Abstract
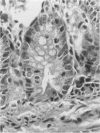
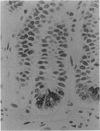
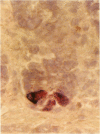
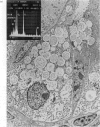
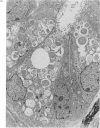
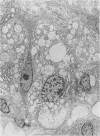
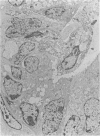
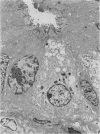
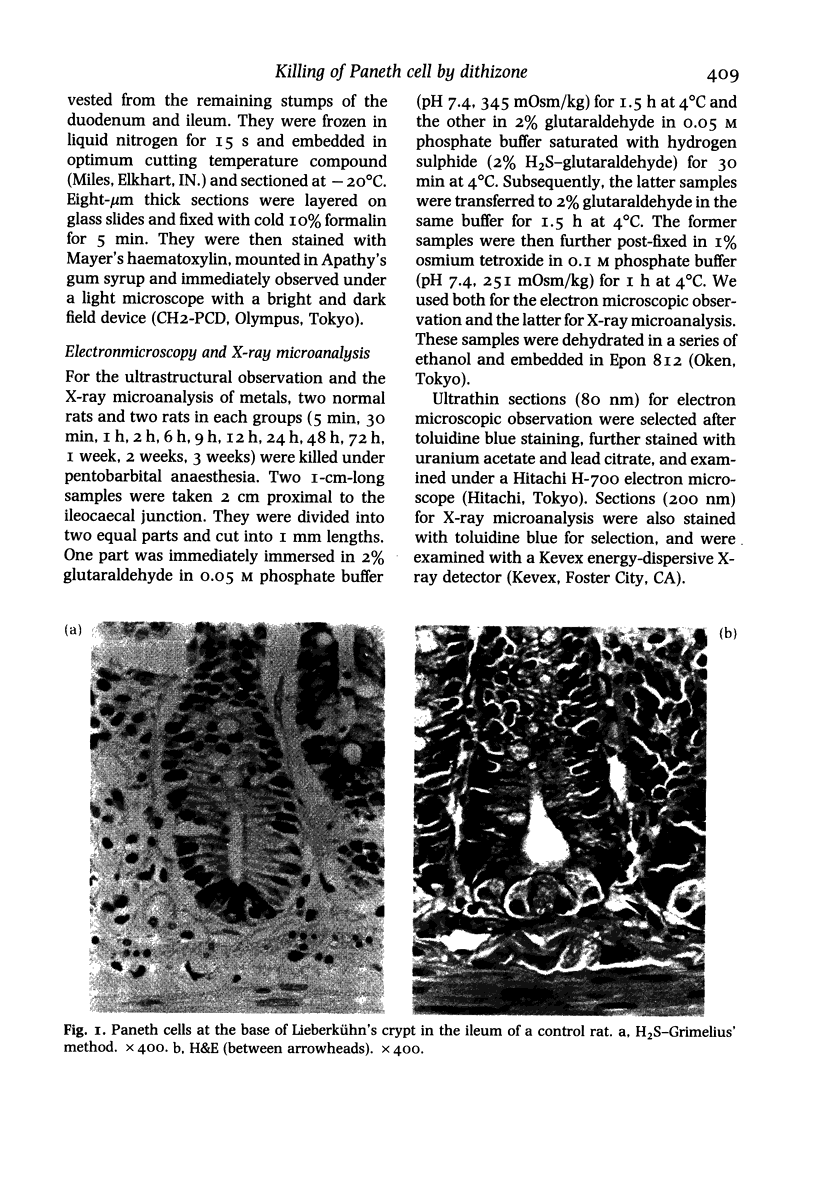
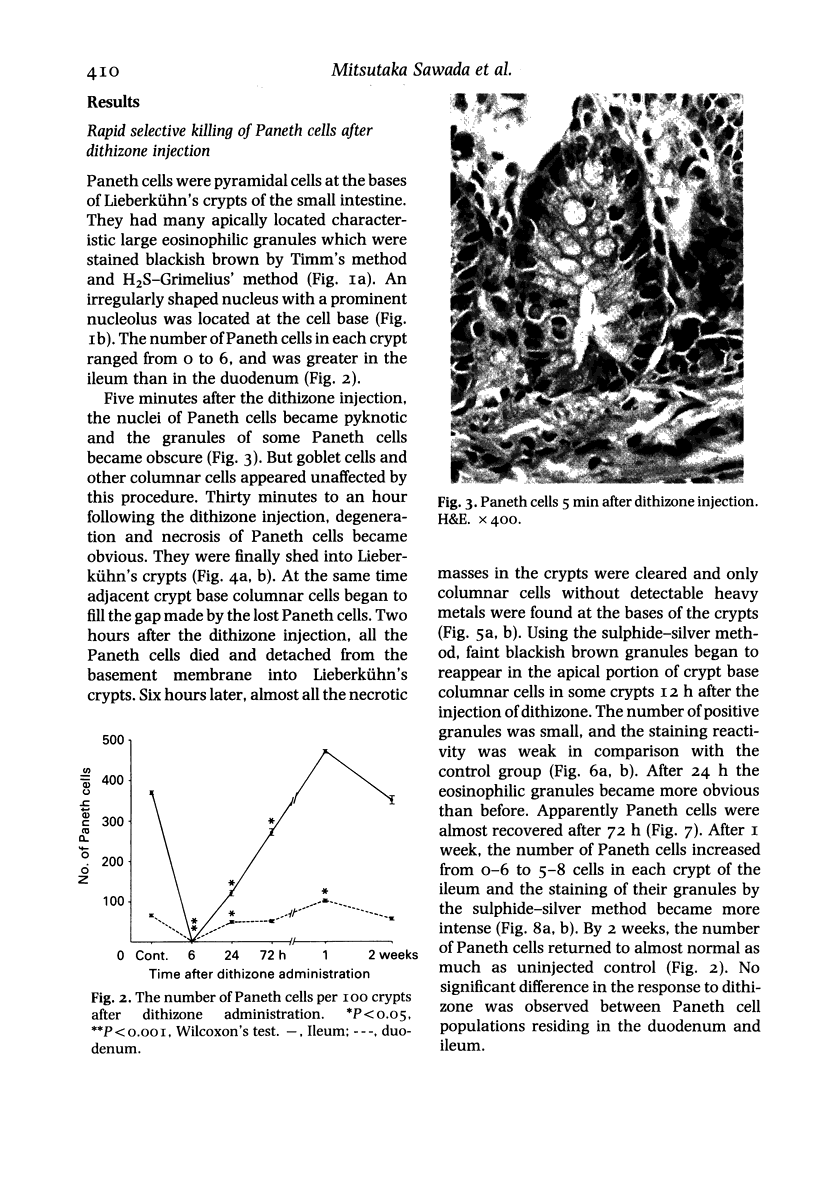
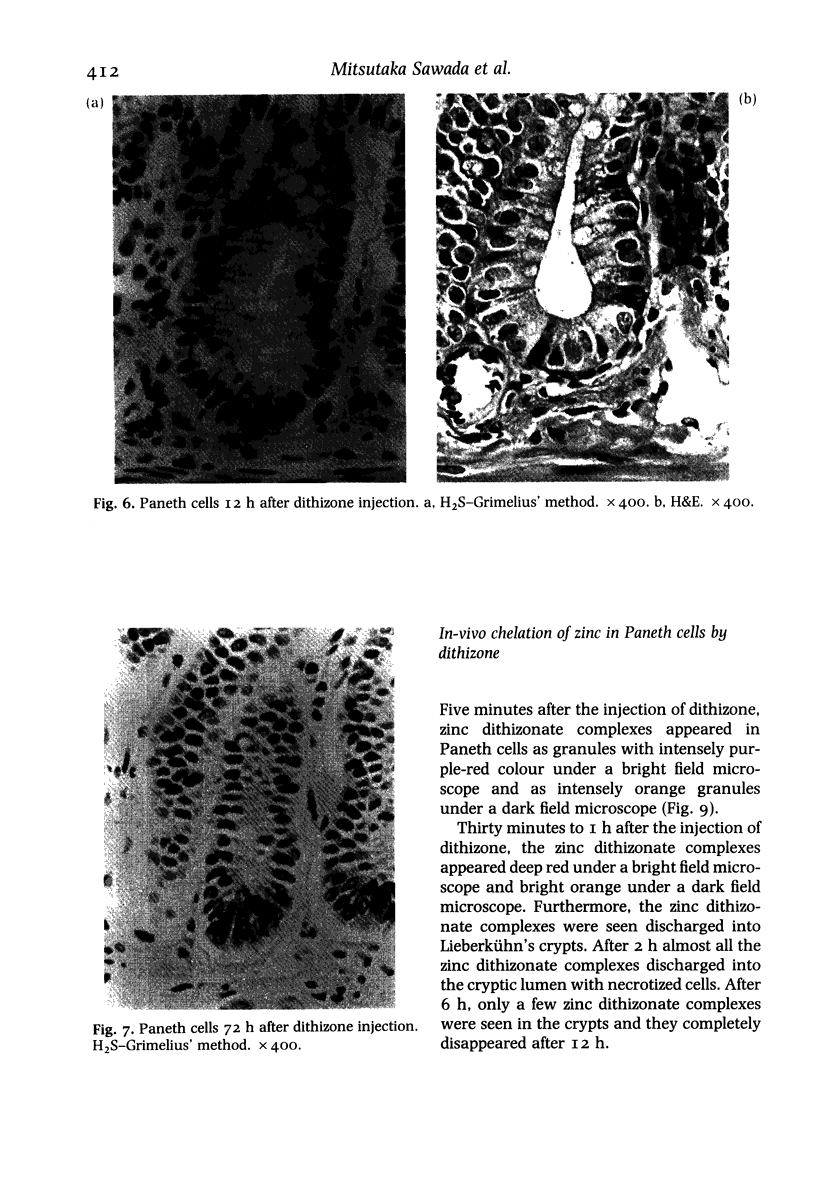
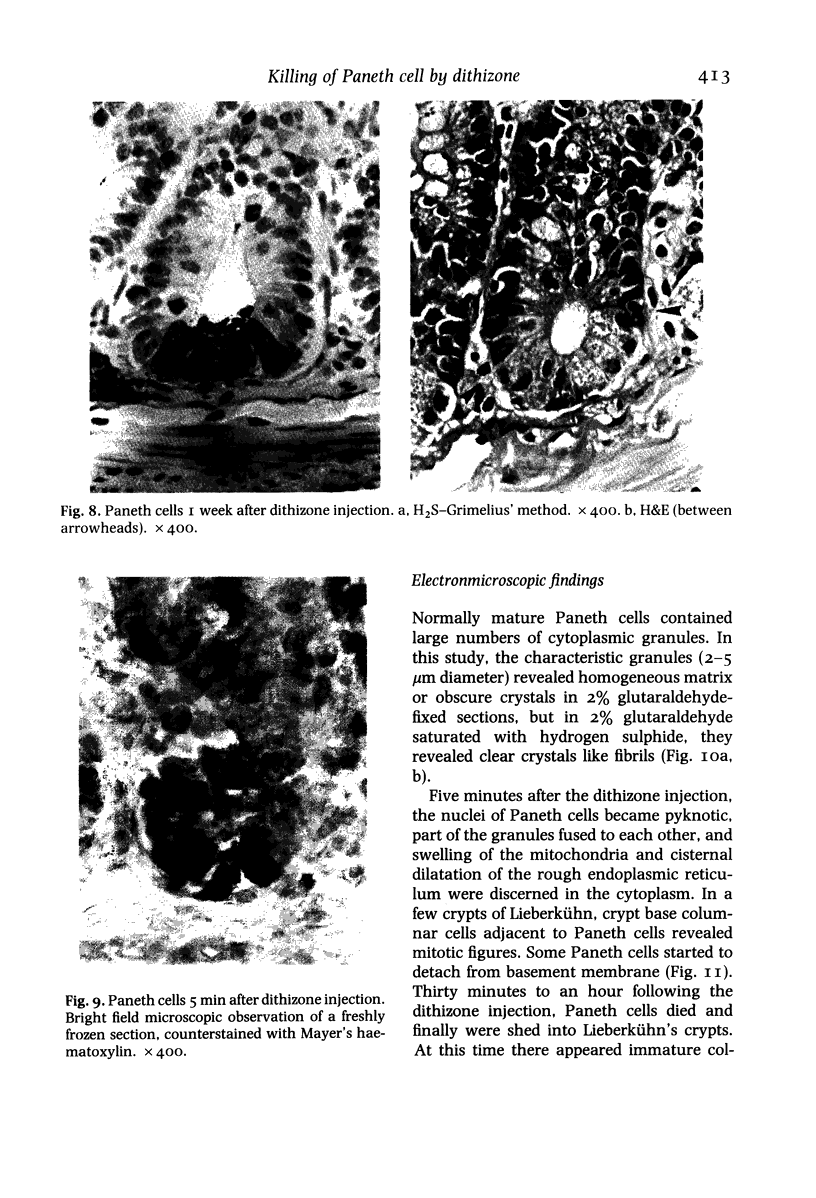
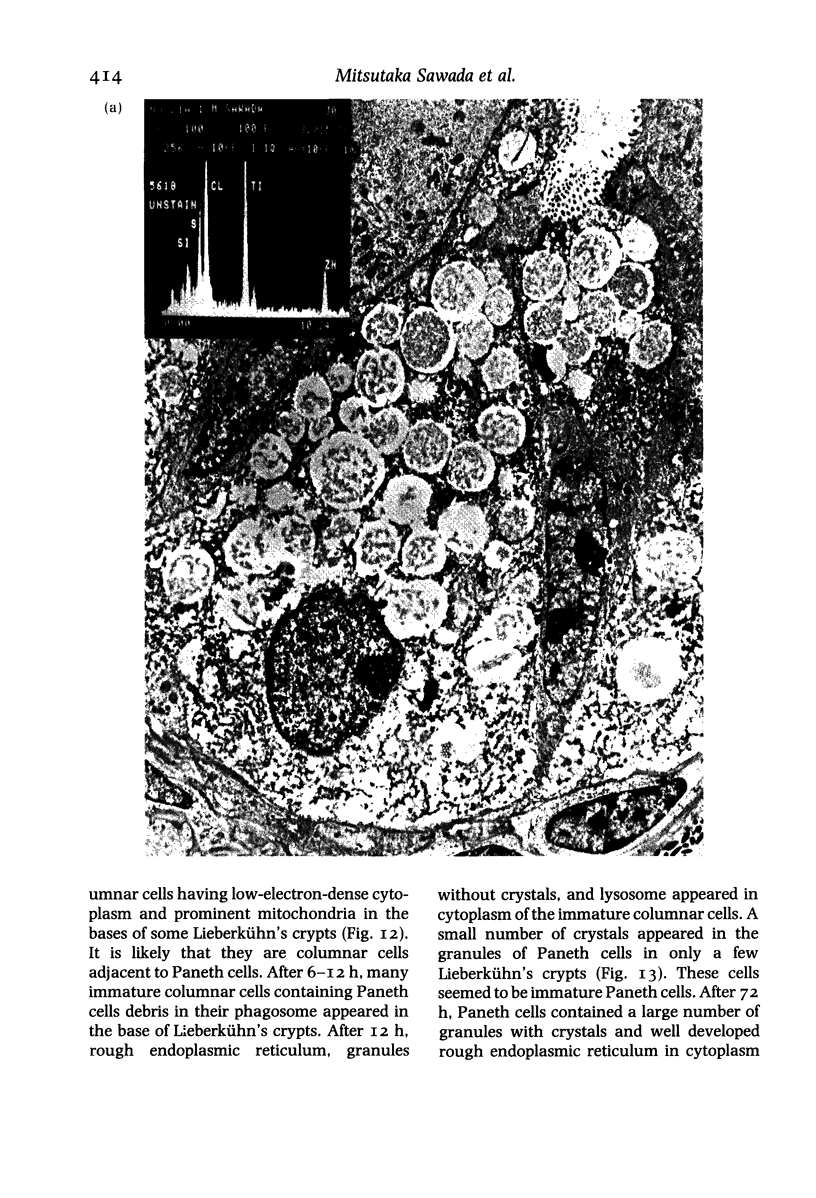
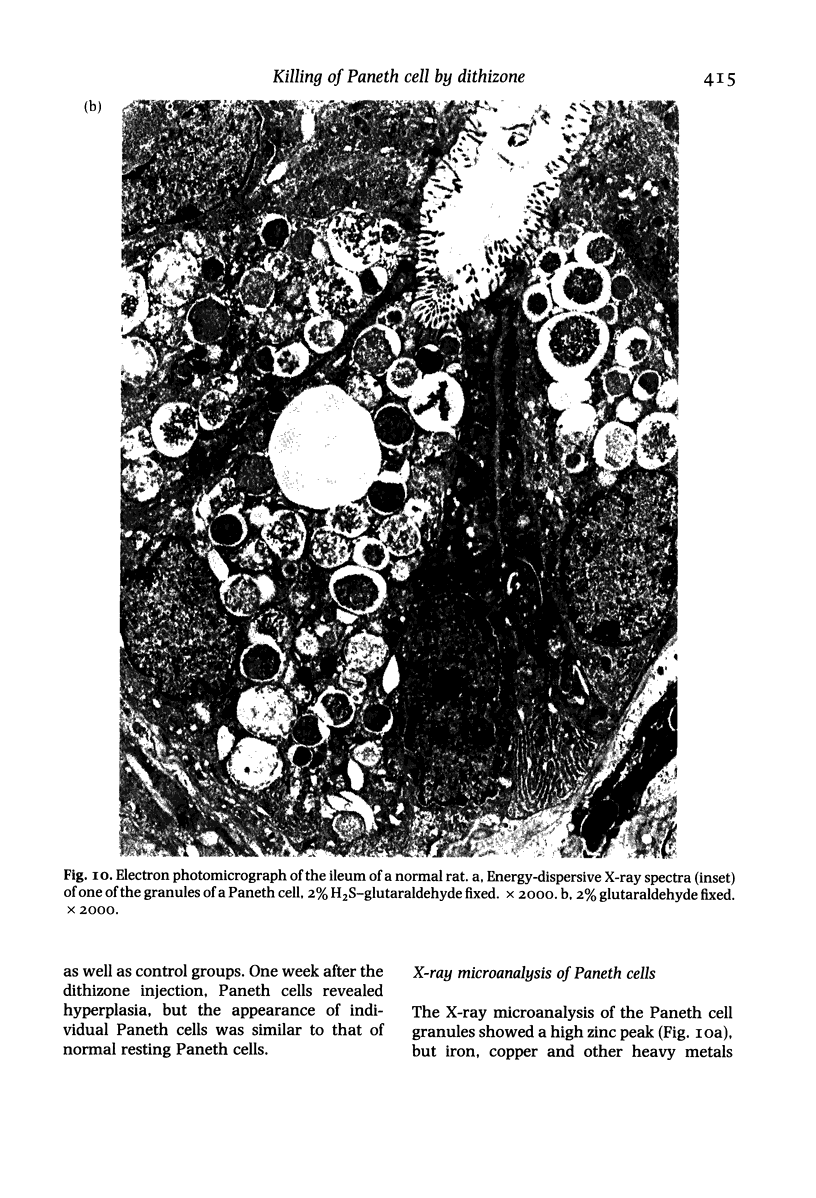
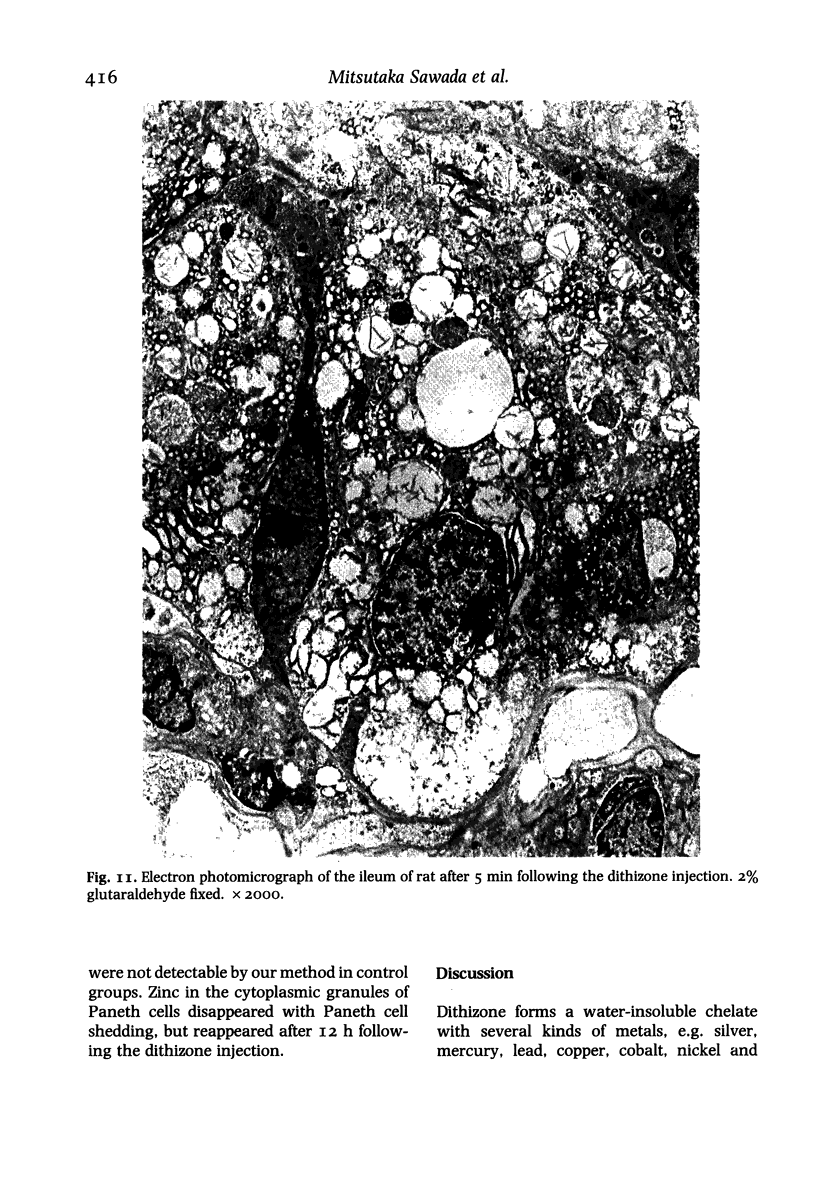
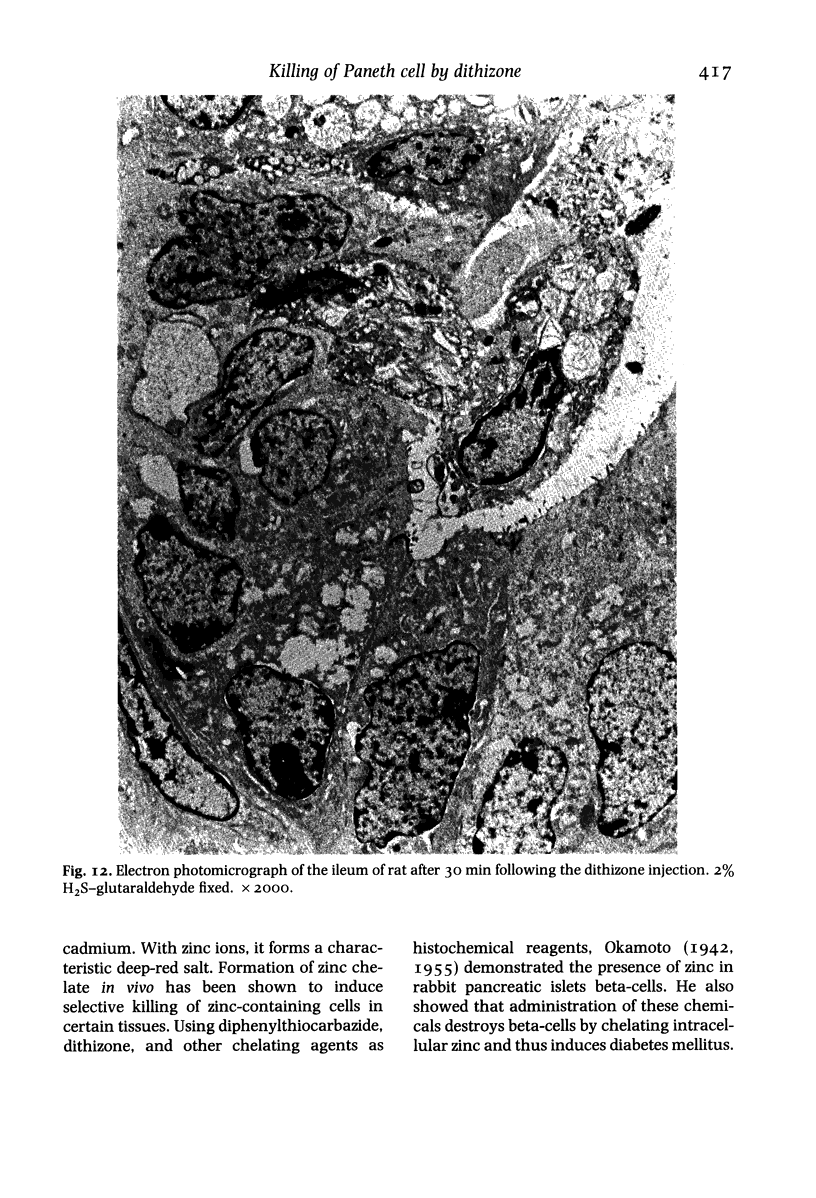
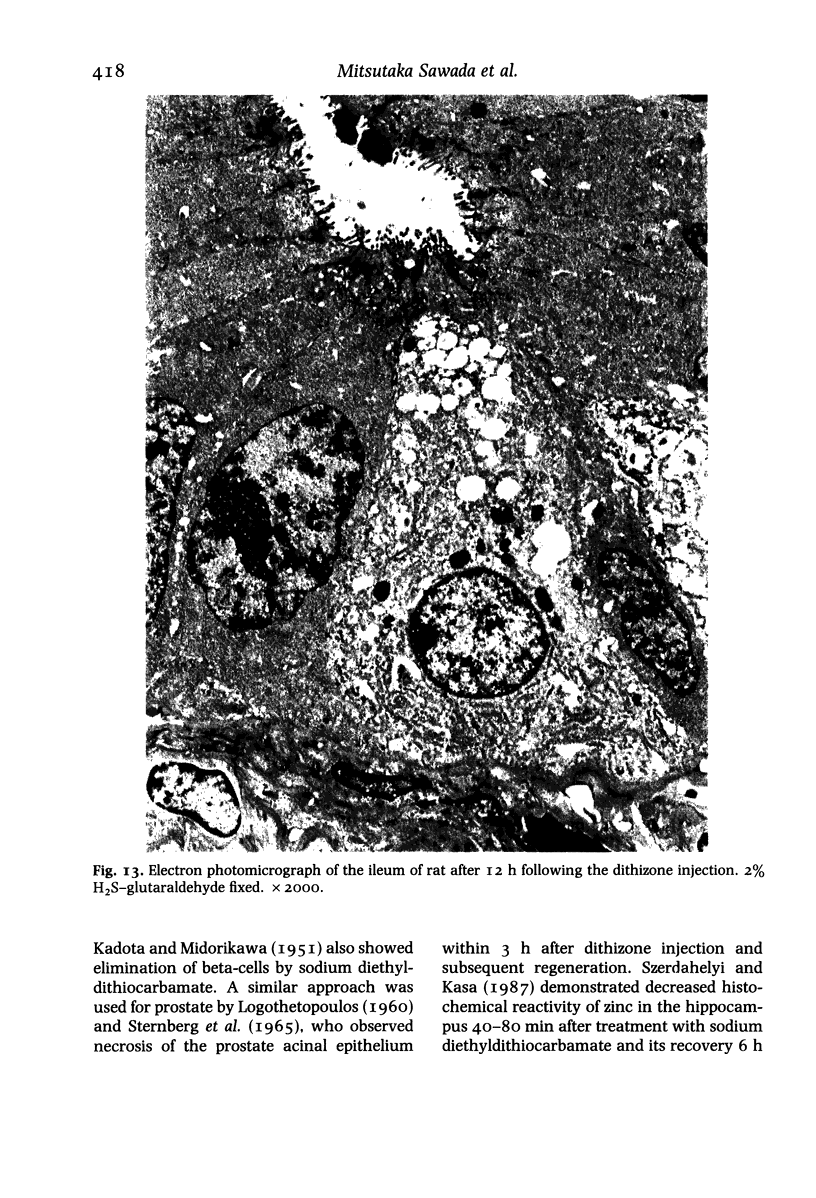
Paneth cells are zinc-containing cells widely distributed in Lieberkühn's crypts of intestine in a variety of species. We found that rapid selective killing of Paneth cells took place after the intravenous (i.v.) injection of diphenylthiocarbazone (dithizone), a chelator forming a zinc dithizonate complex, in the rat. As soon as 5 min after the i.v. injection of dithizone, degeneration of Paneth cells occurred. At this stage, zinc dithizonate complexes were observed as purple-red granules in bright field microscopy. Thirty to 60 min later, Paneth cells were detached from the basement membrane and shed into the cryptic lumen. After 6 h, the cell debris in the crypts was no longer seen and the crypts once housing Paneth cells were now occupied by neighbouring crypt base columnar cells. Histochemically demonstrable zinc totally disappeared. After 12-24 h, however, definite Paneth cells began to resume. Histochemical staining for zinc was again positive at the apex of these cells. One week after dithizone administration, the number of Paneth cells increased twice as much as in uninjected control and histochemical staining for zinc was highly positive. After 2 weeks, Paneth cell hyperplasia subsided. X-ray microanalysis revealed that zinc was the most abundant metal in Paneth cells. We concluded that chelation of zinc and formation of zinc-dithizone complexes in Paneth cells' cytoplasm would be responsible for the selective degeneration observed after dithizone administration.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dinsdale D., Biles B. Postnatal changes in the distribution and elemental composition of Paneth cells in normal and corticosteroid-treated rats. Cell Tissue Res. 1986;246(1):183–187. doi: 10.1007/BF00219016. [DOI] [PubMed] [Google Scholar]
- Dinsdale D. Ultrastructural localization of zinc and calcium within the granules of rat Paneth cells. J Histochem Cytochem. 1984 Feb;32(2):139–145. doi: 10.1177/32.2.6693753. [DOI] [PubMed] [Google Scholar]
- Hertzog A. J. The Paneth Cell. Am J Pathol. 1937 May;13(3):351–360.1. [PMC free article] [PubMed] [Google Scholar]
- MIDORIKAWA O., EDER M. [Comparative histochemical studies on zinc in the intestine]. Z Zellforch Microsk Anat Histochem. 1962;2:444–472. doi: 10.1007/BF00736501. [DOI] [PubMed] [Google Scholar]
- Mathan M., Hughes J., Whitehead R. The morphogenesis of the human Paneth cell. An immunocytochemical ultrastructural study. Histochemistry. 1987;87(1):91–96. doi: 10.1007/BF00518730. [DOI] [PubMed] [Google Scholar]
- Mottet N. K., Body R. L. Primate paneth cell degeneration following methylmercury hydroxide ingestion. Am J Pathol. 1976 Jul;84(1):93–110. [PMC free article] [PubMed] [Google Scholar]
- SPEECE A. J. HISTOCHEMICAL DISTRIBUTION OF LYSOZYME ACTIVITY IN ORGANS OF NORMAL MICE AND RADIATION CHIMERAS. J Histochem Cytochem. 1964 May;12:384–391. doi: 10.1177/12.5.384. [DOI] [PubMed] [Google Scholar]
- STERNBERG S. S., CRONIN A., PHILIPS F. S. HISTOCHEMICAL DEMONSTRATION OF ZINC IN THE DORSOLATERAL PROSTATE OF THE RAT: STUDIES WITH OXINE AND DITHIZONE. Am J Pathol. 1965 Aug;47:325–337. [PMC free article] [PubMed] [Google Scholar]
- Sandow M. J., Whitehead R. The Paneth cell. Gut. 1979 May;20(5):420–431. doi: 10.1136/gut.20.5.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szerdahelyi P., Kása P. Partial depletion and altered distribution of synaptic zinc in the rat hippocampus after treatment with sodium diethyldithiocarbamate. Brain Res. 1987 Oct 6;422(2):287–294. doi: 10.1016/0006-8993(87)90935-8. [DOI] [PubMed] [Google Scholar]
- TIMM F. Zur Histochemie des Zinks. Dtsch Z Gesamte Gerichtl Med. 1958;47(3):428–431. [PubMed] [Google Scholar]