic necrosis across the lobule. Toxicol Lett. 1991 Feb;55(2):171–178. doi: 10.1016/0378-4274(91)90131-o. [DOI] [PubMed] [Google Scholar]
- Powers J. F., Alroy J., Shuster L. Hepatic morphologic and biochemical changes induced by subacute cocaine administration in mice. Toxicol Pathol. 1992;20(1):61–70. doi: 10.1177/019262339202000108. [DOI] [PubMed] [Google Scholar]
- Roth L., Harbison R. D., James R. C., Tobin T., Roberts S. M. Cocaine hepatotoxicity: influence of hepatic enzyme inducing and inhibiting agents on the site of necrosis. Hepatology. 1992 May;15(5):934–940. doi: 10.1002/hep.1840150530. [DOI] [PubMed] [Google Scholar]
- Shuster L., Quimby F., Bates A., Thompson M. L. Liver damage from cocaine in mice. Life Sci. 1977 Mar 15;20(6):1035–1041. doi: 10.1016/0024-3205(77)90291-0. [DOI] [PubMed] [Google Scholar]
- Thompson M. L., Shuster L., Casey E., Kanel G. C. Sex and strain differences in response to cocaine. Biochem Pharmacol. 1984 Apr 15;33(8):1299–1307. doi: 10.1016/0006-2952(84)90184-9. [DOI] [PubMed] [Google Scholar]
- Wanless I. R., Dore S., Gopinath N., Tan J., Cameron R., Heathcote E. J., Blendis L. M., Levy G. Histopathology of cocaine hepatotoxicity. Report of four patients. Gastroenterology. 1990 Feb;98(2):497–501. doi: 10.1016/0016-5085(90)90845-r. [DOI] [PubMed] [Google Scholar]
- Wojcik E., Dvorak C., Chianale J., Traber P. G., Keren D., Gumucio J. J. Demonstration by in situ hybridization of the zonal modulation of rat liver cytochrome P-450b and P-450e gene expression after phenobarbital. J Clin Invest. 1988 Aug;82(2):658–666. doi: 10.1172/JCI113645. [DOI] [PMC free article] [PubMed] [Google Scholar]